Abstract
2 + 2 Photocycloadditions are idealized, convergent construction approaches of 4-membered heterocyclic rings, including azetidines. However, methods of direct excitation are limited by the unfavorable photophysical properties of imines and electronically unbiased alkenes. Here, we report copper-catalyzed photocycloadditions of non-conjugated imines and alkenes to produce a variety of substituted azetidines. Design principles allow this base metal-catalyzed method to achieve 2 + 2 imine-olefin photocycloaddition via selective alkene activation through a coordination-MLCT pathway supported by combined experimental and computational mechanistic studies.
Similar content being viewed by others
Introduction
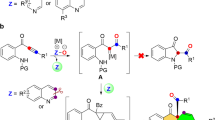
Nitrogen heterocycles are prevalent motifs in small molecule pharmaceuticals and their rapid construction continues to drive new reaction development1. While 3-, 5- and 6-membered N-heterocycles are exceptionally common, made possible by a variety of synthetically accessible routes for their construction, 4-membered heterocycles are scarce by comparison despite the promising biological activity displayed by many azetidine-containing compounds2,3,4,5,6,7. The 2 + 2 photocycloaddition of two π-components is a prototypical approach to 4-membered ring construction. While limited in generality, the Paternò-Büchi reaction forms oxetanes through direct irradiation of a carbonyl C = O double bond followed by C = C double bond capture8,9,10. Aza-Paternò-Büchi11 analogs using imines in place of aldehydes or ketones do not generally result in the formation of analogous azetidines due in part to the low barrier E/Z isomerization available upon excitation of the imine C = N double bond12. Maruoka reported a rare intermolecular azetidine-forming 2 + 2 photocycloaddition example using naphthaldehyde derived N-sulfonyl aldimines and styrene or benzofuran (Fig. 1a)13. However, this reactivity was exclusive to these substrate pairs, enabled by a π-stacking association of the substrates prior to excitation. Photosensitization of cyclic C = N double bonds which cannot undergo E/Z isomerization is one successful strategy for azetidine formation14,15,16. A recent report from Schindler and co-workers used fac-Ir(dFppy)3 (fac = facial; dFppy = 2-(2,4-difluorophenyl)-pyridine) and blue light irradiation as a photocatalyst system to selectively activate the C = N double bond of cyclic glyoxylate imines which coupled to a wide array of alkenes (Fig. 1b)16. Preferential excitation of the alkene component resulted in recent advancements in intramolecular azetidine-forming 2 + 2 photocycloadditions. Sivaguru and co-workers developed an enantioselective xanthone-mediated 2 + 2 photocycloaddition of atropisomeric enamides tethered to imines (Fig. 1c)17. Schindler and co-workers also developed an intramolecular process using [Ir[dF(CF3)ppy]2(dtbbpy)](PF6) (dF(CF3)ppy = 2-(2,4-difluorophenyl)-3-trifluoromethylpyridine; dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine) as a photosensitizer that selectively activated vinyl arenes, leading to fused azetidine products (Fig. 1d)18. Despite these substrate-controlled advances, catalyst-controlled approaches to 2 + 2 imine-olefin photocycloaddition (IOPC) remain limited as general strategies for azetidine construction.
Transition metal compounds capable of photoexcitation via metal to ligand charge transfer (MLCT) to achieve intermolecular electron or energy transfer have gained significant recognition for their use in chemical synthesis19,20,21,22. While the majority of these reports use photocatalysts that access the needed excited states in the absence of any substrate, the formation of the active photocatalytic species in situ through selective substrate engagement is a comparatively underdeveloped approach. Seminal contributions from Salomon, Kochi, and co-workers identified that cyclobutane formation from alkene photodimerization was significantly improved by the addition of Cu(I)-trifluoromethanesulfonate (CuOTf) due to the in situ formation of a Cu(I)-olefin compound serving as the active chromophore23. Meggers and co-workers reported photochemical cyclobutane synthesis by in situ formation of the active chromophore as a chiral at metal, O-bound Rh-enone compound24. We recently reported a Cu-catalyzed 2 + 2 carbonyl-olefin photocycloaddition (COPC) approach for oxetane formation by inverting conventional Paternò-Büchi reactivity and achieved alkene activation via Cu(I)-coordination followed by MLCT25.
