Abstract
Several members of the FGF family have been identified as potential regulators of glucose homeostasis. We previously reported that a low threshold of FGF-induced FGF receptor 1c (FGFR1c) dimerization and activity is sufficient to evoke a glucose lowering activity. We therefore reasoned that ligand identity may not matter, and that besides paracrine FGF1 and endocrine FGF21, other cognate paracrine FGFs of FGFR1c might possess such activity. Indeed, via a side-by-side testing of multiple cognate FGFs of FGFR1c in diabetic mice we identified the paracrine FGF4 as a potent anti-hyperglycemic FGF. Importantly, we found that like FGF1, the paracrine FGF4 is also more efficacious than endocrine FGF21 in lowering blood glucose. We show that paracrine FGF4 and FGF1 exert their superior glycemic control by targeting skeletal muscle, which expresses copious FGFR1c but lacks β-klotho (KLB), an obligatory FGF21 co-receptor. Mechanistically, both FGF4 and FGF1 upregulate GLUT4 cell surface abundance in skeletal muscle in an AMPKα-dependent but insulin-independent manner. Chronic treatment with rFGF4 improves insulin resistance and suppresses adipose macrophage infiltration and inflammation. Notably, unlike FGF1 (a pan-FGFR ligand), FGF4, which has more restricted FGFR1c binding specificity, has no apparent effect on food intake. The potent anti-hyperglycemic and anti-inflammatory properties of FGF4 testify to its promising potential for use in the treatment of T2D and related metabolic disorders.
Similar content being viewed by others
Introduction
The mammalian fibroblast growth factor (FGF) family comprises 18 structurally-related polypeptides (FGF1-FGF10 and FGF16-FGF23); these act in a paracrine or endocrine manner to mediate a plethora of critical events in development, tissue homeostasis/repair, and metabolism1,2. The recent discovery of essential roles for FGFs in the regulation of homeostasis of glucose, lipid, cholesterol, and adipose tissue remodeling has brought FGF signaling to the forefront in terms of drug discovery for type 2 diabetes mellitus (T2D) and related metabolic diseases3,4,5. To date, two endocrine FGFs (i.e., FGF21 and FGF19) and a single paracrine FGF (FGF1) have been shown to exert multiple beneficial effects in obesity and T2D, including potent glucose-lowering activity, improved lipid profiles, and enhanced energy expenditure3,4,5. Notably, these FGFs mitigate a number of complications in T2D4,6 without eliciting many of the side effects associated with current T2D medications; for example, thiazolidinediones (TZDs) tend to engender weight gain, bone loss, and edema7.
FGFs implement their diverse actions by binding, dimerizing, and consequently activating their cognate FGF receptors (FGFRs) in an heparan sulfate (HS)-dependent (in the case of paracrine FGFs)2,8 or an HS and Klotho co-receptor dependent (in the case of endocrine FGFs) manner2,9,10. In mammals, there are four distinct FGFR genes (FGFR1-4)2. Tissue-dependent alternative splicing of FGFR1-3 genes gives rise to epithelial “b” and mesenchymal “c” isoforms with distinct FGF binding specificity thus elaborating the number of principal FGFRs to seven11. Upon activation, FGFR triggers activation of multiple downstream signaling pathways, including PLCγ/PKC, FRS2α/RAS-MAPK, FRS2α/Gab1/PI3 kinase/Akt, CrkL/Cdc42-Rac and CaMKK2/AMPKα and LKB1/AMPKα12,13,14,15, thereby reprogramming the cellular transcriptional landscape that ultimately determines cellular fate/response.
We recently showed that a low threshold of FGF-induced FGF receptor (i.e., FGFR1c) dimerization is sufficient to elicit glucose-lowering activity16,17. We therefore surmised that ligand identity may not matter, and that other cognate paracrine FGFs of FGFR1c might also possess anti-hyperglycemic activity in vivo. If so, these could be repurposed for treating T2D and related metabolic disorders. Indeed, by testing the glucose-lowering activity of representative members from the five paracrine FGF subfamilies in T2D mice, we identified FGF4 as a new anti-hyperglycemic molecule. We show that both paracrine FGF4 and FGF1 elicit more potent and durable anti-hyperglycemic effects than endocrine FGF21. We attribute the superior glycemic control of paracrine FGFs to the ability of these FGFs to stimulate glucose disposal in skeletal muscle, a β-klotho (KLB)-deficient tissue that is off-limits for endocrine FGF21. We further show that paracrine FGFs exert their glycemic control by activating the AMPK signaling pathway in an insulin-independent manner.
Results
Multiple cognate FGFR1c ligands can normalize glucose in T2D mice
In addition to the FGF1 subfamily, the paracrine FGF4, FGF9, and FGF8 subfamilies are cognate ligands of FGFR1c18,19. Accordingly, we expressed and purified three human recombinant FGFs [FGF4 (rFGF4), FGF8 (rFGF8), and FGF9 (rFGF9)]—all founding members of their respective subfamilies (Fig. 1a)—and tested their anti-hyperglycemic activities following intraperitoneal (i.p.) injection into db/db mice. Recombinant FGF1 (rFGF1) (the founding member of the FGF1 subfamily) and FGF7 (rFGF7) (the founding member of the FGF7 subfamily that does not recognize FGFR1c) were used as positive and negative controls, respectively. Reminiscent of rFGF1—an established anti-hyperglycemic ligand5—rFGF4, rFGF8, and rFGF9 all elicited a glucose-lowering effect (Fig. 1b), with rFGF4 exerting the greatest response. In contrast, rFGF7 was devoid of any such property (Fig. 1b).
a Schematic of paracrine FGF subfamilies; those that can bind FGFR1c are highlighted in yellow. b Changes in blood glucose levels in db/db mice 6 h after a single intraperitoneal (i.p.) injection of rFGF1, rFGF4, rFGF8, rFGF9, or rFGF7 (each at 1.0 mg/kg body weight) (n = 6). c–e Blood glucose levels in db/db (c) (n = 6), DIO (d) (PBS (n = 6), rFGF4 (n = 7), rFGF1 (n = 5), rFGF21 (n = 6)), and ob/ob (e) (PBS (n = 5), rFGF4 (n = 4), rFGF1 (n = 5), rFGF21 (n = 5)) mice at various times after acute i.p. injection of rFGF4, rFGF1, rFGF21 (each at 1.0 mg/kg body weight) or PBS buffer control. The statistical comparisons in these figures are PBS vs FGF4, PBS vs FGF1, and PBS vs FGF21, respectively. f–h Food intake of db/db (f), DIO (g), and ob/ob (h) mice measured 24 h after acute i.p. injection with rFGF4, rFGF1, rFGF21 (each at 1.0 mg/kg body weight), or with PBS as a buffer control (n = 3). Data are presented as mean +/− SEM. Statistical comparison in (b) is unpaired two-tailed test. Statistical comparisons in (c–e) are two-way ANOVA tests with Tukey’s multiple comparisons tests. Statistical comparisons in (f–h) are one-way ANOVA tests with Tukey’s multiple comparisons tests. Source data are provided as a Source data file.
We next compared the anti-hyperglycemic effects of paracrine rFGF4 and rFGF1 and endocrine rFGF21 (all at 1.0 mg/kg body weight) in db/db, DIO, and ob/ob mice over the course of 36 h. Both rFGF4 and rFGF1 elicited significant glucose-lowering effects 3 h after administration and normalized the glucose level by 6 h in these mice (Fig. 1c–e). These anti-hyperglycemic effects persisted for at least 24 h. However, the corresponding effect was much weaker in both amplitude and duration in rFGF21-treated mice, tapering off after 6 h (Fig. 1c–e). Notably, as previously reported for rFGF2120,21, rFGF4 had no impact on food intake (Fig. 1f–h) whereas rFGF1 did inhibit food intake consistent with previously reported data5 (Fig. 1f–h). We also found that administration of either rFGF1 or rFGF4 potently remedied high glucose in fasted db/db mice (Supplementary Fig. 1a). As in the case of FGF15, a single dose of rFGF4 rescued hyperinsulinemia in db/db mice (Supplementary Fig. 1b) but had no significant effect on either blood glucose or insulin levels in chow-fed wild type mice (Supplementary Fig. 1c, d), indicating that exogenous FGF4 does not stimulate pancreatic β-cell insulin release. Moreover, a bolus of rFGF4 as high as 3 mg/kg body weight did not induce hypoglycemia in db/db mice (Supplementary Fig. 1e). Examination of the pharmacokinetics of rFGF4 following a single injection into Sprague Dawley (SD) rats showed the in vivo half-life to be ~4.4 h, which was approximately 1.8 times greater than that of rFGF1 (~2.5 h) (Supplementary Table 1).
