Abstract
The geochemical cycle of mercury in Earth’s surface environment (atmosphere, hydrosphere, biosphere) has been extensively studied; however, the deep geological cycling of this element is less well known. Here we document distinct mass-independent mercury isotope fractionation (expressed as Δ199Hg) in island arc basalts and mid-ocean ridge basalts. Both rock groups show positive Δ199Hg values up to 0.34‰ and 0.22‰, respectively, which deviate from recent estimates of the primitive mantle (Δ199Hg: 0.00 ± 0.10‰, 2 SD)1. The positive Δ199Hg values indicate recycling of marine Hg into the asthenospheric mantle. Such a crustal Hg isotope signature was not observed in our samples of ocean island basalts and continental flood basalts, but has recently been identified in canonical end-member samples of the deep mantle1, therefore demonstrating that recycling of mercury can affect both the upper and lower mantle. Our study reveals large-scale translithospheric Hg recycling via plate tectonics.
Similar content being viewed by others
Introduction
Mercury (Hg) is a unique heavy metal that has an active redox chemistry, high volatility, strong bioaccumulation, and extreme toxicity2. Both natural and anthropogenic activities emit large amounts of Hg to the atmosphere, mainly in the form of elemental Hg (Hg0)3. Gaseous Hg0 has a lifetime of ~1 year in the atmosphere, allowing its global transport before deposition into terrestrial and oceanic ecosystems via both wet and dry deposition pathways4. Due to its toxicity to humans and wildlife, the geochemical cycle of Hg in Earth’s surface environment (atmosphere, hydrosphere, and biosphere) has been extensively studied2. However, the cycling of Hg in Earth’s interior reservoirs (e.g., crust and mantle) remains less studied1,5.
Mercury ore deposits are predominantly located in active continental margin settings, with two well-known mercuriferous belts—the Pacific rim metallogenic belt and the Alpine-Himalayan belt4, implying a causal link between Hg metallogenesis and plate subduction. Mercury in oceanic reservoirs (e.g., sediments, seawater) may be carried by subducting ocean slabs into the asthenospheric mantle, and can then be emitted during arc magmatism to form Hg-enriched hydrothermal ore deposits in active continental margin settings6. A recent study, based on Hg isotopes in ocean island basalts, suggests that crustal Hg is also recycled into the lower mantle1.
Mercury is the only metal that exhibits both significant isotopic mass-dependent fractionation (MDF, defined as δ202Hg) and mass-independent fractionation (MIF, defined as Δ199Hg, Δ200Hg, Δ201Hg, and Δ204Hg)7,8. Hg-MDF is produced during essentially all biological, chemical, and physical processes involving Hg7. MIF of 200Hg and Δ204Hg is detected mainly in atmospherically derived samples, and values in rocks are generally too small to enable source tracing8. MIF of 199Hg and 201Hg mainly occurs during photochemical processes with little contribution from other reactions, and therefore provides clear source constraints7. On Earth’s surface, photochemical processes result in negative Δ199Hg (−0.6 to 0‰) in terrestrial reservoirs (e.g., soil and vegetation) and positive Δ199Hg (0 to 0.4‰) in oceanic reservoirs (e.g., seawater and marine sediments)7,8. It has been estimated that the primitive mantle has near-zero Δ199Hg (Δ199Hg: 0.00 ± 0.10‰, 2 SD) and negative δ202Hg (−1.7 ± 1.2‰, 2 SD), based on Hg isotope analyses of 3He-rich lavas1. A recent study also found significantly positive Δ199Hg (0 to 0.4‰) in hydrothermal ore deposits associated with arc magmatism, implying the recycling of Hg from marine sediments into arc lavas6.
Distinct Sr-Nd-Pb isotopic compositions of mid-ocean ridge basalts (MORBs), island arc basalts (IABs), ocean island basalts (OIBs), and continental flood basalts (CFBs) demonstrate a pronounced mantle isotope heterogeneity, which is generally accepted to result from recycling of crustal materials into the mantle via plate subduction9,10. MORBs are derived from partial melting of the upper mantle during the ascent of the asthenosphere beneath mid-ocean ridges. IABs result from partial melting of the mantle wedge at convergent margins due to the addition of subducted crustal materials (including volatile components and/or melts). OIBs and CFBs are genetically related to mantle plumes, which originate from the lower mantle or the core-mantle boundary11. A landmark study recently observed deviations in Δ199Hg in three well-characterized mantle endmembers (EM-1: −0.45 to 0.05‰; EM-2: 0.23‰; HIMU: −0.14‰), compared to the estimate of the primitive mantle (Δ199Hg: 0 ± 0.1‰, 2 SD) based on Hg isotope analyses of 3He-rich lavas, highlighting the great potential of using Hg isotopes to trace crustal Hg recycling into the mantle1.
