Abstract
As the simplest alkyne and an abundant chemical feedstock, acetylene is an ideal two-carbon building block. However, in contrast to substituted alkynes, catalytic methods to incorporate acetylene into fine chemicals are quite limited. Herein, we developed a photoredox-catalyzed synthetic protocol for diverse C2-linked molecules via a molecular glue strategy using gaseous acetylene under mild conditions. Initiated by addition of an acyl radical to acetylene, two cascade transformations follow. One involves a double addition for the formation of 1,4-diketones and the other where the intermediate vinyl ketone is intercepted by a radical formed from a heterocycle. In addition to making two new C-C bonds, two C-H bonds are also created in two mechanistically distinct ways: one via a C-H abstraction and the other via protonation. This system offers a reliable and safe way to incorporate gaseous acetylene into fine chemicals and expands the utility of acetylene in organic synthesis.
Similar content being viewed by others
Introduction
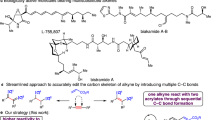
As the simplest alkyne, acetylene has traditionally been viewed more as a fuel than as an economical chemical feedstock, though it is widely used in chemical industry, with an estimated annual goal production of over one million tons1. The high-volume industrial use of acetylene includes the production of vinyl-containing monomers, such as vinyl amine, vinyl chloride, acrylic acid and its derivatives, used for polymeric materials, in other chemical commodities and as feedstocks2,3,4. The spatial orthogonality of the two independent π-systems in alkynes can be used for the discovery, design and control of the new cascade transformations5. However, in contrast to other substituted alkynes, a very limited number of catalytic protocols directly incorporate acetylene into fine chemicals. This is probably due to the greater inherent strength of the π-bonds5, higher activation energies for reactions5 and, especially, apprehensions about handling an explosive6 and flammable gaseous reagent. In fact, reactions exploiting acetylene at atmospheric pressure (1 atm) are uncommon1,2,3,4,7,8,9,10,11,12,13,14. Moreover, the instability of its possible intermediates, the terminal vinyl radical15,16 and, especially, the cation17 has traditionally restricted its conversion. Circumvention of these obstacles would undoubtedly boost the use of acetylene as a reagent in modern organic synthesis involving complex small molecules. Currently, the catalytic transformations of acetylene into fine chemicals are mainly focused on simple monofunctionalizations, such as vinylation2,3,7,8,9,10,13,14,18,19, the Sonogashira coupling reaction20,21, and cyclization2,22. In addition, construction of other molecules with different levels of molecular complexity11,12,21,22,23 is of interest (Fig. 1a). To effectively introduce acetylene in synthesis, competing reactions, such as the generation of more reactive substituted alkynes or alkenes from acetylene, need to be avoided. Despite elegant synthetic developments, effective catalytic strategies to incorporate acetylene gas into fine chemicals remain scarce.
a Present situation of acetylene transformations. b Representative C2-linked molecules with bioactivity. c Quickly assembling functional molecules via a molecular glue strategy with acetylene. d Design for synthesis of C2-linked molecules with acetylene. e Diverse synthesis of C2-linked molecules with acetylene. DCM, Dichloromethane.
A variety of structurally complex and biologically active molecules or their precursors can be regarded as two components linked with a saturated two-carbon unit24,25,26 (Fig. 1b). Ideally, it would be desirable to bridge two or more simple molecules simply through their exposure to a C2-synthon in the presence of a catalyst. This would provide an efficient platform to quickly construct the target molecules and offer molecular diversity via the molecular glue strategy27. As the simplest and abundant unsaturated two-carbon molecular building units, ethylene12,28,29 and acetylene2,3,7,8,9,10,11,12,18,19,20,21,22,23 have been employed as C2 building blocks for the formation of fine chemicals. In fact, due to the presence of two addressable π-systems, acetylene can be used as a carbon glue30, a type of molecular glue, that connects molecules through sequential transformations of the two π-bonds, which mutually complements the ethylene transformation field12,28,29,31,32 (Fig. 1c). Given 1,4-diketones have great importance as versatile intermediates for the synthesis of some bioactive molecules33, natural products and related compounds34,35, along with the ubiquity of bioactive molecules containing a carbonyl group linked to a heterocycle moiety26 by a CH2-CH2 bridge, developing a general methodology to quickly access these compounds via a molecular glue strategy from readily available molecules and abundant feedstock would be highly desirable, especially in drug discovery chemistry36.
Photocatalysis has recently emerged as a powerful platform for the direct functionalization and activation of organic compounds via open-shell pathways under mild conditions37,38,39,40,41,42. As one of the most widely exploited transformations within the realm of open-shell chemistry, the direct addition of carbon radicals to carbon-carbon π-bonds has been broadly leveraged to effect carbon-carbon bond formation with alkenes43,44. Encouraged by these developments, we wondered if it is feasible to utilize the highly reactive terminal vinyl radical, generated through the acyl radical addition to acetylene, to accomplish the C-H abstraction from suitable hydrogen donors, followed by a Giese radical addition45,46,47,48,49, resulting in the formation of 1,4-diketones or connecting the carbonyl group to heterocycles via a two-carbon unit (Fig. 1d). However, the direct addition of most common radicals to unactivated alkynes, especially acetylene, to obtain the terminal vinyl radical, is a really big challenge due to (i) the diminished rate of C-C bond formation owing to increased activation barriers15,50,51 and (ii) the in situ generation of high-energy vinyl radical intermediates that are highly unstable, have a short lifetime, and readily participate in various undesirable open-shell pathways. To the best of our knowledge, direct addition to acetylene producing a terminal vinyl radical followed by functionalization is rarely reported52. In addition, the various radicals formed can react with the acetylene or the newly formed alkene generating undesired products. Importantly, how to selectively generate the desired product is also a big problem. Using the polarity matching effect, a subtle yet important element in radical addition process53, the generated product may be controlled by varying the electronic properties of the hydrogen donors. In addition, side reactions may be limited by quenching the radical intermediate formed by the C-H abstraction from the hydrogen donor.