Acute rFGF4 treatment upregulates GLUT4 expression and translocation in skeletal muscle
To understand the underlying mechanism(s) for the dramatic differences in glucose-lowering potencies between paracrine FGFs and endocrine FGF21, we studied their target organ(s) via micro positron emission computed tomography (microPET-CT). To do so, db/db mice were treated acutely (i.e., single bolus) with rFGF4, rFGF1, rFGF21, or buffer (as a control) for 2 h, then infused with radiolabeled fluodeoxyglucose (18F-FDG) and its uptake monitored for 1 hr by whole body positron emission tomography (PET). We found a pronounced enrichment of 18F-FDG in skeletal muscle (including in both the back (erector spinae muscles) and lower limb muscles (thigh muscles)) of rFGF4 and rFGF1 treated animals (Fig. 2a–c). In contrast, rFGF21 did not cause any such enrichment in muscle tissues (Fig. 2a–c). These results were corroborated upon measuring the radioactivity of excised organs via gamma-counting: 18F-FDG uptake in skeletal muscle was robust and comparable in both rFGF4 and rFGF1-treated db/db mice, whereas rFGF21-treated animals showed no such enrichment (Fig. 2d).
a–c PET imaging (a) and uptake of 18F-FDG into db/db mouse organs 30 min (b) and 60 min (c) after infusion with radiolabeled fluodeoxyglucose (18F-FDG) through the lateral tail vein. All these animals received i.p. injection of rFGF4, rFGF1, rFGF21 (each at 1.0 mg/kg body weight), or buffer controls 2 h prior to infusion with 18F-FDG (n = 5). Images in (a) are lateral views; red, high uptake; blue, low uptake. BM: back muscle; LLM: lower limb muscle. d 18FDG uptake by liver, LLM, BM, subcutaneous white adipose tissue (SWAT) and epididymal white adipose tissue (Epi-WAT) of db/db mice 3 h after i.p. injection of buffer (control), rFGF4, rFGF1, or rFGF21 (each at 1.0 mg/kg body weight) as measured in excised tissues by gamma-ray emission (n = 4). e, f Translocation of GLUT4 in LLM of db/db mice 6 h after i.p. injection of PBS buffer (control), rFGF4, rFGF1, or rFGF21 (each at 1.0 mg/kg body weight) shown by immunofluorescence staining with an antibody to GLUT4 (e) (n = 5). Scale bars, 50 μm. Data were quantified using ImageJ software (f). g Expression of GLUT4 in LLM of db/db mice 6 h after i.p. injection of PBS buffer (control), rFGF4, rFGF1, or rFGF21 (each at 1.0 mg/kg body weight) measured by western blot analysis using an anti-GLUT4 antibody and an antibody to GAPDH as a loading control (upper panel) (n = 4). Data were quantified using ImageJ software (lower panel). h mRNA level of β-klotho (KLB) in liver, skeletal muscle and epididymal WAT (WAT) of WT mice determined by real-time PCR analysis (n = 6). Data are presented as mean +/− SEM. Statistical comparisons in (b–d) and (f–h) are one-way ANOVA test with Tukey’s multiple comparisons tests. Source data are provided as a Source data file.
The glucose transporter GLUT4 is the principal mediator of glucose uptake in skeletal muscle22,23. We therefore examined the effect of single-dose FGF administration on the expression and cell surface translocation of GLUT4 in db/db mice. Indeed, we found that administration of rFGF4 or rFGF1 induced a profound upregulation of GLUT4 transcription 2 h post administration (Supplementary Fig. 2a), which manifested in an enhancement in total GLUT4 expression that persisted for at least 6 h after administration (Fig. 2e, g and Supplementary Fig. 2b). These effects were accompanied by a conspicuous translocation of GLUT4 to the skeletal muscle cell membrane (Fig. 2e, f). Notably, administration of the GLUT inhibitor phloretin22 abolished rFGF4’s acute glucose-lowering effects in db/db mice (Supplementary Fig. 2c). In contrast to rFGF4 and rFGF1, rFGF21 failed to upregulate GLUT4 expression/translocation in skeletal muscle (Fig. 2e–g). These results are consistent with the fact that this tissue lacks expression of KLB (Fig. 2h), an obligatory co-receptor for FGF2110. We conclude that paracrine FGF1 and FGF4 mediate their potent anti-hyperglycemic effects by targeting KLB-deficient skeletal muscle.
rFGF4 induces GLUT4 expression and translocation by activating the AMPKα pathway
GLUT4 expression and translocation in skeletal muscle is regulated by activation of AMPKα or by insulin signaling24,25. We detected no significant changes in insulin signaling in the skeletal muscle of db/db mice 6 h post-administration of a single dose of rFGF4 or rFGF1 as measured by phosphorylation of IRS1 and/or AKT—two known reporters for insulin signaling (Supplementary Fig. 3a, b). Surprisingly, both rFGF4 and rFGF1 induced robust activation of AMPKα in db/db mouse skeletal muscle (Fig. 3a and Supplementary Fig. 3c), implying that these ligands stimulate GLUT4 expression and translocation by activating AMPKα independently of insulin signaling. To test this conjecture, we pretreated db/db mice with the selective AMPKα antagonist compound C26 prior to administration of rFGF4 or rFGF1. This pretreatment diminished the ability of rFGF4 or rFGF1 to induce GLUT4 expression and translocation (Supplementary Fig. 4a, b and Supplementary Fig. 4d, e) as well as anti-hyperglycemic activity (Supplementary Fig. 4c and Supplementary Fig. 4f). These data were further corroborated using mice lacking AMPKα2, the principal AMPK isoform in skeletal muscle24. In these animals, the acute glucose-lowering effect of rFGF4 or rFGF1 was dramatically compromised (Fig. 3b and Supplementary Fig. 5a), and was accompanied by a marked reduction in GLUT4 expression and translocation (Fig. 3c, d and Supplementary Fig. 5b, c).
a Phosphorylation levels of FGFR1 and AMPKα hosphorylatdb/db mice 6 h after i.p. injection of buffer (control) or rFGF4 (1.0 mg/kg body weight) as determined by western blotting (left panel) and quantitated using ImageJ software (center and right panels) (n = 6). b Blood glucose levels 6 h after i.p. injection of rFGF4 (1.0 mg/kg body weight) into WT and AMPKα2 knockout mice (AMPKa2−/−) (n = 7). All mice were fed with a high-fat diet for 12 weeks. c Expression and translocation of GLUT4 in LLM of AMPKα Expressiut mice 6 h after i.p. injection of rFGF4 (1 mg/kg body weight) shown by immunofluorescence staining using an anti-GLUT4 antibody. Data are representative of 6 mice from each group. Scale bars, 50 μca. d Expression of AMPKαE (n = 5) and GLUT4 (n = 6) in LLM of AMPKα in LLM of AMPKAMP h after i.p. injection of buffer (control) or rFGF4 (1.0 mg/kg body weight) as measured by western blotting (left panel) and quantitated using ImageJ software (center and right panels). e mRNA level of FGFR1c (n = 9), FGFR2b (n = 7), FGFR2c (n = 9), FGFR3b (n = 9), FGFR3c (n = 9), and FGFR4 (n = 9) in skeletal muscle of WT mice determined by real-time PCR analysis. f FGFR1 and GLUT4 expression and AMPKαFGFR1 and GLUT4 expression and AMPinducible skeletal muscle FGFR1 knockout (imKO) mice 6 h after i.p. injection of buffer (control) or rFGF4 (1.0 mg/kg body weight) as determined by western blotting (left panel) and quantitated using ImageJ software (right panels) (n = 4). g Expression and translocation of GLUT4 in LLM of imKO mice 6 h after i.p. injection of rFGF4 (1 mg/kg body weight) shown by immunofluorescence staining with an anti-GLUT4 antibody. Data are representative of 5 mice from each group. Scale bars, 50 μca. h Blood glucose levels 6 h after i.p. injection of rFGF4 (1.0 mg/kg body weight) into imKO mice, with FGFR1flox/flox mice (WT) as controls (n = 5). All mice were fed with a high-fat diet for 12 weeks. Data are presented as mean +/− SEM. Statistical comparison in (a) is unpaired two-tailed test. Statistical comparisons in (b) and (h) are two-way ANOVA tests with Tukey’s multiple comparisons tests. Statistical comparisons in (d–f) are one-way ANOVA tests with Tukey’s multiple comparisons tests. Source data are provided as a Source data file.