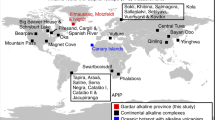
To aid in understanding the deep Hg cycle and to probe possible mantle Hg isotope heterogeneity, we measured the Hg isotopic compositions of a variety of types of basalts at a global scale (Fig. 1). Samples include MORBs from the Mid-Atlantic Ridge (MAR, n = 5), Southwest Indian Ridge (SWIR, n = 3), and East Pacific Rise (EPR, n = 7); IABs (n = 9) from the southern part of the Mariana Island Arc; HIMU-like OIBs (n = 2) and EM1-like OIBs (n = 5) from the Pako guyot of the Magellan Seamount Chain in the West Pacific Seamount Province; and CFBs (n = 17) from the Siberian Trap, the world’s largest flood basalt province. The geological background of these samples and analytical methods are given in the Materials and Methods section in detail. The sample information and analytical results are summarized in Supplementary Tables 1 and 2. We observed diverse Hg-MIF signals in these basalts, providing evidence of oceanic Hg recycling into the mantle.
Ten MORB (mid-ocean ridge basalt) samples are from the East Pacific Rise (1), Southern Mid-Atlantic Ridge (2), and Southwest Indian Ridge (3); nine IAB (island-arc basalt) samples are from the southern part of the Mariana Island Arc (4); seven OIB (ocean-island basalt) samples are from the Pako guyot of the Magellan Seamount Chain (5); and 17 CFB (continental flood basalt) samples are from the end-Permian Siberian Traps (6). The global map is modified after the World ocean bathymetric map by worldinmaps.com.
Results and discussion
Hg concentrations and mass-dependent fractionation of Hg isotopes
In this study, total Hg (THg) concentrations vary from 0.65 to 2.35 ng/g for MORBs, from 0.54 to 1.28 ng/g for IABs, from 0.48 to 2.08 ng/g for OIBs, and from 0.91 to 4.29 ng/g for CFBs, with mean values of 1.48 ng/g, 2.25 ng/g, 1.63 ng/g, and 0.80 ng/g, respectively (Fig. 2A). These concentrations at the low ng/g level are in the range of mafic-ultramafic rocks worldwide (i.e., 0.2–7.0 ng/g for basalts and peridotite)12,13.
Values for A THg, B δ202Hg, and C Δ199Hg in mid-ocean ridge basalts (MORB), island arc basalts (IAB), continental flood basalts (CFB), and ocean island basalts (OIB) were investigated in this study. Analytical uncertainties (2 SD) are 0.11‰ for δ202Hg and 0.07‰ for Δ199Hg. Analytical uncertainties (RSD) for THg are <9%.
A large variation of δ202Hg was observed for all basalts studied (−2.13 to 0.13‰, Fig. 2B), which is ~20 times larger than the 2 SD analytical uncertainty for δ202Hg (±0.11‰). Specifically, MORBs and IABs have δ202Hg ranging from −1.58 to −0.50‰ and −1.72 to 0.13‰, with similar mean values of −0.93 ± 0.62‰ (2 SD) and −0.80 ± 1.22‰ (2 SD), respectively. OIBs and CFBs have relatively lower δ202Hg values of -2.13 to −1.60‰ and −2.13 to −1.48‰, with mean values of −1.85 ± 0.30‰ (2 SD) and −1.66 ± 0.54‰ (2 SD), respectively.
The large variation of δ202Hg in the studied basalts could be caused by MDF during magmatic processes. Lighter Hg isotopes may be preferentially volatilized during Hg degassing14, and thus generate enrichment of heavy Hg isotopes in the residue phases. The lower δ202Hg of fumarolic gas (−1.74 ± 0.36‰, 2 SD) compared to condensed particles (−0.74 ± 0.18‰, 2 SD) in an active volcano (Vulcano Island, Italy) strongly supports this view14. In our study, the more negative values of δ202Hg in OIBs and CFBs (that are genetically related to the lower mantle) relative to those of MORBs and IABs (that originate from the upper mantle), as shown in Fig. 2B, could possibly be explained by more severe Hg degassing during the eruption of the latter compared to the former.