In this work we show that azetidines may be accessed by substrate coordination and selective activation via MLCT with the alkene π-component in a 2 + 2 imine-olefin photocycloaddition (Fig. 1e).
Results
Reaction optimization
We irradiated a 1.1:1 mixture of N-butyl-2-methylpropan-1-imine (1) and norbornene with catalytic hydrotris(pyrazolyl)borate copper(I) (TpCu) using a 100 W Hg lamp for 24 h resulting in formation of the corresponding azetidine 1A in 55% yield with exclusive exo selectivity (Table 1, entry 1). Removing either TpCu or light resulted in no formation of 1A (entries 2 and 3). Using a threefold excess of 1, produced 1A in 79% yield (entry 4). Application of a 300 nm cut-on filter resulted in a significant decrease in the yield of 1A to 39% even after a prolonged 36 h reaction time suggesting that product forming irradiation occurred at wavelengths shorter than 300 nm (entry 5). Using CuCl or CuOTf in place of TpCu did not result in any detectable quantities of 1A (entries 6 and 7), underscoring the importance of the coordination environment of the Cu(I) ion source.
Reaction scope
In exploration of imines that undergo Cu-catalyzed 2 + 2 IOPC with norbornene, we found that aldimines from N-allyl amine condensation with isobutyraldehyde, 3-(methylthio)propanal, or melonal were all successfully converted to their corresponding azetidines, 2, 3, and 4, respectively, in good to excellent yields (Fig. 2). Imines bearing cyclopropyl groups participated in this 2 + 2 IOPC to produce azetidines exclusively with no detection of cyclopropyl ring-opened products (5 and 6)26, 27. While imines derived from acetophenone, benzaldehyde, or aniline did not participate in this 2 + 2 IOPC, N-phenethylamine isobutyraldimine, produced azetidine 7, suggesting that arenes are tolerated when not conjugated to the imine C = N double bond. Heterocycles including tetrahydrofuran (8), thiophene (9), and morpholine (10) as well as an acyclic 3° amine (12) were all well incorporated in this process. Productive 2 + 2 IOPC was particularly notable in the presence of a γ-lactam (11), showcasing the chemoselectivity of olefin activation even in the presence of other potentially competitive chromophores. Imines derived from ketones also reacted efficiently to produce the corresponding azetidines even when the imine also contained Lewis-basic 3° amine (13), acetal (14), or ether (15) functionalities.
Summary of imine and alkene substrate variation. All reactions carried out with 1 equiv alkene, 1.1 equiv imine, 20 mol % TpCu, in diethyl ether (0.15 M) and irradiated with a 100 W Hg lamp in borosilicate tubes; all yields are of isolated material following purification via basic alumina chromatography and diastereomeric ratios were determined via gas chromatography of crude reaction mixtures; all products detected as >95:5 exo:endo diastereomers. Yield in parentheses obtained in the absence of TpCu. a3 equiv of imine used.
Biologically active azetidines such as penaresidin A, nicotianamine, medicanine, and mugineic acid bear N-alkyl or no substitution, but access to 2° azetidines can be challenging using existing cyclization methods that require N-substituents that are stubborn or not amenable to cleavage. This Cu-catalyzed 2 + 2 IOPC is uniquely capable of engaging N-alkyl imines directly as well as N-substituents that are easily removed. N-Allyl azetidine 13 underwent deallylation to reveal the corresponding 2° azetidine in 82% yield using 2 mol % Pd(PPh3)4 and 2-mercaptobenzoic acid. Alternatively, O-methyl oxime-derived azetidine 19 underwent facile reductive N-O bond cleavage using activated Zn dust in aqueous acetic acid to provide the corresponding unsubstituted azetidine in 93% yield.
Diversification of the olefin component allowed norbornene derivatives containing acetyl, vinyl, cyano, and tert-butyltrimethyl silyl ether groups to be successfully coupled with N-allyl cyclopentanimine (Fig. 2, 20–24). The identity of azetidine 23 was unambiguously confirmed through determination of the solid-state structure of its hydroiodic salt using X-ray diffraction (23-HI salt).