FGFR1c is the most abundantly expressed FGFR isoform in skeletal muscle (Fig. 3e)19,27, implying that rFGF4 and rFGF1 upregulate GLUT4 expression/translocation and induce anti-hyperglycemic activity by binding and activating FGFR1c. Indeed, rFGF4 incurred a major loss in its ability to upregulate AMPKα activation and GLUT4 expression/translocation when administered to mice containing a muscle-specific FGFR1 deletion (Fig. 3f, g). Accordingly, rFGF4 failed to correct hyperglycemia in these mice (Fig. 3h). Consistent with these data, the FGFR1 kinase antagonist PD16686628 blocked rFGF4’s acute glucose-lowering effect (Supplementary Fig. 5d). Importantly, PD166866 also abolished the anti-hyperglycemic activity of rFGF1 implying that rFGF1 also activates FGFR1c to stimulate GLUT4 translocation and AMPKα activation (Supplementary Fig. 5e-g).
Consistent with the observed insulin-independent effects of rFGF4 on skeletal muscle in vivo, we found that rFGF4 is sufficient to induce glucose uptake in differentiated rat myoblasts cells (L6) (Fig. 4a). In addition, dose-dependent rFGF4 treatment led to the phosphorylation and activation of FGFR1 and AMPKα, as well as an upregulation of GLUT4 expression in these cells (Fig. 4b). Indeed, rFGF4 noticeably increased the surface abundance of GLUT4 (Fig. 4c). This observation was also replicated in the context of 3T3-L1 adipocytes transfected with Myc-GLUT4-GFP expression plasmid (Supplementary Fig. 6a, b) (functioning as a model cell), in which rFGF4 robustly promoted the translocation of GLUT4 to cell membrane (Fig. 4d). Moreover, either FGFR1 inhibition by PD166866 or AMPKα2 gene silencing by siRNA significantly blocked these rFGF4-induced activities (Fig. 4e, f). Finally, incubation with rFGF4 did not enhance the ability of insulin to induce AKT phosphorylation in either normal or insulin-resistant rat L6 myocytes (Fig. 4g, h), while exposure of L6 myocytes to insulin did enhance GLUT4 expression and translocation in an FGFR1/AMPK signaling-independent manner (Fig. 4b–d). This presumably reflects activation of the classical IRS1/AKT/GLUT4 pathway29. Taken as a whole, the data from these experiments lead us to conclude that a single dose of rFGF4 upregulates GLUT4 expression and translocation by activating AMPKα signaling operating downstream of FGFR. Notably, as consistent with its neutral effect on blood glucose in wild-type mice, rFGF4 did not activate AMPK or induce any change in the expression and translocation of GLUT4 in skeletal muscle in these animals (Supplementary Fig. 1f).
a Glucose uptake in differentiated rat myoblasts cells (L6) stimulated with rFGF4 (0.1 or 0.5 μg/mL) or a buffer control (vehicle) for 1 h with insulin (100 nM) served as a positive control. Data from four independent measurements are presented as mean +/− SEM. b Semi-quantitative western blot analyses of phosphorylation levels of FGFR1 and AMPKα together with expression levels of GLUT4 in L6 cells exposed to rFGF4 (0.1 or 0.5 μg/mL), insulin (50 nM) or a buffer control for 20 min. Data from three independent measurements are presented as mean +/− SEM. c Semi-quantitative western blot analyses of plasma membrane GLUT4 protein levels in L6 cells exposed to rFGF4 (0.5 μg/mL), insulin (50 nM) or a buffer control for 20 min. Na, K ATPase was used as a plasma membrane fraction marker. Data from four independent measurements are presented as mean +/− SEM. d Plasma membrane translocation of GLUT4 in 3T3-L1 adipocytes transfected with Myc-GLUT4-GFP expression plasmid and stimulated with rFGF4 (0.5 μg/mL) (n = 8), insulin (100 nM) (n = 10) or a buffer control (vehicle) (n = 15) for 20 min shown by total internal reflection fluorescence (TIRF) images. The fluorescent area from GLUT4 dots on the plasma membrane was quantified as mean pixel intensity per unit area and represented as % relative to insulin treatment. Scale bars, 20 μm. Data are presented as mean +/− SEM. e, f Semi-quantitative western blot analyses of phosphorylation levels of AMPKα together with expression levels of GLUT4 in L6 cells either pretreated with PD166866 (20 μM) for 30 min and then exposed to rFGF4 (0.5 μg/mL) for an additional 20 min (e) or transfected with control or AMPKα2 siRNAs and exposed to rFGF4 (0.5 μg/mL) for 20 min (f). Data from three independent measurements are presented as mean +/− SEM. g Phosphorylation levels of AKT in L6 myoblasts pretreated with rFGF4 (0, 0.1, or 0.5 μg/mL) for 20 min and stimulated with insulin (50 nM) for another 15 min. The images are representative of three independent experiments with similar results. h L6 myoblasts were pretreated with either insulin alone (200 nM), insulin plus rFGF4 (0.5 μg/mL -pre) or a buffer control for 2 h, and then stimulated with either insulin (50 nM), rFGF4 (0.5 μg/mL), or insulin plus rFGF4 for 20 min. The images are representative of three independent experiments with similar results. Phosphorylation levels of AKT in these cells were measured by western blotting. Statistical comparisons in (a–f) are one-way ANOVA tests with Tukey’s multiple comparisons tests. Source data are provided as a Source data file.
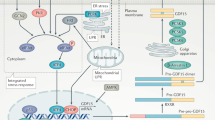
rFGF4 activates AMPKα by triggering Ca2+/CaMKK2 signaling downstream of FGFR1
PLCγ acts downstream of FGFR1 to hydrolyze phosphatidylinositol 4,5-bisphosphate, thereby generating diacyl glycerol and inositol 1, 4, 5-triphosphate (IP3) second messengers30,31. The latter induces the release of Ca2+ from intracellular stores31,32. The increase in intracellular [Ca2+] could lead to activation of Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2, also known as CaMKKβ), and consequently of AMPKα33,34. We therefore surmised that rFGF4-FGFR1 signaling might activate AMPKα via the Ca2+/CaMKK2 pathway. Indeed, we found that rFGF4 induced a marked upregulation of intracellular [Ca2+] in mouse embryonic fibroblasts after 10 min treatment (Supplementary Fig. 7a, b). Consistent with these data, the acute treatment of db/db mice with rFGF4 robustly enhanced the activity of calmodulin-dependent protein kinase I (CaMKI) (a known reporter for CaMKK2 activity35) in skeletal muscle (Supplementary Fig. 7c). This activity was mostly abrogated by muscle-specific deletion of FGFR1 (Supplementary Fig. 7d). These results were further validated in L6 myocyte-based experiments in which treatment with rFGF4 led to significant activation of CaMKI, an effect that was abrogated in the presence of PD166866 (Supplementary Fig. 7e). Moreover, the selective pharmacological inhibitor of CaMKK2 (STO-609)36 significantly blocked rFGF4-induced activation of CaMKK2 and AMPKα (Supplementary Fig. 7f). These cell-based data were further corroborated using CaMKK2 knockout (CaMKK2-/-) mice. Specifically, rFGF4 failed to reduce hyperglycemia in high fat diet fed CaMKK2-/- mice (Supplementary Fig. 7g). We conclude that rFGF4-induced AMPKα activation is relayed by Ca2+/CaMKK2 signaling acting downstream of FGFR1.
Chronic rFGF4 treatment ameliorates insulin resistance in db/db and DIO mice
We next explored the effects of prolonged (i.e., chronic) administration of FGF4 by i.p. injection of db/db mice with rFGF4 (1 mg/kg body weight) on alternate days over the course of 37 days. Consistent with the results of our single-dose treatment experiments, this chronic treatment elicited a sustained glucose-lowering effect without causing any significant change in body weight or undesired induction of hypoglycemia (Fig. 5a, b). Similar results were observed in DIO mice receiving 15 days of rFGF4 (1 mg/kg body weight) treatment (Fig. 5c, d). Notably, rFGF4 administration did not affect energy expenditure as measured by rates of oxygen consumption (VO2) or carbon dioxide production (VCO2), heat production, food intake, or in total or ambulatory activity (Supplementary Fig. 8a-g). Epididymal white adipose tissue (Epi-WAT) mass, adipocyte size, lean mass, fat mass, and organ mass were not impacted (Supplementary Fig. 9a-d and Supplementary Table 2). Additionally, chronic rFGF4 administration had no deleterious impact on either bone mineral density (BMD) or bone mineral content (BMC) (Supplementary Fig. 9e, f). Finally, db/db mice receiving long-term rFGF4 treatment showed no overt signs of hepatic hyperplasia as revealed by intensities of markers including proliferating cell nuclear antigen (PCNA) and Ki67 (Supplementary Fig. 10a, b).
