Abstract
Few methods have been reported for intermolecular arylamination of alkenes, which could provide direct access to important arylethylamine scaffolds. Herein, we report an intermolecular syn-1,2-arylamination of unactivated alkenes with arylboronic acids and O-benzoylhydroxylamine electrophiles with Ni(II) catalyst. The cleavable bidentate picolinamide directing group facilitates formation of stabilized 4-, 5- or 6-membered nickelacycles and enables the difunctionalization of diverse alkenyl amines with high levels of regio-, chemo- and diastereocontrol. This general and practical protocol is compatible with broad substrate scope and high functional group tolerance. The utility of this method is further demonstrated by the site-selective modification of pharmaceutical agents.
Similar content being viewed by others
Introduction
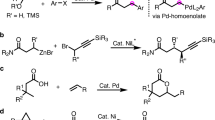
C–C and C–N bonds are two of the most omnipresent bonds in nature, and the arylamination of olefins represents a powerful and attractive synthetic tool for the simultaneous introduction of vital aryl and amino groups across alkenes to enable the rapid increase in molecular complexity from abundant and readily available materials1,2,3,4. Intramolecular arylamination with an alkene acceptor tethered to either the aryl halide or active nitrogen functionality using transition-metal catalysts has been developed for the synthesis of nitrogen-containing cycles5,6,7,8,9,10,11,12,13,14. In comparison, intermolecular arylamination providing access to arylethylamine-based acyclic molecules is particularly difficult and remain rare, owing to their high entropic cost and the problems of controlling the chemoselectivity of multicomponent reactions15,16,17,18,19,20.
The arylethylamine scaffold exists in many bioactive molecules and pharmaceuticals, such as dopamine agonists21,22. Only a few methods have been reported for the synthesis of this important motif via 1,2/2,1-arylamination of alkenes. Liu and coworkers disclosed an enantioselective Cu-catalyzed 2,1-arylamination of terminal styrenes using the N-fluoro-N-alkylsulfonamide as the amine reagent via a radical process15. In 2018, Stephenson16 demonstrated a two-component photocatalytic 2,1-arylamination of vinyl arenes with arylsulfonylacetamides as the bifunctional reagents. For the more challenging arylamination of unactivated alkenes with low reactivity, reduced polarization, and high tendency toward β-hydride elimination, the directing group strategy has been utilized to address the issues. Recently, Engle achieved an anti-2,1-arylamination of 8-aminoquinoline tethered alkenyl carbonyls with aryl iodides and nitrogen nucleophiles via aminopalladation (Fig. 1a)18. After the pioneering work, this group reported a Ni-catalyzed 1,2-carboamination of alkenes with organozinc nucleophiles and O-benzoylydroxylamine (N–O) electrophiles in a syn-addition (Fig. 1b)19. However, the arylzinc reagent underwent arylamination with low efficiency. In this context, it is highly desirable to develop a protocol to enable regio- and stereoselective 1,2-arylamination of unactivated alkenes in terms of efficiency, alkene, and amine substrate scope, functional group tolerance.
Recently, functionality-tolerant and operationally simple arylboron has been used in the catalytic intermolecular dicarbofunctionalization of alkenes, such as diarylation23,24,25,26 and alkylarylation27,28. We, therefore, hypothesized that arylboron may be used with the N–O electrophiles to realize Ni-catalyzed arylamination of unactivated alkenes. However, to the best of our knowledge, intermolecular olefin arylamination with these two reagents remain scarce17. The development of this protocol for arylamination of unactivated alkenes was limited by three fundamental issues: (1) undesired competitive cross-couping between arylboron reagents and O-benzoylhydroxylamines29,30, (2) the low binding affinity of unactivated alkenes to metal centers, especially for internal alkenes, and (3) the difficulty to control regioselectivity, such as arylamination vs aminoarylation, and 1,2-arylamination vs 1,n-arylamination via chain-walking isomerization31,32,33.
Inspired by the flexibility of the coordinating directing group, we anticipated that the Ni-Ar species could be generated by transmetalation with arylboron reagents with a Ni(II) catalyst under the assistance of a bidentate directing group. Subsequent 1,2-migratory insertion followed by a sequential electrophilic amination reaction will yield the arylamination product34,35,36,37,38,39. Critical to the success of this process, the reaction must be initiated by a transmetalation step to form the Ni-Ar species, which is rarely reported and challenging because of the fact that most of the three-component difunctionalization reactions utilizing arylboron reagents were initiated by oxidative addition with electrophiles to Ni(0) catalyst, thus furnishing products that incorporate electrophile at the terminal position and aryl at the internal position.