In this work, we developed a photoredox-catalyzed synthetic protocol for diverse C2-linked molecules via a molecular glue strategy that employed gaseous acetylene under mild conditions (Fig. 1e). Formally, aryl ketones were linked to aryl ketones, or linked with heterocycles by CH2-CH2 bridges, resulting in the formation of two C-C bonds and two C-H bonds. Mechanistic experiments demonstrate that the two C-H bonds are created in two mechanistically distinct ways, one via a C-H abstraction and the other via protonation.
Results and discussion
Reaction development
To start our investigation, the synthetic method for 1,4-diketones was explored with the commercially available α-oxocarboxylic acid 1a as the model substrate, acetylene gas as the C2-linker reagent, K2HPO4 as the base, H2O as the hydrogen source and Ir[dF(CF3)ppy]2(dtbpy)PF6 as the visible-light photocatalyst at room temperature under the irradiation of blue LEDs. From a mechanistic perspective, the highly reactive vinyl radical should abstract a hydrogen atom from a solvent Csp3-H bond because of the Csp2-H’s higher bond dissociation energy (BDE)54,55. To our delight, the reaction in a solution of DCM/H2O (v/v) (3/2) afforded the desired 1,4-diketone 2a in 10% yield (Table 1, entry 1). Examination of a range of photocatalysts revealed that Ir[dF(CF3)ppy]2(phen)PF6 was superior with respect to reaction efficiency, yielding 2a in 16% yield (entries 2-4). Various solvents, such as acetone, N,N-dimethylformamide, acetonitrile, and tetrahydrofuran, were used instead of dichloromethane (entries 5-8) and the results indicated dichloromethane was more suitable for this transformation. The concentration of 1a and the amount of water may affect the reaction efficiency. When 1a was decreased to 0.05 M, 38% isolated yield of 2a was obtained (H2O (5 equiv.), 24 h) (entry 9). Prolonging the reaction time (36 h) did not improve the yield of 2a (entry 10). Further reducing 1a to 0.025 M improved the yield to 51% (entry 11). A slight decrease in the yield was observed when the concentration was further reduced (entry 12). Using other inorganic bases did not positively affect the reaction efficiency (entries 13-15). Gratifyingly, the yield of 2a could be greatly improved to 79% by increasing H2O (20 equiv.) (entry 16). Further increasing the amount of water decreased the yield (entry 17), which means the amount of water has an important effect on the yield.
Reaction scope investigation
With the optimal reaction conditions in hand (Table 1, entry 16), we next investigated the scope of a variety of α-oxocarboxylic acids summarized in Fig. 2. The o-, m- and p-methyl groups on phenyl α-oxocarboxylic acids were tolerated (2b-d). A number of phenyl α-oxocarboxylic acids bearing electron-donating groups, including not only 1°-alkyl groups but also more hindered 2°- and 3°-alkyl groups, were converted into the corresponding 1,4-diketones in good yields (2e-l). The 3,5-dimethyl substituted substrates was transformed into the desired compound in 68% yield (2m). Halogenated phenyl substrates were incorporated into the corresponding compounds with slightly lower yields (43-66%) (2n-q). The phenyl α-oxocarboxylic acid bearing a strong electron-withdrawing group underwent smoothly to afford 2r in 50% yield. Notably, 2-(2,3-dihydro-1H-inden-5-yl)-2-oxoacetic acid 1s and 2-oxo-2-(5,6,7,8- tetrahydronaphthalen-2-yl) acetic acid 1t provided the corresponding 2s and 2t in moderate yields. Importantly, the methoxy-substituted long chain substrate 1u was also suitable for this transformation giving 2u in moderate yield. Moreover, the 2-adamantanol-derived α-oxocarboxylic acid 1v and L-menthol-derived α-oxocarboxylic acid 1w reacted smoothly under the standard conditions to furnish the corresponding desired compounds 2v and 2w in moderate yields. These results show a great potential for the structural modification of an array of complex biological molecules in medicinal chemistry. Notably, various experiments with substrates containing redox non-innocent substituents, such as amino-, phenolic hydroxyl-, vinyl-, alkynyl- and hydroxyl-substituted, were also performed under the standard conditions. Unfortunately, these functional groups were not compatible with our system.
Standard conditions: α-Oxocarboxylic Acid (0.3 mmol), Ir[dF(CF3)ppy]2(Phen)PF6 (0.01 equiv.), H2O (20 equiv.) in a solution of DCM (0.025 M) under the irradiation of blue LEDs under acetylene gas for 24 h at room temperature. Isolated yield of products. dF(CF3)ppy, 3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl; phen, o-Phenanthroline; DCM, Dichloromethane; equiv., equivalent.