An alternative explanation for the variation of δ202Hg in the basalts studied is the mixing of Hg sources with distinct δ202Hg values. The continental crust, in particular sedimentary rocks and sediments, displays large variations of δ202Hg (−3 to 1‰), with a mean value of −0.7 ± 1.6‰ (2 SD) (reviewed by Blum et al.7). The large variations of δ202Hg in MORBs and IABs in our study, coupled with their higher mean δ202Hg values compared to OIBs and CFBs, may be explained by the recycling of crustal rocks into the upper mantle. However, many different processes could trigger Hg-MDF7, which hinders the full understanding of Hg-MDF during magmatic processes. Thus, δ202Hg is not considered to be highly diagnostic of Hg sources. Instead, we focus on the Hg-MIF signal as a more reliable source tracer, as discussed below.
Mass independent fractionation of Hg isotopes
All samples investigated show limited or no MIF of 200Hg, with Δ200Hg ranging from −0.08 to 0.08‰, which is nearly within the analytical uncertainty for Δ200Hg (±0.06‰). However, large variations of Δ199Hg (−0.08 to 0.34‰) and Δ201Hg (−0.09 to 0.23‰) are observed in the samples studied, compared to the analytical uncertainties for Δ199Hg (±0.07‰) and Δ201Hg (±0.06‰). MIF of 199Hg and 201Hg is mainly produced during aqueous Hg(II) photoreduction on Earth’s surface with little contribution from other processes7,15. Magmatic degassing could produce limited Hg-MIF of <0.1‰ in Δ199Hg and Δ201Hg5. Hg(II) photoreduction imparts negative Δ199Hg in the product gaseous Hg(0) and positive Δ199Hg in the residual Hg(II) phase15, with Δ199Hg/Δ201Hg of ~1. For this reason, terrestrial vegetation mostly shows negative Δ199Hg (−0.6 to 0‰, Fig. 3A), due to the primary accumulation of Hg(0) during uptake by foliage16,17,18. Soil receives a substantial amount of Hg from litterfall16,19,20,21, and thus is characterized by negative Δ199Hg (−0.6 to 0‰, Fig. 3A). In comparison, the oceans mainly receive Hg through wet deposition of Hg(II), resulting in positive Δ199Hg in seawater (0 to 0.4‰) and marine sediments22,23,24 (−0.1 to 0.4‰, Fig. 3A). Terrestrial and marine reservoirs both show Δ199Hg/Δ201Hg of ~1 (Fig. 3B), suggesting that Hg-MIF is mainly caused by Hg(II) photoreduction.
A δ202Hg versus Δ199Hg diagram for mid-ocean ridge basalts (MORB, this study), island arc basalts (IAB, this study), continental flood basalts (CFB, this study), and ocean island basalts (OIB, this study and Moynier et al1.), compared with reference data for soil16,19,20,21, vegetation16,17,18, seawater22, marine sediments23,24, arc-related low-temperature hydrothermal deposits (Deng et al6. and references therein). The gray shaded area corresponds to the terrestrial reservoir (soil and vegetation) with negative Δ199Hg. The light blue shaded area represents the complementary marine reservoir (marine sediments and seawater) with positive Δ199Hg. The light orange shaded area represents the primitive mantle with Δ199Hg ≈ 0. B Δ201Hg versus Δ199Hg diagram. The correlation trend with a slope of 1 identifies photochemical reduction of Hg (II) as the process which controls mass-independent fractionation of mercury (Blum et al7.). Analytical uncertainties (2 SD) for the data are 0.11‰ for δ202Hg and 0.07‰ for Δ199Hg and Δ201Hg.
Crustal Hg recycling into the lower mantle
OIBs and CFBs are petrogenetically related to the lower mantle. As shown in Fig. 3A, OIBs and CFBs investigated in this study show negative δ202Hg (−1.80 ± 0.42‰, 2 SD) and near-zero Δ199Hg values (−0.01 ± 0.08‰, 2 SD), which are within the range of the estimate of the primitive mantle (δ202Hg: −1.7 ± 1.2‰; Δ199Hg: 0 ± 0.1‰) based on Hg isotope analyses of 3He-rich lavas1. The recently observed significant Δ199Hg signals in two EM-1 OIBs (−0.45 and −0.11‰), an EM-2 OIB (0.23‰), and a HIMU OIB (−0.14‰)1, imply that Hg in oceanic and continental crustal materials can be recycled into the lower mantle. However, the amount of recycled Hg is likely small with respect to the total Hg pool in the lower mantle, given the fact that OIBs and CFBs in this study as well as 16 previously studied OIBs (including 11 3He-rich lavas)1 show near-zero Δ199Hg values (Fig. 3A).