a, b Blood glucose (a) and body weight (b) of ad libitum-fed db/db mice injected with rFGF4 (1.0 mg/kg body weight) (n = 8) on alternate days for 37 days with PBS buffer (db/db) (n = 8); db/m mice served as controls (n = 14). c, d Blood glucose (c) and body weight (d) of ad libitum-fed DIO mice injected with rFGF4 (1.0 mg/kg body weight) on alternate days for 15 days with PBS buffer (DIO) as controls (n = 5). e Left: OGTT over the course of 120 min done following 37 days of chronic administration of rFGF4 (1.0 mg/kg body weight) to db/db mice (n = 5). Right: integrated AUC for changes in blood glucose levels. f Left: OGTT over the course of 120 min done following 15 days of chronic administration of rFGF4 (1.0 mg/kg body weight) to DIO mice (n = 6). Right: integrated AUC for changes in blood glucose levels. g, h Blood glucose levels (g) and glucose infusion rate (GIR) (h) during a hyperinsulinemic euglycemic clamp for 14 days in rFGF4-treated (1.0 mg/kg body weight) db/db mice (n = 6) with PBS buffer (db/db) served as controls (n = 7). i–k Glucose infusion rate (GIR) (i), glucose disposal rate (GDR) (j), and insulin stimulated glucose disposal rate (IS-GDR) (k) during a hyperinsulinemic euglycemic clamp for 14 days in rFGF4-treated (1.0 mg/kg body weight) DIO mice (n = 5) with PBS buffer (DIO) served as controls (n = 7). Data are presented as mean +/− SEM. Statistical comparisons in (a, c, e (left penal) and f (left penal)) are two-way ANOVA tests with Tukey’s multiple comparisons tests (a and e) or Šídák’s multiple comparisons tests (c, f and h). Statistical comparison in (e (right penal) is one-way ANOVA test with Tukey’s multiple comparisons test. Statistical comparisons in (f (right penal) and i–k) are unpaired two-tailed tests. Source data are provided as a Source data file.
The diminished mitogenic character of rFGF4 observed in vivo was rather expected, as rFGF4 carries an engineered N-terminal truncation (residues Ala67 to Leu206) (Supplementary Fig. 10c-e) that we intentionally introduced in order to induce decrease in the mitogenic activity predicted to be associated with the wild-type parent molecule (i.e., mFGF4) (Supplementary Fig. 10f). Specifically, the truncation in rFGF4 removes several N-terminal residues in mFGF4 necessary for stable FGFR binding and dimerization. As a result, the stability/longevity of rFGF4 induced FGFR dimer is below the threshold necessary for eliciting a mitogenic response but still sufficient for a metabolic response17. Indeed, surface plasmon resonance (SPR) spectroscopy experiments showed that compared to mFGF4, the binding affinity of the N-terminally truncated rFGF4 to FGFR1c isoform is weaker (Supplementary Fig. 10g, h). Accordingly, proximity ligation assay (PLA) experiments showed that relative to its wild-type parent molecule, truncated rFGF4 had reduced capacity to induce dimerization of FGFR1c ectopically expressed on the surface of L6 myoblasts (Supplementary Fig. 10i).
We further corroborated the diminished mitogenic character of rFGF4 in the context of wild type mice by treating these mice daily for 15 days. In parallel experiments, we administered mFGF4 and rFGF19 as positive controls; in both cases, these proteins are known to induce full proliferative activity2,4,37. Consistent with published literature38,39, rFGF19 treatment led to dramatic increase in the expression of hepatic proliferation markers (Supplementary Fig. 10j, k). Relative to buffer control, mFGF4 also caused significant increase in the expression of hepatic proliferation biomarkers albeit to significantly lesser degree than rFGF19. Consistent with the data in db/db mice, the intensities of proliferation markers in the rFGF4 group were not statistically different from the PBS control group (Supplementary Fig. 10j, k). Notably, the in vivo half-life of rFGF4 (~4.4 h) remained similar to that of mFGF4 (~4.3 h) (Supplementary Table 1) ruling out the possibility that a difference in mitogenic potentials between these molecules was due to reduced biostability of rFGF4. We conclude that rFGF4 is a potent and potentially clinically useful glucose-lowering agent, and that it causes neither hypoglycemia nor other overt adverse effects under our experimental conditions.
To further assess the ability of chronic rFGF4 administration to exert glycemic control, db/db and DIO mice were subjected to an oral glucose tolerance test (OGTT). In both cases, we found a sustained reduction in serum glucose level over the course of 2 h (Fig. 5e, f). These results were corroborated via a hyperinsulinemic/euglycemic clamp test in which db/db and DIO mice were treated for 14 days with rFGF4. Under hyperinsulinemic conditions, db/db mice required more exogenous glucose to maintain euglycemia, as shown by the glucose infusion rate (GIR) (Fig. 5g, h). Moreover, in similarly treated DIO mice, there were evident increases in GIR, glucose disposal rate (GDR) and insulin-stimulated GDR (IS-GDR) (Fig. 5i-k) without any significant change in insulin level or suppression of free fatty acids (FFA) (Supplementary Fig. 11). Taken together, these data demonstrate that chronic treatment with rFGF4 substantially alleviates hyperglycemia and improves insulin resistance in T2D and obese mice.
Chronic rFGF4 treatment promotes skeletal muscle glucose disposal
As in the case of acute treatment, chronic treatment with rFGF4 led to sustained phosphorylation of AMPKα and increased expression of GLUT4 in the skeletal muscle of db/db or DIO mice (Fig. 6a, e). Consistent with improved insulin resistance, AKT activity in skeletal muscle was also enhanced in these experiments (Fig. 6a, e). This contrasts with the data observed in mice receiving single-dose treatment, in which the insulin signaling was not activated. Activation of the AMPKα and insulin signaling pathways led to increased skeletal muscle glucose disposal and diminished phosphorylation of glycogen synthase (GS) (Fig. 6a, e), an effect that was mirrored by enhanced glycogen storage (Fig. 6b, c and f). Muscle glucose metabolic activity was also enhanced as revealed by an increase in transcription of two key glycolytic enzymes (i.e., Pkm and HK2), a tricarboxylic acid cycle enzyme (Sdha), an enzyme involved in oxidative phosphorylation (Pdha1, a subunit of pyruvate dehydrogenase (PDH)), and a decrease in the transcription of Pdk4 (a negative regulator of PDH) (Fig. 6d, g)40. We conclude that long-term administration of rFGF4 maintains glucose homeostasis through sustained activation of AMPKα and amelioration of insulin resistance in skeletal muscle, whereas in the case of short-term treatment, rFGF4 maintains glucose homeostasis solely via AMPKα activation.
a–d db/db mice were treated every other day for 37 days with rFGF4 (1.0 mg/kg body weight) or a buffer control; littermate db/m mice served as additional controls. a Phosphorylation levels of AMPKα, GS, and AKT and expression levels of GLUT4 as determined by western blotting (left panel) and quantitation using ImageJ software (right panel) (n = 4). b, c Representative periodic acid–Schiff staining (PAS; magenta denotes glycogen) (b) and glycogen content (c) of skeletal muscle in db/db mice (n = 6). d Real-time PCR analysis of expression of Pkm, Hk2, Shda, Pdha1, and Pdk4 mRNAs (n = 6). Scale bars, 100 μm. e–g DIO mice were treated every other day for 30 days with rFGF4 (1.0 mg/kg body weight) or a buffer control. e Phosphorylation levels of AMPKα, GS, and AKT and expression levels of GLUT4 were determined in skeletal muscle by western blotting (left panel) and quantified using ImageJ software (right panel) (n = 6). f Glycogen content of skeletal muscle in DIO mice (n = 5). g Real-time PCR analysis of expression of Pkm, Hk2, Shda, Pdha1, and Pdk4 mRNAs (n = 6). Data are presented as mean +/− SEM. Statistical comparisons in (a, c) are one-way ANOVA tests with Tukey’s multiple comparisons tests. Statistical comparisons in (d–g) are unpaired two-tailed tests. Source data are provided as a Source data file.