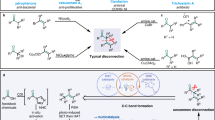
Considering these results, we turned our attention to test other hydrogen donors, specifically nucleophilic hydrogen donors. These donors would generate radical intermediates from C-H abstractions by the highly reactive vinyl radicals. The resulting C-H abstracted radical intermediate reacted well with the electron-deficient alkenes44,45,46,47,48 to furnish the desired compounds containing the carbonyl group and nucleophilic component linked via a CH2-CH2 bridge. In 2016, Knowles56 reported an elegant intermolecular C-H functionalization to construct similar molecules in moderate yields using stoichiometric N-ethyl-4-methoxybenzamide as the abstractor. This abstractor has the potential to serve as a structurally modular catalyst for radical C-H functionalization. Though powerful, the preparation of the vinyl ketone and additional reagents to activate the substrate were required for the reported method. If the vinyl radical generated in our system could be used as the abstractor, it would be highly desirable. Due to the prevalence of furan-containing compounds57,58, THF was subsequently explored as a hydrogen donor and furan source, as well as the solvent. In fact, 13% yield of the tetrahydrofuran linked compound was isolated during the optimization (Table 1, entry 8). A considerable increase in yield was obtained with a slight variation of the reaction conditions (Fig. 3). With respect to the 2-aryl-2-oxocarboxylic acid partner, we observed moderate to good yields of the desired products, which represent an important skeleton in a variety of bioactive molecules11. 2-Oxo-2-phenyl-acetic acids bearing both electron-donating and electron-withdrawing substituents on the phenyl ring are suitable substrates (3a-3ab). Relatively lower yields were observed when an electron-withdrawing group was attached to the phenyl ring (3r-u), which could be ascribed to the reduced reductive quenching ability toward the photoexcited photocatalyst. Notably, 2-aryl-2-oxocarboxylic acids with synthetic handles, such as halides, were readily incorporated into the products (3t-u), which highlights the potential for the incorporation of these scaffolds into more complex targets. Evaluation of substrates containing reactive groups, such as the chemically and biologically abundant amide and ester, provided the corresponding products in moderate yield (3x-3z). The starting material, bearing an easily-oxidized thioether, is also tolerated in this system, furnishing the desired product 3aa in moderate yield. In addition, 2-naphthyl- and 1-fluorene-substituted glyoxylic acids are both suitable substrates, albeit in slightly lower yield (3ac-3ad). The reactive benzylic C-H bonds in the starting material, or in the corresponding product 3ad, remain intact under the reaction conditions. The simple benzoylformic acid was transformed to the corresponding product 3ae in 69% yield. Moreover, the menthol-derived α-oxocarboxylic acid and 2-adamantanol-derived α-oxocarboxylic acid reacted smoothly under the standard conditions to furnish the desired compounds 3af and 3ag in moderate yield. These results show the potential for the structural modification of an array of bioactive molecules.
Standard conditions: α-Oxocarboxylic Acid (0.3 mmol), Ir[dF(CF3)ppy]2(Phen)PF6 (0.01 equiv.), H2O (20 equiv.), MeOH (20 equiv.) in a solution of THF (0.025 M) under the irradiation of blue LEDs under acetylene gas balloon for 24 h at room temperature. Isolated yield of products. dF(CF3)ppy, 3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl; phen, o-Phenanthroline; THF, tetrahydrofuran.
Considering the experimental results above and the requirements for construction of other heterocycle-containing compounds as well as the extension of this approach, some hydrocarbons, especially, heterocycles instead of tetrahydrofuran were subsequently tested (Fig. 4). It was found that this strategy could also be adapted for use with other five-membered heterocycle-based hydrogen donors (Fig. 4a). When substituted tetrahydrofuran was examined, the regioisomers 4 and 4′ were provided in moderate yield with a 4/1 ratio. N-Boc pyrrolidine reacted smoothly to furnish the corresponding compound 5 in 31% yield. Moreover, tetrahydrothiophene provided the desired compound 6 under similar conditions in 30% yield. These results suggest that the weak C-H bonds adjacent to heteroatoms in the five-membered heterocycles could be directly functionalized with acetylene using our system. However, the less reactive cyclopentane (a five-membered carbocycle) could not serve as the hydrogen donor to provide the desired product 7. In addition to five-membered heterocycles, we also examined the feasibility of other heterocycles with different ring sizes (Fig. 4b). The experiments showed that the three-membered cyclic ether, 1,2-epoxypropane, could not be glued with acetylene to the carbonyl group for the formation of 8 or 8′, which might be attributed to the epoxides easily opened ring. To our delight, the four-membered cyclic ether, oxetane, was successfully connected with acetylene, furnishing the desired ketone 9 in 26% yield, accompanied by 19% yield of 2a. As shown in Fig. 3, THF, a five-membered cyclic ether, gave a good result with 69% yield of 3ae. When the ring size was further increased from five to six, the corresponding ketone 10 tethering with tetrahydropyran (six-membered cyclic ether) could be isolated as well, but with a lower yield (17%). However, no desired product 11 could be detected when the seven-membered cyclic ether, oxepane, was used as the substrate. It seems that the larger membered heterocycles (over six members) are not suitable partners. Linear ether (isopropyl ether) failed to provide the desired product 12, which is in line with the trend demonstrated in Fig. 4b. Interestingly, connecting 1,3-dioxolane with the carbonyl group was possible, giving regioisomers 13 and 13′ in a reasonable yield with a 5/1 ratio (Fig. 4c). The observed regioselectivity of H-abstraction in 1,3-dioxolane may be attributed to the anomeric lowering of the C-H BDE59 (BDEα(C-H) = 86.8 Kcal/mol vs BDEβ(C-H) = 88.2 Kcal/mol60). After deprotection, 1,4-ketoaldehyde 14 was obtained efficiently from the crude regioisomers. Similar regioisomers 15 and 15′, which are an ideal precursor of unsymmetric 1,4-diketones, were also obtained by switching from 1,3-dioxolane to 2-methyl-1,3-dioxolane. These outcomes illustrate that unsymmetric 1,4-dicarbonyl compounds could be accessed by using acetylene as a carbon glue, which greatly expands the synthetic scope of 1,4-dicarbonyl compounds. Compared to Knowles’ strategy56, our protocol utilized the vinyl radical generated in situ, to activate the weak Csp3-H bond of heterocycles avoiding the need for the additional abstractor and the preparation of the corresponding vinyl ketones as substrates. Our strategy provides alternative access to this type of compound from abundant feedstock under mild conditions.