Recycling of oceanic Hg into the upper mantle
MORBs and IABs have mostly positive Δ199Hg values of 0.05 to 0.22‰ and -0.01 to 0.34‰ (Figs. 2C, 3A), with Δ199Hg/Δ201Hg of ~1 (Fig. 3B). These values fall within the range of marine sediments and seawater, suggesting that a substantial amount of Hg in these rocks is of marine origin. Their positive Δ199Hg values may result from the fixation of seawater Hg during seawater-rock reactions. MORBs and IABs investigated here were collected from the seafloor and show variable weight loss on ignition (LOI) ranging from −0.9 to 0.4 wt% and 6.2 to 8.0 wt%, respectively (Supplementary Table 2). The very low LOI of the MORBs and their pristine chemical and mineralogical composition argue against seawater overprinting (Supplementary Table 2). Although IABs have high LOI, no correlations are observed between LOI and THg or Δ199Hg (Fig. 4), indicating that seawater-rock reactions are unlikely to be the mechanism responsible for the positive Δ199Hg in IABs. The OIB samples studied here were also collected from the seafloor. Although these OIBs have relatively higher LOI values (2.0 to 8.6 wt%, Supplementary Table 2) than MORBs, they have near-zero Δ199Hg values. Given the low Hg concentration in seawater (<0.02 to 2 ng/L)25, seawater-rock reactions can be further precluded as a reason for the positive Δ199Hg values in MORBs and IABs.
We attribute the positive Δ199Hg signals in the studied IABs to recycled marine sediments. At convergent margins, subduction of the oceanic slab carries a substantial amount of Hg from marine sediments into subduction zones. The downgoing slab undergoes dehydration and metamorphism and releases fluids and melts that in turn metasomatize the mantle wedge. Partial melting of such metasomatized mantle could generate IABs with positive Δ199Hg. Notably, given the low volatilization temperature of Hg, subducted Hg may be mostly released from the downgoing slab and returned to the Earth’s surface through arc volcanism. This is supported by the positive Δ199Hg signal observed in volcanic arc-related epithermal Hg-Au deposits in NE China and the Pacific rim (0 to 0.4‰, Fig. 3A, B, Deng et al.6 and references therein). The most recent estimate of present-day global volcanic Hg flux (76 ± 30 t/year)26 is slightly lower than the total mass of Hg in global subducted sediments (~87.4 t/year), calculated from the mass of global subducted sediment (1.4 × 1015 g/year)27 multiplied by the Hg concentration of average marine sediments (62.4 ng/g)28.
The positive Δ199Hg of MORBs could also be the result of the recycling of oceanic crust. In subduction zones, the subducted marine Hg may not be completely released via arc volcanism. The remaining Hg in the subducted slab could continue to descend into the asthenospheric mantle. This fraction of Hg is recycled into the asthenosphere and could be brought back to Earth’s surface via basaltic magmatism at mid-ocean ridges. The upper mantle is heterogeneous in Hg isotopes, apparently due to the variable involvement of recycled crustal Hg. Such isotopic heterogeneities are consistent with large variations in Sr-Nd-Pb isotope compositions for MORBs from different mid-ocean ridges (e.g., MAR, SWIR, and EPR), which supports the model of recycling of crustal materials into the upper mantle29,30,31. The recycling of oceanic Hg into the upper mantle is also favored by the positive Δ199Hg values (0.07 to 0.27‰) observed in hotspring and sinter samples from the Yellowstone Plateau volcanic field32, which might be explained by upwelling asthenospheric mantle flow induced by the subducting Pacific plate33.
Geodynamic implications
Based on our current knowledge of Hg isotopic variation in mantle-derived rocks, the deep cycling of Hg is illustrated in Fig. 5. Photochemical reactions produce significant negative Δ199Hg values in terrestrial reservoirs (e.g., soil and vegetation) and significant positive Δ199Hg values in marine reservoirs (e.g., seawater and marine sediments). Mercury from terrestrial and marine reservoirs is subducted at convergent margins. Due to the low volatilization temperature of Hg, much of the subducted Hg is likely released back to the surface environment through arc volcanism, forming IABs and arc-related epithermal Hg deposits with significantly positive Δ199Hg values. The remaining Hg in the subducted slab is recycled into the asthenosphere and released at mid-ocean ridges by MORBs, which are characterized by significantly positive Δ199Hg values.