rFGF4 ameliorates inflammation by direct action on macrophages
Adipose tissue macrophage (ATM) infiltration and inflammation play primary roles in the etiology of insulin resistance41,42. In obesity, F4/80+CD11c+ macrophages account for the majority of infiltrating ATMs41,43,44. We therefore assessed the inflammatory status of Epi-WAT in chronically rFGF4-treated db/db mice by immunostaining with antibodies to CD68 (Cluster of Differentiation 68, a protein highly expressed in macrophages) and F4/80. We noted reduced expression of CD68 and monocyte chemotactic protein 1 (MCP-1) in Epi-WAT excised from rFGF4-treated animals (Fig. 7a–c). Consistent with these observations, we found a substantial decrease in F4/80+ macrophages in rFGF4-treated db/db mice relative to controls (Fig. 7d). Immunofluorescence using F4/80 and CD11c-specific antisera showed a marked increase in F4/80+CD11c+ macrophages in db/db mice, a feature that was greatly diminished by treatment with rFGF4 (Fig. 7d, e). There was also a corresponding decrease in transcription of several pro-inflammatory genes in Epi-WAT, including KC, Galectin-3, IL-1β, IL-6, CCl2, CCl3, CCl5, Cxcl10, and TNF-α (Fig. 7f).
a–j Analysis of Epi-WAT tissue and sera from db/db mice treated over the course of 37 days with rFGF4 (1.0 mg/kg body weight). a Immunohistochemical staining with an anti-CD68 antiserum. Data are representative of 4 mice from each group. Scale bars, 50 μm. b, c Western blot analyses showing expression of CD68 (b) and MCP-1 (c) in Epi-WAT tissue and their quantitation (shown at right) (n = 6). d Immunofluorescence staining with antibodies to either F4/80 or CD11C (n = 4). Scale bars, 100 μm. e Quantification of immunofluorescence staining with antibodies to either F4/80 or CD11C in panel d using ImageJ software. f Real-time PCR analysis of expression of F4/80, CD11c, KC, Galectin-3, IL-1β, IL-6, CCl2, CCL3, CCl5, CXCl10, and TNF-α mRNAs (n = 5). Littermate db/m mice served as controls. g Schematic of in vivo macrophage tracking experiments. h, i FACS analysis of total CD45+CD11b+GFP+ monocytes (h) and total CD45+CD11b+ GFP+F4/80+CD11c+ macrophages (i) in mouse stromal vascular cells (n = 4). j Serum concentrations of TNF-α and IL-6 determined by ELISA (n = 5). k Western blot analyses of bone marrow-derived macrophages (BMMs) pretreated with rFGF4 (1, 10, or 100 ng/mL for 1 hr) and exposed to either low glucose (LG) or high glucose (HG) (36 mM) for 2 h. The images are representative of three independent experiments with similar results. Antisera used were against phosphorylated or unphosphorylated forms of FGFR1, AMPKα, JNK, IKBα, and IKKα/β; an antibody to GAPDH was used as a loading control. l Immunofluorescence staining showing nuclear import of NF-κB subunit P65 (red) in BMMs incubated with LG or HG and treated with increasing concentrations of rFGF4. The images are representative of three independent experiments with similar results. Nuclei were stained with DAPI (blue). Scale bars, 25 μm. m Expression of proinflammatory cytokine IL-6 as determined by ELISA in BMMs pretreated with rFGF4 (1, 10, or 100 ng/ml) for 1 h, followed by treatment with HG (36 mM) for an additional 24 h. Data from three independent measurements are presented as mean +/− SEM. Statistical comparisons in (b, c and e) are unpaired two-tailed tests. Statistical comparisons in (f, h, i, j and m) are one-way ANOVA tests with Tukey’s multiple comparisons tests. Source data are provided as a Source data file.
To corroborate these findings, we did an in vivo macrophage chemotaxis assay which entailed injecting GFP-labeled monocytes into db/db mice treated with either buffer alone or rFGF4 (Fig. 7g). In this experiment, rFGF4 administration resulted in a substantial decrease in the number of GFP-labeled adipose tissue monocytes (Fig. 7h). Moreover, ATM infiltration and accumulation in Epi-WAT was greatly reduced by chronic rFGF4 treatment, an observation presumably attributable to a decrease in F4/80+CD11c+ macrophages (Fig. 7i). Consistent with reduced inflammation in Epi-WAT in rFGF4 treated db/db mice, we found reduced expression of inflammatory genes in skeletal muscle and in liver (Supplementary Fig. 12a, b). rFGF4 treatment also resulted in reduced serum levels of some pro-inflammatory cytokines (i.e., IL-6 and TNF-α) (Fig. 7j), as well as of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Supplementary Fig. 12c) in db/db mice. Reminiscent of the db/db mice subjected to chronic treatment with rFGF4, there were also decreases in expression of CD68 and inflammatory genes in Epi-WAT as well as reduced serum levels of IL-6 and TNF-α in long-term rFGF1-treated db/db mice (Supplementary Fig. 13a-c).
Given that macrophages endogenously express FGFR1c45 and that AMPKα activation in macrophages ameliorates adipose tissue inflammation46, we considered the possibility that rFGF4 might act directly on these cells to attenuate the inflammatory response via activation of the AMPKα pathway. To test this idea, we exposed cultured primary macrophages to high glucose and examined the phosphorylation of FGFR1, AMPKα, IKKα/IKKβ, IκBα, and JNK in response to rFGF4 treatment. We found that rFGF4 enhanced phosphorylation of FGFR1 and AMPKα, while reducing phosphorylation levels of JNK and IKKα/β in a dose-dependent manner (Fig. 7k). This was accompanied by a corresponding suppression in dissociation of the IκBα/NF-κB complex, and by phosphorylation of IκBα (Fig. 7k). Moreover, the immunofluorescence assay showed that nuclear import of the NF-κB subunit P65 was strongly suppressed by rFGF4 treatment (Fig. 7l), with a reduction of IL-6 in the macrophage culture medium following incubation with rFGF4 (Fig. 7m). These data demonstrate that rFGF4 can alleviate ATM infiltration and inflammation in diabetes by acting directly on macrophages.
Discussion
FGF4 plays indispensable roles during embryonic development, including implantation, morphogenesis, and organogenesis. In adults, it is expressed at low level in duodenum, ileum, and colon where it is proposed to maintain intestinal stem cells27. At the cellular level, it acts by regulating cell proliferation and differentiation of embryonic and tissue stem cells in a paracrine fashion2,37,47. It exerts its diverse functions by binding, dimerizing, and hence activating its multiple cognate FGFRs including FGFR1c, FGFR2c, FGFR3c splice isoforms, and FGFR4 with the assistance of HS2,37,47. FGF4 shows greatest activity towards FGFR1c followed by FGFR2c, FGFR3c, and FGFR419.
In this study, we screened multiple paracrine FGFs in a search for those that possess a glucose-lowering effect in T2D mice. This search resulted in the discovery of FGF4 as a potent anti-hyperglycemic factor, with glucose-lowering effects rivaling those of FGF1 (Fig. 1 and Supplementary Fig. 1). Our findings expand the potential armamentarium of FGFs for the treatment of T2D and related metabolic disorders. Notably, unlike FGF1, which causes a phasic restriction of food intake, FGF4 does not affect food intake (Fig. 1 and Supplementary Fig. 8e). This may be attributed to the fact that FGF1 is the most promiscuous FGFR ligand capable of binding and activating all seven principal FGFRs, including the ‘b’ splice isoforms19,48,49. In contrast, FGF4 does not cross-react with ‘b’ splice isoforms at all.
We found via micro-PET CT that short-term administration of either rFGF4 or rFGF1 in mice led to conspicuous glucose uptake in skeletal muscle, and to a lesser extent in liver and in WAT (Fig. 2a–c). Because skeletal muscle lacks β-klotho (a mandatory co-receptor of endocrine FGF214), rFGF21 is unable to induce 18F-FDG uptake in this tissue (Fig. 2a–c). These results are consistent with previous reports that endocrine FGF21 and FGF19 mediate their glucose-lowering activities principally in liver and WAT, but not in muscle4,10,50. Therefore, the inability of FGF19 and FGF21 to stimulate glucose uptake in skeletal muscle—the main site of insulin-mediated glucose disposal (up to ~75%)51,52—accounts for the inferior potency of these endocrine FGFs relative to paracrine FGF4/FGF1, which can target this β-klotho deficient tissue.