While maleic anhydride is known to undergo selective cyclobutane forming 2 + 2 photocycloaddition with ethylene using acetophenone as a photosensitizer28, direct excitation of maleic anhydride with N-allyl cyclopentanimine resulted in the formation of azetidine 25 in 51% yield along with a complex mixture of unidentified byproducts. Subjecting the same reactant pair to irradiation in the presence of 20 mol % TpCu resulted in the exclusive formation of 25 in 98% yield. Similarly, other imines were converted to the corresponding maleic anhydride derived azetidines (26 and 27) and use of TpCu uniformly increased both reaction efficiency and selectivity compared to uncatalyzed reactivity. N-Methyl maleimide was also successfully converted to the corresponding azetidine 28 with N-allyl cyclopentanimine in 49% yield.
Mechanism investigations
We initially hypothesized that 2 + 2 IOPC could occur through activation of either the imine or alkene π-bond. Through a combination of 1H NMR and electronic absorption spectroscopies we observed the requirement of alkene coordination to TpCu for successful azetidine formation. Three olefin adducts of TpCu were successfully prepared and isolated through equimolar mixing experiments (Fig. 3a). TpCu-NB was isolated as a white crystalline solid and the solid-state structure determined by single crystal X-ray diffraction (Fig. 3b). The coordination geometry is best described as pseudo-tetrahedral about the Cu-ion with the carbon-carbon bond distance of the coordinated olefin (1.387 Å) in the range of other Cu(I)-norbornene compounds (1.36-1.39 Å) indicative of a predominating σ-bonding, rather than π-back-bonding, metal-olefin coordination interaction29,30,31,32. Examination of the Cu-N(pyrazolyl) bond distances show that while two were nearly equivalent (Cu-N(2) = 1.999(2), Cu-N(4) = 2.010(2)), the third was significantly longer (Cu-N(6) = 2.274(2)), breaking the C3 symmetry axis of the hydrotris(pyrazolyl)borate chelate.
a Synthesis of hydrotris(pyrazolyl)borate copper olefin compounds. b X-ray structures of the norbornene and maleic anhydride complexes TpCu-NB and TpCu-MA. c Electronic absorption spectra of reaction components (TpCu, MA = maleic anhydride, imine = 1) and transmission spectrum of reaction vessels used. Pink line = stoichiometric mixture of TpCu and maleic anhydride; orange line = stoichiometric mixture of TpCu, maleic anhydride, and 1; gold line = stoichiometric mixture of TpCu and 1; green line = TpCu; light blue line = maleic anhydride; dark blue line = 1. Gray shaded area indicates transmission spectrum of the borosilicate glass reaction vessels used. A.U. = arbitrary units.
The solid-state structure of TpCu-MA shows an analogous coordination geometry to TpCu-NB and while the carbon-carbon bond length of the coordinated maleic anhydride (1.390 Å) is elongated compared to unligated maleic anhydride (1.3032 Å)33, it is significantly shortened compared to the only other structurally characterized Cu(I)-maleic anhydride compound supported by a strongly donating iminophosphanamide chelate (1.49 Å)34. This suggests that despite the electronic disparities between maleic anhydride and norbornene, the bonding in TpCu-MA is analogous to that observed in TpCu-NB. While ligand exchange was observed by 1H NMR spectroscopy from TpCu-NB in the presence of the imines used in our study, isolation of a TpCu-imine coordination compound was unsuccessful likely due to the observed rapid, reversible ligand exchange. However, TpCu-MA did not undergo ligand exchange when subjected to imines of this study. We observed that irradiating equimolar amounts of TpCu-MA, N-allyl cyclopentanimine, and norbornene for 24 h resulted in the exclusive formation of azetidine 25 in 95% yield. This suggests that olefin coordination is necessary for 2 + 2 IOPC to proceed and that the coordinated alkene is selectively incorporated into the cycloadduct produced. We noted throughout our investigations that when additional strained, cyclic alkenes were assayed, azetidine formation was not observed when TpCu-alkene coordination was not detected by 1H NMR.
The transmission spectrum of the borosilicate glass reaction vessels used in this study showed that light below 280 nm is effectively filtered from reaching the reaction mixture (Fig. 3c, gray area) and the electronic absorption spectra of ethereal solutions of most individual 2 + 2 IOPC reaction components showed no distinct absorption features 250–400 nm (Fig. 3c). While neither TpCu (green line), 1 (dark blue line), nor norbornene (not pictured) showed significant absorptions above 250 nm, maleic anhydride did (light blue line), explaining the small amount of non-selective background photocycloaddition observed with this alkene. Conversely, a stoichiometric solution mixture of TpCu and maleic anhydride (pink line) resulted in an electronic absorption spectrum with a distinctive broad feature with a maximum at 263 nm, which is similar to the spectrum observed of TpCu-NB which shows a maximum at 272 nm25, and is nearly identical to the spectrum of a stoichiometric mixture of TpCu, maleic anhydride, and imine 1 (orange line). In combination with the diminished azetidine formation when a 300 nm cut-on filter was applied (Table 1, entry 6), these data support azetidine formation occurring via excitation between 280–300 nm.