Photoreduction of Hg(II) produces gaseous Hg(0) with negative Δ199Hg values and gaseous Hg(II) with positive Δ199Hg values. Gaseous Hg (0) is preferentially accumulated in soil and vegetation (light gray area), whereas gaseous Hg(II) is readily deposited into the marine reservoir (light blue area). In subduction zones, oceanic crust (white-gray area) is subducted underneath the continental crust (dark gray area) and delivers large amounts of Hg from marine sediments (light blue circles) into the mantle. Most of the subducted Hg is released and cycled back to the surface environment through arc volcanism, forming island arc basalts (IAB, purple area) with positive Δ199Hg values. The remaining Hg in the subducting slab is cycled into the upper mantle (light orange area) and released at mid-ocean ridges by mid-ocean ridge basalt volcanism (MORB, highlighted in blue), which displays positive Δ199Hg values. Some of the subducted Hg may also be cycled into the lower mantle (orange area), resulting in Δ199Hg signals in some ocean island basalts (OIB)1; however, given that OIBs and CFBs mostly show near-zero Δ199Hg values (highlighted in red), this recycled Hg may represent only a small proportion of Hg in the lower mantle.
Some of the subducted Hg may also be recycled into the lower mantle, consistent with the recently observed significant Δ199Hg signals in some OIB samples1; however, given that most OIBs and CFBs show near-zero Δ199Hg values, this recycled Hg likely represents only a small proportion of Hg in the lower mantle, and may be mostly located in the source of the mantle end-members. In the future, analysis of OIBs and CFBs from additional sites will be needed to better quantified the amount of recycled Hg in the lower mantle.
Overall, our study demonstrates mantle Hg isotopic heterogeneity and reveals large-scale translithospheric Hg recycling via plate tectonics. Hg tends to be depleted in volcanic rocks derived from the mantle (e.g., several ng/g in basalts) and enriched in crustal sedimentary rocks (e.g., 62.4 ng/g in average marine sediments28). Given the significantly large Δ199Hg values and high Hg concentrations in crustal materials, we infer that the crustal signature cannot easily be erased by the primitive mantle signature (Δ199Hg ~ 0). We conclude that Hg-MIF (Δ199Hg) can be a useful tool for studying crust-mantle interactions.
Methods
Samples and geological background
The sample locations are shown in Fig. 1 and summarized in Supplementary Table 1. MORBs were collected from the EPR (n = 7), MAR (n = 5), and SWIR (n = 3), during the DY115-21, DY115-22, DY125-30, and DY135-40 cruises, by the Chinese R/V DayangYihao using TV grab (grab system controlled via a TV camera). The three regions have different spreading rates (EPR: 80 mm/yr; MAR: 35 mm/yr; SWIR: 14 mm/yr), representing a broad spectrum of crustal accretion modes34,35.
IABs (n = 9) were collected from the southern part of the Mariana Island Arc, using the submersible Jiaolong, during the R/V Xiangyanghong 09 Dayang 37th cruise. The Mariana Island Arc is a classic young island arc in the western Pacific Ocean. Most of the islands and underwater volcanoes in the southern part of the Mariana Island Arc were formed during the Eocene to Miocene36.
The Pako guyot was formed by the large-scale eruption of a hotspot at 120–90 Ma37. Seven OIBs were collected from the Pako guyot of the Magellan Seamount Chain in the West Pacific Seamount Province (Fig. 1), during Chinese R/V Dayang Yihao and Xiangyanghong 09 cruises (DY105 and DY31), using the submersible Jiaolong38. Two samples (JL-Dive80-ST01-S01-1 and JL-Dive80-ST03-S05-1) show HIMU-like Sr-Nd-Pb-Hf isotopic compositions indicating recycled ancient oceanic crust in their mantle source, while the others display EM1-like isotopic compositions consistent with recycled sub-continental lithospheric components in their mantle source.
CFBs (n = 17) were sampled from the Norilsk region at the northwestern margin of the Siberian platform, which is a part of the world’s largest flood basalt province, the Siberian Traps (Fig. 1). The eruption of these CFBs occurred from the latest Permian to the Early Triassic (i.e., 248–252 Ma) and was associated with the break-up of Gondwanaland39. Trace element and Sr-Nd isotope data indicate that CFBs from the Norilsk region were formed by the interaction of plume-generated picritic magmas with the lithosphere40.