GLUT4 is the key modulator that facilitates glucose uptake in the skeletal muscle22,23. Indeed, dysregulation of GLUT4 has been associated with insulin resistance in skeletal muscle. Although GLUT4 level is moderately decreased in skeletal muscle of obese rodents, impaired insulin-dependent GLUT4 translocation in this tissue is considered as one of the main reasons for obesity-induced insulin resistance53,54,55,56. Targeted overexpression of GLUT4 in the skeletal muscle is considered as a potential approach to improve insulin resistance23,57. It has been suggested by a number of studies that recruitment of GLUT4 to the cell surface of skeletal muscle is acutely activated by signals including insulin and AMPK24,25,29,58,59. Insulin elicits GLUT4 translocation via PI3-kinase-dependent mechanisms whereas exercise relies on the activation of AMPK signaling29,59. Exercise activates AMPK probably through increasing the AMP/ATP ratio or activating LKB1 or Ca2+ associated CaMKK259,60. In addition to its role in GLUT4 translocation, activated AMPK is thought to be translocated to the nucleus, where it stimulates binding of MEF2 to the GLUT4 promotor61 or phosphorylates histone deacetylase 562 to upregulate GLUT4 expression. Here we found that acute treatment of db/db mice with rFGF4 triggers AMPKα activation rather than activating the insulin-dependent pathway to subsequently upregulate GLUT4 expression and translocation to the cell membrane (Fig. 3 and Supplementary Fig. 3). Consistent with our observations, activation of AMPKα has recently been shown to induce an acute glucose-lowering effect without enhancing insulin signaling40,63. Furthermore, we found that rFGF4 activates AMPKα via activation of CaMKK2 probably as a result of intracellular Ca2+ release following engagement of FGFR1 (Supplementary Fig. 7). Indeed, suppression or elimination of skeletal muscle AMPKα2 in insulin-resistant mice abrogated FGF4-mediated GLUT4 translocation and expression as well as FGF4’s glucose-lowering effect (Fig. 3 and Supplementary Fig. 4).
It has been reported that activation of AMPKα in adipose tissue reduces macrophage infiltration and associated inflammation64. A particularly striking finding with regard to the anti-diabetic effect of rFGF4 is its direct action on macrophages, where it blocks the inflammatory response and tissue inflammation in liver, muscle, and adipose tissue (Fig. 7 and Supplementary Fig. 12), likely via activation of AMPKα signaling. Because inflammatory cytokines block insulin signaling and induce insulin resistance directly65, our observed decrease in macrophage infiltration and inflammation explains the enhanced insulin-sensitizing effect of chronic rFGF4 treatment. Given the ability of rFGF4 to induce glucose uptake in skeletal muscle and to ameliorate systemic inflammation, our data offer promise that rFGF4 may yield a clinically useful agent for the treatment of T2D.
Methods
Expression and purification of FGFs and FGFR1c ectodomain
Recombinant full length mature human FGF1 (rFGF1) (Met1-Asp155), full length mature form human FGF4 (mFGF4) (Ala31-Leu206) and its N-terminally truncated human FGF4 (rFGF4) (Ala67-Leu206), full length mature human FGF7 (rFGF7) (Cys32-Thr194), FGF8 (rFGF8) (Gln23-Arg215), FGF9 (rFGF9) (Met1-Ser208), FGF19 (rFGF19) (Leu25-Lys216), FGF21 (rFGF21) (His29-Ser209) and soluble extracellular D2–D3 region of human FGFR1c (Asp142-Arg365) were all expressed in E. coli (BL21) and purified as described in Supplementary experimental procedures.
Surface plasmon resonance spectroscopy
All real-time biomolecular interactions were performed on a BIAcore T200 system (GE Healthcare, Piscataway, NJ) in HBS-EP buffer (10 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% Surfactant P20) as described in Supplementary experimental procedures.
Animals and animal welfare
All animal protocols used in our studies were approved by the Animal Care and Use Committee of Wenzhou Medical University, China. We have complied with all relevant ethical regulations for animal testing and research.
Four- to eight-week-old male db/db (C57BLKS/J-leprdb/leprdb) mice and their non-diabetic db/m littermates, male ob/ob (C57BL/6J-lepob/lepob) mice, male EGFP transgenic (STOCK-Tg (CAG-EGFP)/Nju) mice, male C57BL/6J mice (wild type mice) and male Sprague Dawley (SD) rats were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). Male and female FGFR1flox/flox (B6.129S4-Fgfr1tm5.1Sor/J) mice were purchased from The Jackson Laboratory. Male and female AMPKα2 knockout mice66 were obtained from Dr. Louise D. McCullough at the Department of Neurology, McGovern Medical School, University of Texas Health Science Center at Houston (UTHealth), Houston, TX, USA. Four- to six-week-old male CaMKK2 knockout mice were obtained from Dr. Shengcai Lin at the School of Life Sciences, Xiamen University, Xiamen, Fujian, China. To confirm genotypes, genomic DNA prepared from tail snips was analyzed using PCR. All protocols used in our studies were approved by the Animal Care and Use Committee of Wenzhou Medical University, China. All animals were acclimatized to our laboratory environment before use and housed in a specific pathogen-free (SPF) animal facility with controlled environment (22 ± 2 °C, 50–60% humidity, 12-h light/dark cycle, lights on at 7 AM) and free access to food and water. Diet-induced obese (DIO) mice were established by feeding male C57BL/6J mice, AMPKα2 knockout mice, or CaMKK2 knockout mice with a high-fat diet (20% protein; 60% fat; 20% carbohydrates; Cat. D12492; Research Diets, Inc, New Brunswick, NJ) and with free access to water from an age of 4–6 weeks. After 12 weeks feeding on either a standard or high-fat diet, mice were randomly assigned by body weight and fed blood glucose levels determined using a FreeStyle complete blood glucose monitor (Abbott Diabetes Care Inc., Alameda, CA).
Four- to six-week-old male FGFR1flox/flox mice were fed with a high-fat diet for 8 weeks and randomly assigned by body weight and fed blood glucose levels. Conditional skeletal muscle FGFR1 knockout mice were established by local gastrocnemius muscle injection of HBAAV2/9-CMV-cre-GFP viruses with HBAAV2/9-GFP viruses as controls (purchased from Hanbio Biotechnology Co., Ltd., Shanghai, China). These mice received ~2 × 1010 viral particles per injection and eight injections per limb, and were continuously fed with a high-fat diet for 4 weeks.
Glucose-lowering studies of rFGF1, rFGF4, rFGF7, rFGF8, and rFGF9 in db/db mice
For analysis of the acute (single dose) effects of rFGF1, rFGF4, rFGF7, rFGF8, and rFGF9 on glucose levels in db/db mice, 30 animals were placed into 5 groups of 6 mice each and each group injected intraperitoneally (i.p.) with rFGF1, rFGF4, rFGF7, rFGF8, or rFGF9 (1.0 mg/kg body weight). Blood samples were taken from tail veins 6 h after treatment and blood glucose was measured as described above.
Functional evaluation of rFGF1, rFGF4, and rFGF21 in diabetic mice
Before each study, mice were randomized based on body weight and blood glucose level. All drugs and buffer controls were delivered by i.p. injection into mice unless specified otherwise. Full details of animal protocols (i.e., acute and chronic efficacy evaluation) are described in Supplementary experimental procedures.
Indirect calorimetry
db/db mice were treated with rFGF4 (1.0 mg/kg body weight) on alternate days over a 37-day period with buffer (PBS) as a control. The animals were then acclimated for 24 h in metabolic cages before monitoring. Both light cycle and dark cycle measurements of all animals were recorded at 49-minute intervals for 4 days. Oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange ratio (RER), heat production, food intake, and total and ambulatory activity were measured by an indirect calorimetry system (Oxymax, Columbus Instruments, Columbus, OH).
Hyperinsulinemic-euglycemic clamp study
db/db or DIO mice received either rFGF4 (1.0 mg/kg body weight) or buffer alone as a control on alternate days over 2 weeks. Hyperinsulinemic-euglycemic clamp tests were performed as previously described42 with minimal modification. Briefly, one catheter (Silastic 508-001, Dow Corning) was implanted in the right jugular vein of anesthetized mice, then tunneled subcutaneously to the back of the neck and exteriorized. Mice were allowed to recover for 4 days prior to clamp experiments. Mice losing <5% of their pre-surgery weight were included in the test, described in full in Supplementary experimental procedures.
RNA extraction, cDNA synthesis, and quantitative RT-PCR
Total RNA was extracted from mouse tissues with TRIzol reagent (Thermo Fisher Scientific, Waltham, MA). Details of cDNA synthesis and qRT-PCR are described in Supplementary experimental procedures.
Micro positron emission computed tomography (microPET-CT) imaging and analysis
Overnight fasted db/db mice were injected i.p. with rFGF1, rFGF4, rFGF21 (each at 1.0 mg/kg body weight), or PBS (as a control). After 2 h, mice received a single dose (177.6 +/− 20.65 μCi, i.v.) of 18F-FDG through the lateral tail vein and were anaesthetized (via inhalation of 1.5% isoflurane in oxygen at 2 L/min) and scanned during a 1-h period in an Inveon preclinical multimodal PET-CT (Siemens, Munich, Germany). Images were reconstructed using a three-dimensional ordered subsets expectation maximum (3D OSEM) algorithm followed by Maximum a Posteriori (MAP). 3D regions of interest (ROIs) were drawn over the liver and muscle guided by CT images; radiological activity of corresponding tissues was measured using an Inveon Research Workplace (Siemens, Munich, Germany). After scanning, mice were sacrificed by cervical dislocation. The liver, lower limb muscles (thigh muscles), back muscle (erector spinae muscles), Epi-WAT, and SWAT were excised and radiotracer uptake (ID%/g) in these tissues was measured by γ-counting. Aliquots of the injected dose were set aside and counted contemporaneously with tissue samples to correct for radioactive decay.