a Exploration of different five-membered heterocycles and cyclopentane. 2-Me-THF was used as solvent for 4 and 4’. N-Boc pyrrolidine (5 equivalent) and 1,2-dichloroethane (solvent) were used for 5. Tetrahydrothiophene (5 equivalent) and acetonitrile (solvent) were used for 6. Cyclopentane was used as solvent for 7. dF(CF3)ppy, 3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl; phen, o-Phenanthroline; DCE, 1,2-Dichloroethane; eq, equivalent; 2-Me THF, 2-Methyltetrahydrofuran. b Exploration of molecules with different ring size. All the heterocycles were used as solvent or cosolvent, see supplementary information for details. c Attempt to synthesize unsymmetric 1,4-ketoaldehyde and 1,4-diketone. The heterocycles were used as solvent.
To illustrate the synthetic utility of our strategy, a series of experiments were conducted (Fig. 5). On a preparative scale, 1,4-diketone 2q was isolated in 60% yield, which was slightly higher than that of the 0.3 mmol scale, suggesting that large-scale production might be feasible. Synthesis of enantiomerically enriched Ombitasvir 1933, an orally bioavailable and potent inhibitor, was achieved using chiral 18 which was prepared in 4 steps from 2q via sequential asymmetric reduction, methanesulfonation, nucleophilic cyclization33 and coupling steps (73% yield for 4 steps, dr 5/1) (Fig. 5a). Moreover, simple transformations of the 1,4-dicarbonyl moiety afforded a range of synthetically useful scaffolds (Fig. 5b). For instance, heterocyclic molecules, such as substituted furan 20, substituted thiophene 21 and substituted pyrroles 22-23 were obtained via the cyclization of 2a under acidic condition or in the presence of Lawesson’s reagent, ammonium acetate or benzylamine. The 1,4-dicarbonyl compound was easily transformed to naphthalene-2,3-diylbis(phenylmethanone) 24 in 98% yield under basic conditions with o-phthalaldehyde. Additionally, alkene 25 was accessed through a Wittig reaction with methyltriphenylphosphonium bromide. The derived 1,3-butadiene 26 was also obtained through a sequential reduction and elimination processes. Furthermore, the 1,4-dicarbonyl compound 2a was transformed to cyclobutene 27 in good yield according to the known literature61.
a The 1,4-diketone 2q could be prepared in gram-scale with no decrease in isolated yield. The approved drug Ombitasvir could be efficiently constructed with our strategy. DCM, Dichloromethane. MsCl, Methanesulfonyl chloride. DMF, N,N-Dimethylformanide. TFA, Trifluoroacetic acid. HOBT, 1-Hydroxybenzotriazole hydrate. EDCI, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride. NMM, N-methylmorpholine. b diverse high-valued compounds could be prepared from 1,4-diketone 2a.
Mechanistic studies
To further gain mechanistic insights, control experiments were performed (Fig. 6). In the presence of the radical trap TEMPO, the reaction completely shut down (Fig. 6a, top), indicating that a radical intermediate might be involved in this transformation. The reaction of 1a was performed for 2 h under standard conditions, affording 2a in 24% yield (Fig. 6a, bottom). Additionally, no change in the yield of 2a was observed when the same reaction was conducted for 2 h and then for an additional 22 h without light (Fig. 6a, bottom). These experiments indicate that the reaction is a visible-light photocatalysis process. Considering benzaldehyde was detected as a by-product during the reaction condition optimizations, we explored the reaction of 1d and benzaldehyde under the standard conditions (Fig. 6b, top) to verify whether benzaldehyde was the reaction intermediate. No cross-over product 28 was observed, strongly suggesting that benzaldehyde is not likely an intermediate of this transformation. According to our initial assumption and the inherent reactivity of the vinyl radical, direct abstraction of a hydrogen atom from hydrogen donors to produce the vinyl phenyl ketone is reasonable. As a result, the reaction of stoichiometric vinyl phenyl ketone 29 and 2-(4-methylphenyl)-2-oxoacetic acid 1d was performed under the standard conditions without acetylene, affording the expected product 28 in 26% yield (Fig. 6b, bottom). The result showed that the corresponding vinyl phenyl ketone is the key intermediate in our system. To get further insight into the hydrogen source, chloroform-d was used as the solvent instead of dichloromethane in the reaction of vinyl phenyl ketone 29 and 2-(4-methylphenyl)-2-oxoacetic acid 1d (Fig. 6c, top) and furnished 28 in 64% yield with 0% D-incorporation in the α-position of the carbonyl group according to 1H NMR analysis. In sharp contrast to this phenomenon, > 90% D was incorporated into the molecule 30a when dichloromethane-d2 was used in the model reaction (Fig. 6c, entry 1), indicating one of the hydrogens next to the carbonyl group comes from the solvent. When the corresponding potassium salt of benzoylformic acid 1aa was tested under the standard conditions using dichloromethane-d2 as the solvent and D2O as an additive, the corresponding product 31a bearing > 90% D-incorporation in each methylene site was isolated without 30a being detected (Fig. 6c, entry 2). Additionally, > 90% D-incorporation was verified in one of the methylene sites using D2O as the additive (Fig. 6c, entry 3), meaning another hydrogen adjacent to the carbonyl group originates from water. To further exclude that the deuterated product was generated from 2a under standard conditions through H/D exchange with D2O, 2a was subjected to reaction conditions with D2O instead of H2O (Figure 6c, entry 4). Interestingly, no deuterated products 30a or 31a were found with the 96% recovery of 2a. This experiment suggested that the CH2 of 2a cannot be deuterated through H/D exchange with D2O under standard conditions. Taken together, these results demonstrated the water and solvent are both hydrogen sources but used in the different stages of the reaction process. This is further supported by the mechanistic experiments for 3ae (see Supplementary Fig. 6 and Supplementary Fig. 7).