Chemical analyses
The samples were cut to expose fresh surfaces, washed by 18.2 MΩ cm water, air-dried, powdered, and homogenized, prior to chemical analysis at the Institute of Geochemistry, Chinese Academy of Sciences (IGCAS). Major elements were analyzed at the ALS Minerals-ALS Chemex, Guangzhou, China, using a PANalytical PW2424 X-ray fluorescence spectrometer. LOI values were measured by combustion loss of weight, with analytical uncertainties (RSD) of <5%. Total Hg (THg) concentration was determined by an RA-915+ Hg analyzer (Lumex, Russia), with a detection limit of 0.01 ng/g. Standard reference materials (GSR-2, andesite; BCR-2, basalt) were measured, yielding Hg recoveries of 94–106% and RSD of <9%.
The samples were prepared for Hg isotope analysis using a double-stage tube furnace coupled with 40% anti aqua regia (HNO3/HCl = 2/1, v/v) trapping solutions41. Standard reference materials (GSR-2 and BCR-2) and method blanks were processed in the same way as the samples. The former yielded Hg recoveries of 90–105% and the latter showed Hg concentrations lower than the detection limit, precluding lab contamination. The preconcentrated solutions were diluted to 0.5 ng/mL with an acid concentration of 10–20% prior to Hg isotope analysis using a Neptune Plus multi-collector inductively coupled plasma mass spectrometry42. Hg isotope ratios were reported following the convention proposed by Blum and Bergquist43. MDF is expressed in δ202Hg notation in units of ‰ referenced to the NIST-3133 (analyzed before and after each sample):
MIF is reported in Δ notation, which describes the difference between the measured δxxxHg and the theoretically predicted δxxxHg value, in units of ‰:
β is 0.252 for 199Hg, 0.5024 for 200Hg, and 0.752 for 201Hg. Hg concentration and acid matrices in the bracketing NIST-3133 solutions were matched with neighboring samples. NIST-3177 secondary standard solutions, diluted to 0.5 ng/mL Hg with 10% HCl, were measured every 10 samples. The overall average and uncertainty of NIST-3177 (δ202Hg: -0.53 ± 0.10‰; Δ199Hg: -0.03 ± 0.04‰; Δ200Hg: 0.00 ± 0.05‰; Δ201Hg: -0.02 ± 0.04‰; 2 SD, n = 26) and GSR-2 (δ202Hg: -1.62 ± 0.11‰; Δ199Hg: 0.04 ± 0.06‰; Δ200Hg: 0.01 ± 0.04‰; Δ201Hg: 0.02 ± 0.06‰, 2 SD, n = 5) and BCR-2 (δ202Hg: -1.89 ± 0.11‰; Δ199Hg: 0.00 ± 0.07‰; Δ200Hg: 0.00 ± 0.06‰; Δ201Hg: 0.01 ± 0.06‰, 2 SD, n = 5) agree well with previous results43,44,45. The larger of the values of standard deviation (2 SD) for either NIST-3177, GSR-2, or BCR-2 are used to reflect maximum analytical uncertainties.
Data availability
All data is available in the main text or supplementary materials.
References
Moynier, F. et al. The mercury isotopic composition of Earth’s mantle and the use of mass independently fractionated Hg to test for recycled crust. Geophys. Res. Lett. 48, e2021GL094301 (2021).
Selin, N. E. Global biogeochemical cycling of mercury: a review. Annu. Rev. Environ. Resour. 34, 43–63 (2009).
Pirrone, N. et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 10, 5951–5964 (2010).
Gustin, M. S. et al. Nevada STORMS project: measurement of mercury emissions from naturally enriched surfaces. J. Geophys. Res.: Atmosph. 104, 21831–21844 (1999).
Moynier, F. et al. Chondritic mercury isotopic composition of Earth and evidence for evaporative equilibrium degassing during the formation of eucrites. Earth Planet. Sci. Lett. 551, 116544 (2020).
Deng, C. et al. Recycling of mercury from the atmosphere-ocean system into volcanic-arc–associated epithermal gold systems. Geology 49, 309–313 (2020).
Blum, J. D., Sherman, L. S. & Johnson, M. W. Mercury isotopes in earth and environmental sciences. Annu. Rev. Earth Planet. Sci. 42, 249–269 (2014).