Pathological, histopathological, immunohistochemical, and immunofluorescent evaluation of mouse tissues
Epi-WAT, liver or skeletal muscle tissues were excised from wild type, FGFR1flox/flox, db/m, db/db, conditional skeletal muscle FGFR1 knockout, or AMPKα2 knockout mice following acute or chronic administration of indicated proteins (all at 1.0 mg/kg body weight) or with PBS buffer as a control and weighed. Epi-WAT, liver, and skeletal muscle were fixed in 4% paraformaldehyde overnight and embedded in either paraffin or using Tissue-Tek OCT compound (Sakura, Tokyo, Japan). After deparaffinization and rehydration, paraffin sections (5 μm) were stained with haematoxylin and eosin (H&E) reagent using standard procedures. Details of immunohistochemistry and immunofluorescence analysis of paraffin sections are described in Supplementary experimental procedures. Glycogen content of skeletal muscle tissue (100–200 mg) from db/db or DIO mice treated for 37 or 30 days with PBS (as a control) or rFGF4 was determined according to the manufacturer’s instructions (Abcam, Cambridge, UK).
Pharmacokinetic evaluation
The in vivo half-lives of rFGF1, rFGF4, and mFGF4 was determined following a single i.p. injection (all at 1 mg/kg body weight) into adult male SD rats (220–250 g). Detailed pharmacokinetic analyses of rFGF1, rFGF4, and mFGF4 are described in Supplementary experimental procedures.
Experiments using L6 muscle cells
L6 rat skeletal myoblasts (Serial number: GNR 4, National Collection of Authenticated Cell Cultures of China, Shanghai, China) were used for myotube formation. Details of cell preparation and stimulation are described in Supplementary experimental procedures.
Proximity ligation assay
L6 rat skeletal myoblasts ectopically expressing human FGFR1c were used for proximity ligation assay. Details of cell preparation and stimulation are described in Supplementary experimental procedures.
Experiments using 3T3-L1 adipocytes
3T3-L1 mouse embryonic fibroblasts (Resource number: ATCC® CRL-3242™, ATCC, Manassas, Virginia) were used for adipocyte differentiation. Details of cell preparation and stimulation are described in Supplementary experimental procedures.
Calcium imaging
Mouse embryonic fibroblasts (MEFs) were established by introducing SV40 T antigen into primary cultured embryonic cells from mice at E13.5. For determining the rFGF4-induced calcium influx, MEFs grown on 35-mm glass-bottom dishes (70% confluence) were treated with Fluo-4-AM (final concentration, 5 μM) (Molecular probes, Thermo Fisher Scientific, Waltham, MA) for 30 min, washed twice with PBS, and incubated in fresh medium. The fluorescent intensity at time zero was recorded. Cells were then treated with rFGF4 (0.5 μg/ml) or buffer control for 10 min and change in fluorescence intensity was recorded using a confocal microscope (Zeiss, Oberkochen, Germany).
Cell proliferation assay
HepG2 cells (obtained from Dr. Shengcai Lin at the School of Life Sciences, Xiamen University, Xiamen, Fujian, China) (Resource number: ATCC® HB-8065™, ATCC, Manassas, Virginia) were seeded in 96-well plates at 3 × 103 cells/well in 100 μl medium and maintained in a 5% CO2 incubator for 24 h at 37 °C. After serum starvation for 12 h, the cells were treated with increasing concentration of mFGF4 or rFGF4 (0–500 ng/ml) for 24 h at 37 °C. The cell proliferation assay was performed using cell counting kit-8 (CCK-8, Beyotime Biotechnology, Shanghai, China); 10 μl CCK-8 reagent was added to each well and cells were incubated for 1 h at 37 °C. The absorbance at 450 nm was measured using a microplate reader (Molecular Devices, CA, USA).
In vivo monocyte tracking
EGFP labeled monocytes were obtained from an EGFP transgenic donor mouse (STOCK-Tg (CAG-EGFP)/Nju) and lysed in red blood cell (RBC) buffer. Monocyte subsets were enriched with an EasySep® mouse monocyte enrichment kit according to the manufacturer’s instructions (STEMCELL Tech, Vancouver, BC). Isolated monocytes (2 × 106–5 × 106) were washed once in serum-free DMEM medium, resuspended in PBS buffer, and their densities determined. 0.5 × 106 viable cells were suspended into 0.2 ml PBS and injected into db/m or db/db mice via the tail vein. After treatment with either buffer (PBS) or rFGF4 (1.0 mg/kg body weight) on alternate days for a 10-day period, stromal vascular cells (SVCs) were isolated from Epi-WAT and analyzed by Flow Cytometry.
Stromal vascular cell (SVC) isolation and FACS analysis
Epididymal fat pads from db/m or db/db mice were minced and digested in PBS buffer containing 1 mg/ml type II collagenase (Sigma-Aldrich, St. Louis, MO) and 2% BSA (pH 7.4) at 37 °C for 30 min. The cell suspension was filtered through a 100 μm cell strainer and centrifuged at 1500 × g for 10 min. The floating adipocyte fraction was removed from the SVC pellet. The pellet was incubated with RBC lysis buffer (Thermo Fisher Scientific, Waltham, MA) for 5 min, re-centrifuged at 1500 × g for 10 min, and the pellet resuspended in PBS buffer supplemented with 2% FBS. Finally, SVCs were incubated with FcR blocking reagent (Miltenyi Biotec, Bergisch Gladbach, Germany) at 4 °C for 20 min, followed by an incubation with fluorochrome-conjugated antibodies to CD45 BV510, CD11b PE, CD11c BV421 (Biolegend, San Diego, CA), or F4/80 APC (Thermo Fisher Scientific, Waltham, MA). Labeled cells were analyzed using FACSAria II (BD Biosciences, NewJersey, USA) and data analysis was done using Flow Jo (Tree Star) (Supplementary Fig. 14).
Bone marrow-derived macrophage (BMM) based experiments
The isolation and culture of primary BMM from C57BL/6J mice was performed as previously described;67 experimental details are provided in Supplementary experimental procedures.
Western blot analysis
L6 myoblasts, bone marrow-derived macrophages, or mouse tissues were homogenized in RIPA lysis buffer (25 mM Tris, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) supplemented with protease and phosphatase inhibitors (all from Thermo Fisher Scientific Waltham, MA). Plasma membrane proteins of L6 muscle cells were isolated according to the manufacturer’s instructions provided (Invent Biotechnologies, Inc, Minnesota, USA). Protein concentrations were determined using a BCA Kit (Protein Assay Kit, Beyotime Biotechnology, Shanghai, China). Equal quantities of soluble protein (40 μg) were separated using 8–12% SDS-PAGE and electro-transferred onto a nitrocellulose membrane. Protein blots were probed with antibodies against primary antibodies (refer to Reagent and Resource-Antibody, Supplementary Table 3). Immune-reactive bands were detected by incubating with secondary antibody (Santa Cruz Biotechnology, Dallas, TX; or Biosharp, Hefei, China) conjugated with horseradish peroxidase and visualized using enhanced chemiluminescence (ECL) reagents (Bio-Rad, Hercules, CA). The optical densities of the immunoblots were analyzed using ImageJ image software (version 1.38e, NIH, Bethesda, MD) and normalized to the scanning signals of their respective controls.
Statistical analysis
In vitro experiments were repeated at least in triplicate. The results were expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism 7 built-in tests. For experiments with two groups, Student’s unpaired t tests were used. In the comparison of mean values of more than two groups, one-way or two-way ANOVA with post-tests (Tukey or Šídák) were used. P < 0.05 was considered to be statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data supporting the key findings of this study are included within the main article and its supplementary materials and can be obtained from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Ornitz, D. M. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 16, 205–213 (2005).
Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Disco. 8, 235–253 (2009).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005).
Degirolamo, C., Sabba, C. & Moschetta, A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat. Rev. Drug Discov. 15, 51–69 (2016).
Suh, J. M. et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513, 436 (2014).
Gasser, E., Moutos, C. P., Downes, M. & Evans, R. M. FGF1 - a new weapon to control type 2 diabetes mellitus. Nat. Rev. Endocrinol. 13, 599–609 (2017).