a Radical trapping and light-on-off experiment were performed, suggesting this transformation might proceed via a radical pathway. TEMPO, 2,2,6,6-tetramethylpiperidinooxy. b Exploration of intermediate involved. n.d., not detected. c Exploration of hydrogen source. Both the solvent and water are the hydrogen source of this transformation. DCM, Dichloromethane.
Mechanistic proposal
Based on the previously reported literature54,55,62,63,64,65,66 and the aforementioned mechanistic studies, a plausible mechanism for this transformation is proposed in Fig. 7. Upon irradiation with visible light, the photocatalyst Ir[dF(CF3)ppy]2(phen)PF6 I is known to access the highly oxidizing excited state (ES) species II ([Ir3+]*), Which could be reductively quenched by the anion of α-oxocarboxylic acid 1 to generate an acyl radical 3262,65 through a decarboxylative pathway, and the reduced species III. The generated 32 could be captured by acetylene gas to afford vinyl radical species 33 which is highly unstable and quickly abstracts a hydrogen from dichloromethane to provide the electron-deficient vinyl ketone 34 accompanied by the generation of 35. This proposed process is in line with the fact that the BDE of the Csp2-H bond (~110 kcal/mol)54,55 exceeds that of Csp3-H bond of dichloromethane (~95 Kcal/mol)66. The reduced species III could be oxidized to regenerate the photocatalyst I by 35, furnishing the carbon anion 36 which could be protonated to regenerate dichloromethane. Subsequently a Giese radical addition reaction occurs with another molecule of acyl radical 32, which adds quickly to alkene 34 to produce a new carbon radical 37. Then a single-electron reduction of radical 37 by III affords the carbon anion 38 and regenerates the photocatalyst I. The desired product is obtained after protonation of 38. The phenomenon that nearly no electron-deficient alkene 34 was observed could be attributed to the fact that 34 is more highly reactive than the parent acetylene. Similarly, vinyl radical species 33 could also abstract hydrogen from heterocycles bearing weaker C-H bonds, affording alkene 34 and nucleophilic radical intermediate 39 which is rapidly captured by 34 to produce 40. Then the sequential single electron transfer (SET)/protonation process occurs to provide the final product 3. It is noteworthy that dichloromethane functions as solvent, as hydrogen donor, and as oxidant precursor to facilitate the regeneration of the reduced photocatalyst. In addition to serving as hydrogen donors and solvents (special conditions), heterocycles also work as the nucleophilic components due to the higher nucleophilicity of their corresponding radicals over that of 35.
The catalytic cycle begins with oxidative single electron transfer (SET), generating the acyl radical 32. The intermediate then undergoes addition to acetylene to generate vinyl radical 33. Intermolecular hydrogen atom transfer (HAT) then occurs to afford aryl vinyl ketone 34 accompanied by the generation of 35 or 39. At last, the Giese radical reaction between aryl vinyl ketone and another acyl radical 32 or 39 occur to furnish the final product.
In summary, a diverse synthesis protocol for C2-linked functionalized molecules with gaseous acetylene was developed, which provided an efficient method to quickly access a variety of compounds through connecting two components together via a molecular glue strategy with readily available substrates and abundant acetylene gas under mild conditions. A series of 1,4-diketones, which are important precursors of an array of heterocycles, were quickly constructed. Importantly, the reaction system was expanded to construct heterocycle-containing compounds. These compounds form when the intermediate vinyl ketone is intercepted by a radical intermediate formed from a heterocycle C-H abstraction. In both of these transformations, acetylene is incorporated in the final product as a CH2-CH2 bridge. Additionally, mechanistic studies demonstrated that one of the formed C-H bonds is created via a C-H abstraction from the hydrogen donor, such as dichloromethane or a heterocycle, and the other via protonation from water or methanol. Moreover, this approach provides ready access to interesting, functionalized molecules and expands the utility of acetylene in organic synthesis, which will inspire new perspectives for value-added chemical synthesis using acetylene and promote the renaissance of catalytic transformations for acetylene.
Methods
Materials
Unless otherwise noted, all the materials were obtained commercially and used without further purification. All the solvents were treated according to general methods. Flash column chromatography was performed over silica gel (300-400 mesh). See Supplementary Methods for experimental details.