A calculated electronic absorption spectrum of TpCu-NB best approximated the experimentally observed spectrum using time-dependent density functional theory (DFT) calculations of at the M06 level of theory with the Tamm-Dancoff approximation. This enabled the construction of qualitative molecular orbital diagrams for TpCu-NB at the observed absorption feature 250–300 nm linked to azetidine formation (see the Supporting Information for additional details). The highest occupied molecular orbitals (HOMO-1) and the lowest unoccupied molecular orbital (LUMO) of TpCu-NB are primarily TpCu-based and olefin(π*)-based, respectively (Fig. 4). This is supported by previous DFT calculations which concluded an MLCT of idealized cationic Cu(I)-ethylene compounds was responsible for cyclobutane forming 2 + 2 photocycloadditions35 and recent optical and X-ray transient absorption spectroscopic insights of Cu(I)-mediated photodimerization of norbornene36. The selective imine C = N capture of this excited state affords the cycloadduct azetidine. Chemoselectivity of azetidine formation over alkene dimerization pathways is attributed to the rapid rates of C-centered radical addition to C = N double bonds37 and preference over alkene addition26.
The proposed 2 + 2 IOPC mechanism commences with olefin substrate coordination to TpCu. Ligand exchange experiments indicate that imine substrates undergo rapid and reversible coordination to TpCu. The active TpCu-olefin chromophore then undergoes a MLCT from computationally visualized TpCu-NB HOMO-1 which is primarily Cu-based to TpCu-NB LUMO which is olefin(π*)-based. Capture by the imine C = N double bond leads to azetidine formation and release of TpCu.
Analogous to our observations in 2 + 2 COPC, luminescence data collected from excitation between 280–300 nm did not indicate linear relationships between TpCu and the concentration of added norbornene or imine 1. However, a quenching relationship was observed with maleic anhydride, indicating its potential as a sensitizing agent in this process. While photosensitization cannot be ruled out for all substrates, the impact of olefin coordination ability in azetidine formation and chemoselectivity suggests that a shared sensitization process in all examples is unlikely to be operative. Stereochemical probes often used to infer 2 + 2 photocycloaddition spin states are not applicable in this 2 + 2 IOPC system as identical stereoisomers would be produced irrespective of the proceeding spin state due to the geometric constraints of the cyclic alkenes used.
Discussion
In conclusion, we report herein an intermolecular 2 + 2 photocycloaddition of aliphatic imines and alkenes to form azetidines. The use of hydrotris(pyrazolyl)borate copper(I) as a pre-catalyst allows for coordination of the olefin component and selective absorption of light 280–300 nm. A combination of solution 1H NMR and electronic absorption spectroscopies along with DFT calculations from solid-state structural data supports a Cu(d) to olefin(π*) MLCT pathway to azetidine formation. This work highlights a catalyst controlled 2 + 2 imine-olefin photocycloaddition strategy that selectively activates π-components at red-shifted wavelengths compared to uncoordinated alkenes to access novel azetidine containing compounds.
Methods
Representative procedure
In a nitrogen filled glovebox a borosilicate culture tube was charged with imine (1.1 equiv), TpCu (0.2 equiv), alkene (1.0 equiv), and diethyl ether (0.15 M), capped then sealed with electrical tape and irradiated with a UVP Blak-Ray B-100A UV lamp in a fume hood. After the indicated time, the reaction mixture was quenched by exposure to air for 15 min, then concentrated under reduced pressure. The residue was taken up in methanol and purified by passage through a portion of basic alumina before solvent removal under reduced pressure to afford pure azetidine compounds.