Kung, J. & Henry, R. R. Thiazolidinedione safety. Expert Opin. Drug Saf. 11, 565–579 (2012).
Schlessinger, J. et al. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 6, 743–750 (2000).
Goetz, R. & Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Bio. 14, 166–180 (2013).
Kurosu, H. et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 (2007).
Xie, Y., Zinkle, A., Chen, L. & Mohammadi, M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat. Rev. Rheumatol. 16, 547–564 (2020).
Dailey, L., Ambrosetti, D., Mansukhani, A. & Basilico, C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth F. R. 16, 233–247 (2005).
Eswarakumar, V. P., Lax, I. & Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth F. R. 16, 139–149 (2005).
Kouhara, H. et al. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89, 693–702 (1997).
Chau, M. D., Gao, J., Yang, Q., Wu, Z. & Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl Acad. Sci. USA 107, 12553–12558 (2010).
Huang, Z. et al. Uncoupling the mitogenic and metabolic functions of FGF1 by tuning FGF1-FGF receptor dimer stability. Cell Rep. 20, 1717–1728 (2017).
Zinkle, A. & Mohammadi, M. A threshold model for receptor tyrosine kinase signaling specificity and cell fate determination. F1000Res 7, https://doi.org/10.12688/f1000research.14143.1 (2018).
Ornitz, D. M. et al. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292–15297 (1996).
Zhang, X. et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 281, 15694–15700 (2006).
Xu, J. et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 (2009).
Coskun, T. et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 (2008).
Augustin, R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life 62, 315–333 (2010).
Zisman, A. et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 6, 924–928 (2000).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Huang, S. & Czech, M. P. The GLUT4 glucose transporter. Cell Metab. 5, 237–252 (2007).
Wong, W. T. et al. Adiponectin is required for PPARgamma-mediated improvement of endothelial function in diabetic mice. Cell Metab. 14, 104–115 (2011).
Fon Tacer, K. et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 24, 2050–2064 (2010).
Panek, R. L. et al. In vitro biological characterization and antiangiogenic effects of PD 166866, a selective inhibitor of the FGF-1 receptor tyrosine kinase. J. Pharm. Exp. Ther. 286, 569–577 (1998).
Watson, R. T. & Pessin, J. E. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem. Sci. 31, 215–222 (2006).
Huang, Z. et al. Two FGF receptor kinase molecules act in concert to recruit and transphosphorylate phospholipase Cgamma. Mol. Cell 61, 98–110 (2016).
Ellis, M. V. et al. Catalytic domain of phosphoinositide-specific phospholipase C (PLC). Mutational analysis of residues within the active site and hydrophobic ridge of plcdelta1. J. Biol. Chem. 273, 11650–11659 (1998).
Schlossmann, J. et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature 404, 197–201 (2000).
Anderson, K. A. et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 (2008).
Hawley, S. A. et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 (2005).
Li, M. et al. Transient receptor potential V channels are essential for glucose sensing by aldolase and AMPK. Cell Metab. https://doi.org/10.1016/j.cmet.2019.05.018 (2019).
Tokumitsu, H. et al. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 277, 15813–15818 (2002).
Kosaka, N., Sakamoto, H., Terada, M. & Ochiya, T. Pleiotropic function of FGF-4: its role in development and stem cells. Dev. Dyn. 238, 265–276 (2009).
Wu, X. et al. Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19). Proc. Natl Acad. Sci. USA 107, 14158–14163 (2010).
Niu, J. et al. Curtailing FGF19’s mitogenicity by suppressing its receptor dimerization ability. Proc. Natl Acad. Sci. USA 117, 29025–29034 (2020).
Cokorinos, E. C. et al. Activation of skeletal muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. Cell Metab. 25, 1147–1159 e1110 (2017).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest 112, 1796–1808 (2003).
Li, P. et al. Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance. Cell 167, 973–984 e912 (2016).
Li, P. et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 285, 15333–15345 (2010).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest 117, 175–184 (2007).
Masuda, Y. et al. Expression of Fgf23 in activated dendritic cells and macrophages in response to immunological stimuli in mice. Biol. Pharm. Bull. 38, 687–693 (2015).
O’Neill, L. A. & Hardie, D. G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493, 346–355 (2013).
Itoh, N. & Ornitz, D. M. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J. Biochem. 149, 121–130 (2011).
Travaglio, M. & Ebling, F. J. P. Role of hypothalamic tanycytes in nutrient sensing and energy balance. Proc. Nutr. Soc. 78, 272–278 (2019).
Haan, N. et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci. 33, 6170–6180 (2013).
Fisher, F. M. & Maratos-Flier, E. Understanding the physiology of FGF21. Annu Rev. Physiol. 78, 223–241 (2016).
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Klip, A. & Paquet, M. R. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care 13, 228–243 (1990).
Gurley, J. M., Griesel, B. A. & Olson, A. L. Increased skeletal muscle GLUT4 expression in obese mice after voluntary wheel running exercise is posttranscriptional. Diabetes 65, 2911–2919 (2016).
Sharma, B. R., Kim, H. J. & Rhyu, D. Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 13, 62 (2015).
Miura, T. et al. Impairment of insulin-stimulated GLUT4 translocation in skeletal muscle and adipose tissue in the Tsumura Suzuki obese diabetic mouse: a new genetic animal model of type 2 diabetes. Eur. J. Endocrinol. 145, 785–790 (2001).
King, P. A., Horton, E. D., Hirshman, M. F. & Horton, E. S. Insulin resistance in obese Zucker rat (fa/fa) skeletal muscle is associated with a failure of glucose transporter translocation. J. Clin. Invest. 90, 1568–1575 (1992).
Ren, J. M. et al. Overexpression of Glut4 protein in muscle increases basal and insulin-stimulated whole body glucose disposal in conscious mice. J. Clin. Invest. 95, 429–432 (1995).
Chen, S. et al. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem. J. 409, 449–459 (2008).
Richter, E. A. & Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 93, 993–1017 (2013).
Richter, E. A. & Ruderman, N. B. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem. J. 418, 261–275 (2009).
Jorgensen, S. B. et al. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am. J. Physiol. Endocrinol. Metab. 292, E331–E339 (2007).
McGee, S. L. et al. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 57, 860–867 (2008).
Myers, R. W. et al. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 357, 507–511 (2017).
Dong, J. et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKalpha1/SIRT1. J. Lipid Res. 55, 363–374 (2014).
Bastard, J. P. et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 17, 4–12 (2006).
Viollet, B. et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J. Clin. Invest. 111, 91–98 (2003).
Weischenfeldt, J. & Porse, B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008, pdb prot5080 (2008).
Acknowledgements
This work was supported by Grants from National Key R&D Program of China (2017YFA0506000, 2017YFA0205400) (to X.L., Z.H., and P.L.), Natural Science Foundation of China (92057122, 81874323, 81770800, 81622010 to Z.H. and P.L.), CAMS Innovation Fund for Medical Sciences (2019-12M-5-028, 2016-I2M-4-001, 2021-1-I2M-016 to X.L. and P.L.), Beijing Outstanding Young Scientist Program (BJJWZYJH01201910023028 to P.L.), Key Project from Science Technology Department of Wenzhou (88920006, 2021ZY0064 to Z.H.) and the College Students’ innovation of Science and Technology Activities Plan of Zhejiang Province (2019R413086) (to Y.H.).
Author information
Authors and Affiliations
Contributions
L.Y., L.W., K.G., Y.H., N.L., S.W., X.L., Q.Z., J.Z., L.Z., J.N., C.C., L.S., S.H., L.K., J.R., P.L., and Z.H. researched the data. X.L., J.R., P.L., M.M., and Z.H. contributed to the initial discussion and design of the project. L.Y., M.M., and Z.H. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ying, L., Wang, L., Guo, K. et al. Paracrine FGFs target skeletal muscle to exert potent anti-hyperglycemic effects. Nat Commun 12, 7256 (2021). https://doi.org/10.1038/s41467-021-27584-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-27584-y
This article is cited by
-
Skimmianine attenuates liver ischemia/reperfusion injury by regulating PI3K–AKT signaling pathway-mediated inflammation, apoptosis and oxidative stress
Scientific Reports (2023)
-
Receptor clustering by a precise set of extracellular galectins initiates FGFR signaling
Cellular and Molecular Life Sciences (2023)
-
The CEBPA-FGF21 regulatory network may participate in the T2DM-induced skeletal muscle atrophy by regulating the autophagy-lysosomal pathway
Acta Diabetologica (2023)
-
Advances of nanoparticles in transmucosal drug delivery
Nano Research (2023)
-
Orosomucoid 2 maintains hepatic lipid homeostasis through suppression of de novo lipogenesis
Nature Metabolism (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.