General procedure A for the synthesis of 1,4-dicarbonyl compounds
To an oven-dried 25 mL flask, Ir[dF(CF3)ppy]2(phen)PF6 (0.003 mmol), 2-aryl-2-oxocarboxylic acid (0.3 mmol), K2HPO4 (0.36 mmol), H2O (20 equiv.) and DCM (12 mL) were added sequentially under N2. The flask was degassed through three freeze-pump-thaw cycles under acetylene and then an acetylene gas balloon was attached through a long syringe needle. The reaction mixture was irradiated by 12 W blue LEDs at a distance of 5 cm for 24 h at room temperature. The reaction mixture was filtered through a short pad of silica using ethyl acetate. The filtrate was concentrated in vacuo before it was purified by flash chromatography on silica gel to afford the desired product.
General procedure B for the synthesis of heterocycle-containing compounds
To an oven-dried 25 mL flask, Ir[dF(CF3)ppy]2(phen)PF6 (0.003 mmol), 2-aryl-2-oxocarboxylic acid (0.3 mmol), K2HPO4 (0.36 mmol), H2O/MeOH (1/1, 40 equiv.) and THF (12 mL) were added sequentially under N2. The flask was degassed through three freeze-pump-thaw cycles under acetylene and then an acetylene gas balloon was attached through a long syringe needle. The reaction mixture was irradiated by 12 W blue LEDs at a distance of 5 cm for 24 h at room temperature. The reaction mixture was filtered through a short pad of silica using ethyl acetate. The filtrate was concentrated in vacuo before it was purified by flash chromatography on silica gel to afford the desired product.
Data availability
The authors declare that data relating to the characterization of materials and products, general methods, optimization studies, experimental procedures, mechanistic studies, HRMS data and NMR spectra are available within the article and the Supplementary Information.
References
Doerksen, R. S., Meyer, C. C. & Krische, M. J. Feedstock Reagents in Metal-Catalyzed Carbonyl Reductive Coupling: Minimizing Preactivation for Efficiency in Target-Oriented Synthesis. Angew. Chem. Int. Ed. 58, 14055–14064 (2019).
Trotus, I.-T., Zimmermann, T. & Schüth, F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 114, 1761–1782 (2014).
Voronin, V. V., Ledovskaya, M. S., Bogachenkov, A. S., Rodygin, K. S. & Ananikov, V. P. Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules 23, 2442–2524 (2018).
Ledovskaya, M., Voronin, V., Rodygin, K. & Ananikov, V. Acetylene and ethylene-universal C2 molecular units in cycloaddition reactions. Synthesis 54, 999–1042 (2022).
Alabugin, I. V. & Gold, B. Two functional groups in one package: using both alkyne π-bonds in cascade transformations. J. Org. Chem. 78, 7777–7784 (2013).
U.S. Chemical Safety and Hazard Investigation Board (CSB), Safety Bulletin No. 2005-03-B, January 2006.
Kong, J. R. & Krische, M. J. Catalytic Carbonyl Z-Dienylation via Multicomponent Reductive Coupling of Acetylene to Aldehydes and α-Ketoesters Mediated by Hydrogen: Carbonyl Insertion into Cationic Rhodacyclopentadienes. J. Am. Chem. Soc. 128, 16040–16041 (2006).
Skucas, E., Ngai, M.-Y., Komanduri, V. & Krische, M. J. Enantiomerically Enriched Allylic Alcohols and Allylic Amines via C–C Bond-Forming Hydrogenation: Asymmetric Carbonyl and Imine Vinylation. Acc. Chem. Res. 40, 1394–1401 (2007).
Han, S. B., Kong, J. R. & Krische, M. J. Catalyst-Directed Diastereoselectivity in Hydrogenative Couplings of Acetylene to α-Chiral Aldehydes: Formal Synthesis of All Eight l-Hexoses. Org. Lett. 10, 4133–4135 (2008).
Wang, Z., Lu, X., Lei, A. & Zhang, Z. Efficient Preparation of Functionalized (E,Z) Dienes Using Acetylene as the Building Block. J. Org. Chem. 63, 3806–3807 (1998).
Scharnagel, D. et al. Acetylene as a Dicarbene Equivalent for Gold(I) Catalysis: Total Synthesis of Waitziacuminone in One Step. Angew. Chem. Int. Ed. 59, 4888–4891 (2020).
Tang, S., Wang, D., Liu, Y., Zeng, L. & Lei, A. Cobalt-catalyzed electrooxidative C-H/N-H [4+2] annulation with ethylene or ethyne. Nat. Commun. 9, 798–804 (2018).
Chen, J.-F. & Li, C. Cobalt-catalyzed gem-cross-dimerization of terminal alkynes. Chem. Sci. 10, 3881–3889 (2020).
Härterich, M. et al. Hybrid inorganic-organic cross-metathesis between diborenes and acetylene. J. Am. Chem. Soc. 143, 18339–18345 (2021).
Wille, U. Radical cascades initiated by intermolecular radical addition to alkynes and related triple bond systems. Chem. Rev. 113, 813–853 (2013).
Alabugin, I. V. et al. In search of efficient 5-endo-dig cyclization of a carbon-centered radical: 40 years from a prediction to another success for the baldwin rules. J. Am. Chem. Soc. 130, 10984–10995 (2008).
Vasilyev, A. V. Electrophilic activation of acetylene compounds in Brønsted superacids. Reactions of vinyl type cations. Russ. Chem. Rev. 82, 187 (2013).
Williams, V. M. et al. J. Am. Chem. Soc. 131, 16054–16062 (2009).