Kwon, S. Y. et al. Mercury stable isotopes for monitoring the effectiveness of the Minamata Convention on Mercury. Earth-Sci. Rev. 203, 103111 (2020).
Hofmann, A. W. Mantle geochemistry: the message from oceanic volcanism. Nature 385, 219–229 (1997).
Hofmann, A. W. Sampling mantle heterogeneity through oceanic basalts: isotopes and trace elements. Treatise Geochem. 2, 568 (2003).
White, W. M. Probing the Earth’s deep Interior through geochemistry. Geochem. Perspect. 4, 95–251 (2015).
Canil, D. et al. Mercury in some arc crustal rocks and mantle peridotites and relevance to the moderately volatile element budget of the Earth. Chem. Geol. 396, 134–142 (2015).
Coufalík, P. et al. Model of mercury flux associated with volcanic activity. Bull. Environ. Contamination Toxicol. 101, 549–553 (2018).
Zambardi, T. et al. Mercury emissions and stable isotopic compositions at Vulcano Island (Italy). Earth Planet. Sci. Lett. 277, 236–243 (2009).
Bergquist, B. A. & Blum, J. D. Mass-dependent and-independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 318, 417–420 (2007).
Demers, J. D., Blum, J. D. & Zak, D. R. Mercury isotopes in a forested ecosystem: Implications for air‐surface exchange dynamics and the global mercury cycle. Glob. Biogeochem. Cycles 27, 222–238 (2013).
Yu, B. et al. Isotopic composition of atmospheric mercury in China: new evidence for sources and transformation processes in air and in vegetation. Environ. Sci. Technol. 50, 9262–9269 (2016).
Yin, R., Feng, X. & Meng, B. Stable mercury isotope variation in rice plants (Oryza sativa L.) from the Wanshan mercury mining district, SW China. Environ. Sci. Technol. 47, 2238–2245 (2013).
Biswas, A. et al. Natural mercury isotope variation in coal deposits and organic soils. Environ. Sci. Technol. 42, 8303–8309 (2008).
Zheng, W. et al. Mercury isotope compositions across North American forests. Glob. Biogeochem. Cycles 30, 1475–1492 (2016).
Jiskra, M. et al. Source tracing of natural organic matter bound mercury in boreal forest runoff with mercury stable isotopes. Environ. Sci.: Process. Impacts 19, 1235–1248 (2017).
Štrok, M., Baya, P. A. & Hintelmann, H. The mercury isotope composition of Arctic coastal seawater. Comptes Rendus Geosci. 347, 368–376 (2015).
Yin, R. et al. Anomalous mercury enrichment in Early Cambrian black shales of South China: mercury isotopes indicate a seawater source. Chem. Geol. 467, 159–167 (2017).
Grasby, S. E. et al. Isotopic signatures of mercury contamination in latest Permian oceans. Geology 45, 55–58 (2017).
Lamborg, C. et al. Mercury in the anthropocene ocean. Oceanography 27, 76–87 (2014).
Bagnato, E. et al. First combined flux chamber survey of mercury and CO2 emissions from soil diffuse degassing at Solfatara of Pozzuoli crater, Campi Flegrei (Italy): Mapping and quantification of gas release. J. Volcanol. Geotherm. Res. 289, 26–40 (2014).
Rea, D. K. & Ruff, L. J. Composition and mass flux of sediment entering the world’s subduction zones: implications for global sediment budgets, great earthquakes, and volcanism. Earth Planet. Sci. Lett. 140, 1–12 (1996).
Grasby, S. E. et al. Mercury as a proxy for volcanic emissions in the geologic record. Earth-Sci. Rev. 196, 102880 (2019).
Rehka, M. & Hofmann, A. W. Recycled ocean crust and sediment in Indian Ocean MORB. Earth Planet. Sci. Lett. 147, 93–106 (1997).
Hoernle, K. et al. On-and off-axis chemical heterogeneities along the South Atlantic Mid-Ocean-Ridge (5–11°S): shallow or deep recycling of ocean crust and/or intraplate volcanism? Earth Planet. Sci. Lett. 306, 86–97 (2011).
Zhang, G. L. et al. Geochemical constraints on a mixed pyroxenite–peridotite source for East Pacific Rise basalts. Chem. Geol. 330, 176–187 (2012).
Sherman, L. S. et al. Mercury isotopic composition of hydrothermal systems in the Yellowstone Plateau volcanic field and Guaymas Basin sea-floor rift. Earth Planet. Sci. Lett. 279, 86–96 (2009).