Herein, we report an approach for the 1,2-arylamination of unactivated alkenes under practical and easily handled Ni(II) catalytic system with commercially available arylboronic acids and readily prepared O-benzoylhydroxylamines with excellent regio- and diastereocontrol (Fig. 1c). Under the assistance of picolinamide (PA)-directing group40,41,42, diverse alkenyl amines could be converted into valuable 1-aryl-2,n-diamines, a common structure found in a number of natural products and pharmaceuticals (Fig. 1d). This protocol is compatible with terminal and internal alkenyl amines of different chain lengths, a broad range of primary and secondary amine sources, and exhibits excellent functional group tolerance.
Results
Evaluation of reaction conditions
To check the feasibility of the above scenario, we started this investigation of Ni-catalyzed arylamination of homoallylic amine substrate 1a containing a PA-directing group with phenylboronic acid and piperidino benzoate as coupling partners (Fig. 2). After extensive optimization, we were pleased to find that the reaction in the presence of NiBr2·DME as the catalyst and K3PO4 in tert-butanol at 80 °C gave the desired 1,2-arylamination product 2a as a single regioisomer in 80% isolated yield, accompanying with ≈5% unreacted alkene and trace amounts of hydroarylation byproduct (entry 1). Ni(COD)2 also catalyzed the reaction in moderate yield (54%) (entry 2). NiCl2 or NiBr2 catalyst did not give any desired product, probably due to the lack of stabilizing spectator ligand to promote ligand exchange/transmetalation (entry 3). Replacement of nucleophile PhB(OH)2 by PhBpin led to a slightly lower yield (entry 4). The use of DMF solvent led to a trace amount of product (entry 5), while the use of dioxane or iPrOH solvent provided a lower yield (entries 6–7). Inferior results were obtained when using other bases instead of K3PO4 (entries 8–10) or conducting the reaction at 50 °C (entry 11). Benzoyl protected homoallylic amine and N-methylated PA were subjected to the standard conditions, and both reactions did not occur, indicating that the pyridine N(sp2) and N-H moiety were both indispensable in this transformation. It merits to mention that, the putative 8-aminoquinoline-masked 3-butenoic acid43,44,45,46,47, which was widely used in alkene difunctionalization, did not afford any product under the optimized conditions.
Substrate scope
With the identified conditions in hand, we first sought to define the scope of the arylboronic acid partner (Fig. 3). In general, arylboronic acids bearing a wide range of electronically varied substituents reacted to produce the desired products in good yields. A variety of functional groups were accommodated well, including ethers (2b-c), dimethylamine (2h), trifluoromethyl (2i), fluoro (2j), chloro (2k) and bromo (2l). Notably, sterically hindered ortho-substituted arylboronic acid showed slightly increased reactivity, reacted to afford the desired product in very good yield (2d). In addition, arylboronic acids containing iodide, alkene, aldehyde and ketone (2m-p), which can be further derivatized, were also amenable to this reaction. Gratifyingly, alkenylboronic acid worked well to give the desired product (2q) with moderate yield. On the other hand, alkyl- and alkynylboronic acid coupling partners were ineffective under the reaction conditions.
Subsequently, we examined the N–O electrophile scope of the reaction using PhB(OH)2 as the nucleophilic component (Fig. 4). The reactivities of disubstituted amine sources were first investigated in the arylamination, diethylamine, and N-methylbenzylamine could be introduced under the optimized conditions (3a-b). In addition to acyclic amines, cyclic amines containing a series of heterocyclic scaffolds, including morpholine (3c), thiomorpholine (3d), piperazine (3e), ester group (3f), and acetal group (3g), which are prevalent in biologically active molecules, were competent substrates. Seven-membered hexamethyleneimine was also tolerated, affording the desired product 3h. The scope of monosubstituted amine transfer agents for the synthesis of secondary amines was explored, which usually led to a dramatic drop in reaction efficiency48,49. To our delight, sterically hindered secondary alkylamines are accessible with O-benzoylhydroxylamines derived with tertiary alkyl group (3i-l). However, less sterically hindered primary amine transfer agents gave no desired arylamination product, affording hydroarylation product in moderate yield.
After evaluation of electrophile and nucleophile scope, we turned our attention to the scope of alkene substrates (Fig. 5). The α-substituted homoallylamine was first tested under the standard conditions, the terminal alkene bearing alkyl- or aryl-substitution at the α-position proceeded smoothly to afford the arylamination products (4a-b) in moderate yields with high diastereoselectivity. The relative stereochemistry of the diastereomer of 4b was established by X-ray crystallographic analysis, with the trans orientation of α and γ substituents. This result established the relative stereochemistry of two stereocenters remote from one another, which has been shown to be difficult. The trans orientation of the skipped stereocenters generated from arylamination of α-substituted terminal alkenes presumably arose from the formation of the more stable trans-nickelacycle upon C–N bond reductive elimination (see models in the Supplementary Information). n-Butyl-substituted homoallylamine at the β-position could also be tolerated in the reaction but with lower diastereoselectivity (1.9:1, 4c), and a more sterically bulky group led to a lower yield (4d). 1,1-Disubstitued alkene was also tolerated and successfully afforded desired product containing a quaternary center (4e). We then explored alkene substrates that are typically challenging in alkene carboamination. Gratifyingly, Z- or E- internal substrates could be efficiently converted into the syn-diastereomer under the optimized conditions (4f-l), which was consistent with our proposed mechanism. Both diastereoisomers were accessible based on the cis/trans configuration of the alkene substrate (4f and 4g), suggesting the alkene does not undergo isomerization in the Ni-catalyzed process. In addition, phenyl-, benzyl-, and isopropyl-substituents were well tolerated in the reaction.