Data availability
Experimental data as well as 1H and 13C NMR spectra for all new compounds prepared in the course of these studies are provided in the Supplementary Information of this manuscript. The X-ray crystallographic coordinates for compounds 23-HI, TpCu-NB, TpCu-MA have been deposited at the Cambridge Crystallographic Data Centre with the accession codes 1986944 [https://doi.org/10.5517/ccdc.csd.cc24pkzd], 1986945 [https://doi.org/10.5517/ccdc.csd.cc24pl0g], 1986946 [https://doi.org/10.5517/ccdc.csd.cc24pl1h], respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ddcd.cam.ac.uk/data_request/cif. All other data including synthetic procedures, spectroscopic, crystallographic, and computational data are available in the Supplementary Information Files.
References
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Brandi, A., Cicchi, S. & Cordero, F. M. Novel syntheses of azetidines and azetidinones. Chem. Rev. 108, 3988–4035 (2008).
Mehra, V., Lumb, I., Anand, A. & Kumar, V. Recent advances in synthetic facets of immensely reactive azetidines. RSC Adv. 7, 45763–45783 (2017).
Antermite, D., Degennaro, L. & Luisi, R. Recent advances in the chemistry of metallated azetidines. Org. Biomol. Chem. 15, 34–50 (2017).
Behnke, N. E., Lovato, K., Yousufuddin, M. & Kürti, L. Titanium-mediated synthesis of spirocyclic NH-azetidines from oxime ethers. Angew. Chem. Int. Ed. 58, 14219–14223 (2019).
Bondada, L. et al. Azetidines and spiro azetidines as novel P2 units in hepatitis C virus NS3 protease inhibitors. Bioorg. Med. Chem. Lett. 23, 6325–6330 (2013).
Kirichok, A. A. et al. Synthesis of multifunctional spirocyclic azetidines and their application in drug discovery. Chem. Eur. J. 24, 5444–5449 (2018).
Griesbeck, A. G. & Mattay, J. Synthetic Organic Photochemistry (Molecular and Supramolecular Photochemistry) Vol. 12, Ch. 4 (Marcel Dekker, 2005).
Scharf, D. & Korte, F. Photosensibilisierte cyclodimerisierung von norbornene. Tetrahedron Lett. 13, 821–823 (1963).
Arnold, D. R., Hinman, R. L. & Glick, A. H. Chemical properties of the carbonyl n, p state. The photochemical preparation of oxetanes. Tetrahedron Lett. 5, 1425–1430 (1964).
Richardson, A. D., Becker, M. R. & Schindler, C. S. Synthesis of azetidines by aza Paternò-Büchi reactions. Chem. Sci. 11, 7553–7561 (2020).
Padwa, A. Photochemistry of the carbon-nitrogen double-bond. Chem. Rev. 77, 37–68 (1977).
Sakamoto, R., Inada, T., Skaurai, S. & Maruoka, K. [2+2] Photocycloadditions between the carbon-nitrogen double bonds of imines and carbon-carbon double bonds. Org. Lett. 18, 6252–6255 (2016).
Swenton, J. S. & Hyatt, J. A. Photosensitized cycloadditions to 1,3-dimethyl-6-azauracil and 1,3-dimethyl-6-azathymine. Imine linkage unusually reactive toward photocycloaddition. J. Am. Chem. Soc. 96, 4879–4885 (1974).
Howard, K. A. & Koch, T. H. Photochemical reactivity of keto imino ethers. V. (2+2) Photocycloaddition to the carbon-nitrogen double bond of 3-ethoxyisoindolone. J. Am. Chem. Soc. 97, 7288–7298 (1975).
Becker, M. R., Wearing, E. R. & Schindler, C. S. Synthesis of azetidines via visible-light-mediated intermolecular [2+2] photocycloadditions. Nat. Chem. 12, 898–905 (2020).
Kumarasamy, E., Kandappa, S. K., Raghunathan, R., Jockushch, S. & Sivaguru, J. Realizing an aza Paternò-Büchi reaction. Angew. Chem. Int. Ed. 56, 7056–7061 (2017).
Becker, M. R., Richardson, A. D. & Schindler, C. S. Functionalized azetidines via visible light-enabled aza Paternò-Büchi reactions. Nat. Commun. 10, 5095 (2019).
Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic synthesis. J. Org. Chem. 81, 6898–6926 (2016).
McAtee, R. C., McClain, E. J. & Stephenson, C. R. J. Illuminating photoredox catalysis. Trends Chem. 1, 111–125 (2019).
Salomon, R. G. & Kochi, J. K. Copper(I) catalysis in photocycloadditions. I. Norbornene. J. Am. Chem. Soc. 96, 1137–1144 (1974).