Kincaid, C. et al. Bifurcation of the Yellowstone plume driven by subduction-induced mantle flow. Nat. Geosci. 6, 395–399 (2013).
Chen, C. et al. Calcium isotopic compositions of oceanic crust at various spreading rates. Geochimica et. Cosmochimica Acta 278, 272–288 (2020).
Meijer, A. Pb and Sr isotopic data bearing on the origin of volcanic rocks from the Mariana island-arc system. Geol. Soc. Am. Bull. 87, 1358–1369 (1976).
Koppers, A. A. P. et al. Short-lived and discontinuous intraplate volcanism in the South Pacific: hot spots or extensional volcanism? Geochem. Geophys. Geosyst. 4, 1089 (2003).
Wessel, P. & Lyons, S. Distribution of large Pacific seamounts from Geosat/ERS-1: implications for the history of intraplate volcanism. J. Geophys. Res.: Solid Earth 102, 22459–22475 (1997).
Wei, X. et al. New geochemical and Sr-Nd-Pb isotope evidence for FOZO and Azores plume components in the sources of DSDP Holes 559 and 561 MORBs. Chem. Geol. 557, 119858 (2020).
Lightfoot, P. C. et al. Remobilisation of the continental lithosphere by a mantle plume: major-, trace-element, and Sr-, Nd-, and Pb-isotope evidence from picritic and tholeiitic lavas of the Noril’sk District, Siberian Trap, Russia. Contributions Mineral. Petrol. 114, 171–188 (1993).
Lightfoot, P. C. & Keays, R. R. Siderophile and chalcophile metal variations in flood basalts from the Siberian trap, Norilsk region: implications for the origin of the Ni-Cu-PGE sulfide ores. Economic Geol. 100, 439–462 (2005).
Zerkle, A. L. et al. Anomalous fractionation of mercury isotopes in the Late Archean atmosphere. Nat. Commun. 11, 1–9 (2020).
Yin, R. et al. Effects of mercury and thallium concentrations on high precision determination of mercury isotopic composition by Neptune Plus multiple collector inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 31, 2060–2068 (2016).
Blum, J. D. & Bergquist, B. A. Reporting of variations in the natural isotopic composition of mercury. Anal. Bioanal. Chem. 388, 353–359 (2007).
Geng, H., Yin, R. & Li, X. An optimized protocol for high precision measurement of Hg isotopic compositions in samples with low concentrations of Hg using MC-ICP-MS. J. Anal. At. Spectrom. 33, 1932–1940 (2018).
Wang, X. et al. Oceanic mercury recycled into the mantle: evidence from positive Δ199Hg in lamprophyres. Chem. Geol. 584, 120505 (2021).
Acknowledgements
This work was supported by the Natural Science Foundation of China (41873047, 42076076, and 41873026). R.Y. was also funded by the Chinese Academy of Sciences through the Hundred Talent Plan. We are grateful to the organizers of the 2014 International Platinum Symposium in Yekaterinburg (Russia) for organizing the field trip to collect the Siberian Trap CFB samples. We thank Zhongjie Bai, Liang Liu, Guangyi Sun, and Jingjing Zhu from the Institute of Geochemistry, Chinese Academy of Sciences, for stimulating discussions.
Author information
Authors and Affiliations
Contributions
R.Y. designed research, L.C., X.S., S.Y., C.Z., X.W., and Y.X. provided samples, X.P., L.C., D.C., C.D., and R.Y. measured samples, X.F. provided laboratory supports, R.Y., L.C., J.D.B., and B.L. interpreted the results, R.Y. wrote the paper, with edits from J.D.B. and B.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests, financial or otherwise.
Peer review
Peer review information
Nature Communications thanks Stephen Grasby and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, R., Chen, D., Pan, X. et al. Mantle Hg isotopic heterogeneity and evidence of oceanic Hg recycling into the mantle. Nat Commun 13, 948 (2022). https://doi.org/10.1038/s41467-022-28577-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-28577-1
This article is cited by
-
Applications of mercury stable isotopes for tracing volcanism in the geologic record
Science China Earth Sciences (2024)
-
Phylogenetic and ecophysiological novelty of subsurface mercury methylators in mangrove sediments
The ISME Journal (2023)
-
Mercury isotope evidence for marine photic zone euxinia across the end-Permian mass extinction
Communications Earth & Environment (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.