Hou, J., Xie, J.-H. & Zhou, Q.-L. Palladium-Catalyzed Hydrocarboxylation of Alkynes with Formic Acid. Angew. Chem. Int. Ed. 54, 6302–6305 (2015).
Xue, F. et al. Reaction discovery using acetylene gas as the chemical feedstock accelerated by the “stop-flow” micro-tubing reactor system. Chem. Sci. 8, 3623–3627 (2017).
Kozhemyakin, Y. et al. Synthesis and Properties of Functional Twisted Tolanes. Chem. Eur. J. 23, 9908–9918 (2017).
Biinnemann, H. Cobalt-Catalyzed Pyridine Syntheses from Alkynes and Nitriles. Angew. Chem. Int. Ed. Engl. 17, 505–515 (1978).
Yu, D., Sum, Y. N., Ean, A. C. C., Chin, M. P. & Zhang, Y. Acetylide Ion (C22−) as a Synthon To Link Electrophiles and Nucleophiles: A Simple Method for Enaminone Synthesis. Angew. Chem. Int. Ed. 52, 5125–5128 (2013).
Bolli, M. H. et al. The Discovery of N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N′-propylsulfamide (Macitentan), an Orally Active, Potent Dual Endothelin Receptor Antagonist. J. Med. Chem. 55, 7849–7861 (2012).
Ágai-Csongor, É. et al. Discovery of cariprazine (RGH-188): A novel antipsychotic acting on dopamine D3/D2 receptors. Bioorg. Med. Chem. Lett. 22, 3437–3440 (2012).
Ikejiri, M., Ohshima, T., Fukushima, A., Shimotohno, K. & Maruyama, T. Synthesis and evaluation of 5′-modified 2′-deoxyadenosine analogues as anti-hepatitis C virus agents. Bioorg. Med. Chem. Lett. 18, 4638–4641 (2008).
Wang, P. et al. Molecular glue strategy: Large-scale conversion of clustering-induced emission luminogen to carbon dots. ACS Appl. Mater. Interfaces 11, 19301–19307 (2019).
McLain, S. J., Wood, C. D. & Schrock, R. R. Preparation and characterization of tantalum(III) olefin complexes and tantalum(V) metallacyclopentane complexes made from acyclic α olefins. J. Am. Chem. Soc. 101, 4558–4570 (1979).
Pagar, V. V. & RajanBabu, T. V. Tandem catalysis for asymmetric coupling of ethylene and enynes to functionalized cyclobutanes. Science 361, 68–72 (2018).
Mohamed, R. K. et al. Alkynes as linchpins for the additive annulation of biphenyls: Convergent construction of functionalized fused helicenes. Angew. Chem. Int. Ed. 55, 12054–12058 (2016).
Saini, V., Stokes, B. J. & Sigman, M. S. Transition-metal-catalyzed laboratory-scale carbon-carbon bond-forming reactions of ethylene. Angew. Chem. Int. Ed. 52, 11206–11220 (2013).
Pospech, J. et al. Alternative metals for homogeneous catalyzed hydroformylation reactions. Angew. Chem. Int. Ed. 52, 2852–2872 (2013).
Wagner, R. et al. Highlights of the Structure–Activity Relationships of Benzimidazole Linked Pyrrolidines Leading to the Discovery of the Hepatitis C Virus NS5A Inhibitor Pibrentasvir (ABT-530). J. Med. Chem. 61, 4052–4066 (2018).
Oshima, K., Yamamoto, H. & Nozaki, H. A new route to γ-ketoaldehydes. Application to the synthesis of cis-Jasmone. J. Am. Chem. Soc. 95, 4446–4447 (1973).
Ellison, R. A. Methods for the synthesis of 3-Oxocyclopentenes. Synthesis 7, 397–412 (1973).
Schreiber, S. L. Target-Oriented and Diversity-Oriented Organic Synthesis in Drug Discovery. Science 287, 1964–1969 (2000).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 113, 5322–5363 (2013).
Romero, N. A. & Nicewicz, D. A. Organic Photoredox Catalysis. Chem. Rev. 116, 10075–10166 (2016).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Jiang, H. & Studer, A. Chemistry With N-Centered Radicals Generated by Single-Electron Transfer-Oxidation Using Photoredox. Catal. CCS Chem. 1, 38–49 (2019).
Milligan, J. A., Phelan, J. P., Badir, S. O. & Molander, G. A. Alkyl Carbon–Carbon Bond Formation by Nickel/Photoredox Cross-Coupling. Angew. Chem. Int. Ed. 58, 6152–6163 (2019).
Staveness, D., Bosque, I. & Stephenson, C. R. J. Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer. Acc. Chem. Res. 49, 2295–2306 (2016).
Chu, L., Ohta, C., Zuo, Z. & MacMillan, D. W. C. Carboxylic Acids as A Traceless Activation Group for Conjugate Additions: A Three-Step Synthesis of (±)-Pregabalin. J. Am. Chem. Soc. 136, 10886–10889 (2014).
Capacci, A. G., Malinowski, J. T., McAlpine, N. J., Kuhne, J. & MacMillan, D. W. C. Direct, enantioselective α-alkylation of aldehydes using simple olefins. Nat. Chem. 9, 1073–1077 (2017).
Giese, B. & Dupuis, J. Diastereoselective Syntheses of C-Glycopyranosides. Angew. Chem. Int. Ed. Engl. 22, 622–623 (1983).
Giese, B. Formation of C-C Bonds by Addition of Free Radicals to Alkenes. Angew. Chem. Int. Ed. Engl. 22, 753–764 (1983).