Huang, X. et al. Direct visible-light-excited asymmetric lewis acid catalysis of intermolecular [2+2] photocycloadditions. J. Am. Chem. Soc. 139, 9120–9123 (2017).
Flores, D. M. & Schmidt, V. A. Intermolecular 2+2 carbonyl-olefin photocycloadditions enabled by Cu(I)-norbornene MLCT. J. Am. Chem. Soc. 141, 8741–8745 (2019).
Fallis, A. G. & Brinza, I. M. Free radical cyclizations involving nitrogen. Tetrahedron 53, 17543–17594 (1997).
Togo, H. Advanced Free Radical Reactions for Organic Synthesis Ch. 1 (Elsevier, 2004).
Hernvann, F., Rasore, G., Declerck, V. & Aitken, D. J. Stereoselective intermolecular [2+2]-photocycloaddition reactions of maleic anhydride: stereocontrolled and regiocontrolled access to 1,2,3-trifunctionalized cyclobutanes. Org. Biomol. Chem. 12, 8212–8222 (2014).
Xiaohua, B. & Holt, E. M. Copper(I) halide complexation to norbornene:(CuCl)5(norbornene)4: a complex of novel structure. J. Crystallographic Spectroscopic Res. 20, 339–345 (1990).
Michon, C., Medina, F., Capet, F., Roussel, P. & Agbossou-Niedercorn, F. Inter- and intramolecular hydroamination of unactivated alkenes catalyzed by a combination of copper and silver salts: the unveiling of a Brønstedt acid catalysis. Adv. Synth. Catal. 352, 3293–3305 (2010).
Pasquali, M., Floriani, C., Gaetani-Manfredotti, A. & Chiesi-Villa, A. Interaction of an aliphatic carbon-hydrogen bond with copper in a norbornene(diethlenetriamine)copper(I) cation complex. J. Am. Chem. Soc. 100, 4918–4919 (1978).
Straub, B. F., Eisenträger, F. & Hofmann, P. A remarkably stable copper(I) ethylene complex: synthesis, spectroscopy and structure. Chem. Commun. 2507–2508, https://doi.org/10.1039/A907928I (1999).
Marsh, R. E., Ubell, E. & Wilcox, H. E. The crystal structure of maleic anhydride. Acta Cryst. 15, 35–41 (1962).
Straub, B. F., Gruber, I., Rominger, F. & Hofmann, P. Mechansim of copper(I)-catalyzed cyclopropanation: a DFT study calibrated with copper(I) alkene complexes. J. Organomet. Chem. 684, 124–143 (2003).
Budzelaar, P. H. M., Timmermans, P. J. J. A., Mackor, A. & Baerends, E. J. Bonding in the ground state and excited states of copper-alkene complexes. J. Organomet. Chem. 331, 397–407 (1987).
Jayasekara, G. K. et al. Mechanisms of the Cu(I)-catalyzed intermolecular photocycloaddition reaction revealed by optical and X-ray transient absorption spectroscopies. J. Am. Chem. Soc. 143, 19356–19364 (2021).
Friestad, G. K. Addition of carbon-centered radicals to imines and related compounds. Tetrahedron 57, 5461–5496 (2001).
Acknowledgements
This work was supported by start-up funds provided by the University of California San Diego (D.M.F.), National Science Foundation CAREER Award No. 1945463 (V.A.S.), and the American Chemical Society Petroleum Research Fund grant 60135-DNI1 (V.A.S.). We thank Christine Lee and Jacob Hawver-Patcher for preliminary assistance in imine synthesis, the Tor group at UCSD for usage of their UV-vis spectrometer, and Dr. Glen Junor for assistance with DFT calculations.
Author information
Authors and Affiliations
Contributions
D.M.F. and V.A.S. designed the experiments. D.M.F. carried out the synthetic work, analytical characterization, spectroscopic and computational studies. M.L.N. carried out the crystallographic studies and assisted with computational studies. V.A.S. assisted with data analysis and directed the research. V.A.S. prepared this manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Flores, D.M., Neville, M.L. & Schmidt, V.A. Intermolecular 2+2 imine-olefin photocycloadditions enabled by Cu(I)-alkene MLCT. Nat Commun 13, 2764 (2022). https://doi.org/10.1038/s41467-022-30393-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-30393-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.