Jasperse, C. P., Curran, D. P. & Fevig, T. L. Radical reactions in natural product synthesis. Chem. Rev. 91, 1237–1286 (1991).
Millet, A., Lefebvre, Q. & Rueping, M. Visible-Light Photoredox-Catalyzed Giese Reaction: Decarboxylative Addition of Amino Acid Derived α-Amino Radicals to Electron-Deficient Olefins. Chem. Eur. J. 22, 13464–13468 (2016).
Liu, H. et al. One-Pot Photomediated Giese Reaction/Friedel–Crafts Hydroxyalkylation/Oxidative Aromatization To Access Naphthalene Derivatives from Toluenes and Enones. ACS Catal. 8, 6224–6229 (2018).
Gomez-Balderas, R., Coote, M. L., Henry, D. J., Fischer, H. & Radom, L. What Is the Origin of the Contrathermodynamic Behavior in Methyl Radical Addition to Alkynes versus Alkenes? J. Phys. Chem. A 107, 6082–6090 (2003).
Giese, B. & Lachhein, S. Addition of Alkyl Radicals to Alkynes: Distinction between Radical and Ionic Nucleophiles. Angew. Chem., Int. Ed. Engl. 21, 768–775 (1982).
Gordeev, E. G., Pentsak, E. O. & Ananikov, V. P. Carbocatalytic acetylene cyclotrimerization: a key role of unpaired electron delocalization. J. Am. Chem. Soc. 142, 3784–3796 (2020).
Roberts, B. P. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 28, 25–35 (1999).
Blanksby, S. J. & Ellison, G. B. Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 36, 255–263 (2003).
Xue, X.-S., Ji, P., Zhou, B. & Cheng, J.-P. The Essential Role of Bond Energetics in C-H Activation/Functionalization. Chem. Rev. 117, 8622–8648 (2017).
Choi, G. J., Zhu, Q., Miller, D. C., Gu, C. J. & Knowles, R. R. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature 539, 268–271 (2016).
Sagratini, G. et al. Synthesis and α1-adrenoceptor antagonist activity of derivatives and isosters of the furan portion of (+)-cyclazosin. Bioorg. Med. Chem. 15, 2334–2345 (2007).
Cui, Z.-N. et al. Synthesis and Bioactivities of Novel Pyrazole and Triazole Derivatives Containing 5-Phenyl-2-Furan. Chem. Biol. Drug Des. 79, 121–127 (2012).
Alabugin, I. V. et al. Stereoelectronic power of oxygen in control of chemical reactivity: the anomeric effect is not alone. Chem. Soc. Rev. 50, 10253–10345 (2021).
Nielsen, M. K. et al. Mild, Redox-Neutral Formylation of Aryl Chlorides via Photocatalytic Generation of Chlorine Radicals. Angew. Chem. Int. Ed. 56, 7191–7194 (2017).
Toda, F. et al. A new preparative method for (E)-2,3-diarylbut-2-enedials. J. Chem. Soc. Chem. Commun. 1234–1235 (1984).
Wang, G.-Z., Shang, R., Cheng, W.-M. & Fu, Y. Decarboxylative 1,4-Addition of α-Oxocarboxylic Acids with Michael Acceptors Enabled by Photoredox Catalysis. Org. Lett. 17, 4830–4833 (2015).
Vu, M. D., Das, M. & Liu, X.-W. Direct Aldehyde Csp2−H Functionalization through Visible-Light-Mediated Photoredox Catalysis. Chem. Eur. J. 23, 15899–15902 (2017).
Raviola, C., Protti, S., Ravelli, D. & Fagnoni, M. Photogenerated acyl/alkoxycarbonyl/carbamoyl radicals for sustainable synthesis. Green. Chem. 21, 748–764 (2019).
Yang, B., Li, S.-J., Wang, Y., Lan, Y. & Zhu, S. Hydrogen radical-shuttle (HRS)-enabled photoredox synthesis of indanones via decarboxylative annulation. Nat. Commun. 12, 5257 (2021).
Salamone, M. et al. Bimodal evans-polanyi relationships in hydrogen atom transfer from C(sp3)-H bonds to the cumyloxyl radical. A combined time-resolved kinetic and computational study. J. Am. Chem. Soc. 143, 11759–11776 (2021).
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (grant number 22001079 for B.Y., grant number 22071062 and 21871096 for S.Z.), Guangdong Science and Technology Department (grant number 2018B030308007 for S.Z.), and the China Postdoctoral Science Foundation (grant number 2020M682694 for B.Y.). We also thank Prof. Gary Histand for his assistance with English revision.
Author information
Authors and Affiliations
Contributions
B.Y. and S.Z. designed the experiments. B.Y. performed experiments. S.L. synthesized the substrates. S.Z. and Y.W. codirected the synthetic applications of this method. B.Y. wrote the paper and S.Z. revised-reviewed & edited the paper. All authors discussed the results and commented on the manuscript. S.Z. directed the whole project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, B., Lu, S., Wang, Y. et al. Diverse synthesis of C2-linked functionalized molecules via molecular glue strategy with acetylene. Nat Commun 13, 1858 (2022). https://doi.org/10.1038/s41467-022-29556-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-29556-2
This article is cited by
-
Bifunctional two-carbon reagent made from acetylene via 1,2-difunctionalization and its applications
Science China Chemistry (2024)
-
Thiol-Yne click chemistry of acetylene-enabled macrocyclization
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.