In light of the success in developing a regioselective arylamination of homoallylamines, we continued our survey by applying the protocol to PA-protected allylic and bishomoallylic amines. To our delight, the reaction of allylamine with phenylboronic acid and piperidino benzoate under the optimized conditions furnished the arylamination product in moderated yield, with only a single regioisomer was detected by GC-MS analysis. Moreover, a variety of terminal and internal bishomoallylamines underwent arylamination to regioselectively provide δ-amino benzenepentanamine products. Likewise, both trans- and cis-internal alkenes were effective in this reaction, delivering the desired product with excellent diastereoselectivity (>20:1, 4q). We assumed that four and six-membered nickelacycles were formed and can be stabilized in the catalytic system.
Synthetic potential
We next performed the gram-scale reaction to illustrate the synthetic utility of this methodology (Fig. 6a). The reaction of 1a with phenylboronic acid and morpholine benzoate on a 5 mmol scale afforded 3c in 80% yield. We were able to remove the PA-directing group with NaOH in EtOH at 100 °C, and the primary amine 5 could be generated in nearly quantitative yield. This methodology was also applied to the modification of phamaceutically relevant compounds, as shown in Fig. 6b. Arylboronic acid and O-benzoylhydroxylamine derived from fenofibrate and loratadine independently underwent arylamination, affording corresponding desired products (6a and 6b).
Mechanistic consideration
To elucidate the mechanism, we first conducted the radical clock experiment (Fig. 7a). When the prepared alkene substrate 1b was subjected to standard reaction conditions, only cyclopropane remained product 4s was formed in 64% yield, implying that the cyclopropylmethyl radical intermediate known to ring rupture might not be generated in the catalytic cycle. The effect of radical inhibitors was next examined (Fig. 7b). As it turned out, the arylamination was not largely inhibited by the addition of TEMPO or BHT. This suggested that the arylamination probably did not involve a radical process, although the possibility of radical formation followed by fast recombination with Ni within the solvent cage cannot be ruled out.
Regarding redox manifolds of Ni catalysis, Ni0/NiII and NiI/NiIII catalytic systems were considered as two possible pathways (Fig. 8). In Pathway A, a Ni(II) species 7 was generated from Ni(0) species and O-benzoylhydroxylamine via oxidative addition. Transmetalation followed by migratory insertion of the alkene into the Ni-carbon bond would form 8, which could yield the product by reductive elimination. However, we consider this pathway to be less likely because the selective insertion into the Ni-C rather than Ni–N bond was suspicious and the energy barrier of reductive elimination of NiII amido species is too high under similar catalytic system based on DFT calculations (>50 Kcal/mol)50. An alternative pathway in which the alkene inserted into the Ni–N bond precedes transmetalation and C–C reductive elimination was also considered unlikely, because it would involve the formation of thermodynamically unfavored larger nickelacycles, especially for bishomoallylic amine substrates (seven-membered nickelacycles). Alternatively in Pathway B, the reaction was initiated by a Ni(I) species (I)51,52, which was formed probably from comproportionation between NiBr2 and Ni(0) species53,54. After further transmetalation with arylboronic acids and olefin migratory insertion, nickel-alkyl species (III) was generated. The species stabilized by bidentate PA-directing group underwent oxidative addition with the aminating reagent much faster than protonation with the alcohol solvent or β-hydride elimination, forming NiIII amido species IV, which was believed to be able to undergo facile reductive elimination55,56,57,58. Finally, the active Ni(I)-X catalyst I was regenerated, and the desired product was furnished through the subsequent ligand exchange with the alkene substrate.
To further support our aforementioned mechanism, a series of control experiments were conducted. Stoichiometric reaction of 1a with NiBr2•DME followed by treatment with two equivalents of phenylboronic acid for 6 h was conducted to generate Ni(I) (III-1) complex (Fig. 9a), addition of methanol to the solution of complex III-1 furnished the hydroarylation product 9 (phenyl unit located at terminal position) in 86% yield upon alcoholic protonation. Besides, the III-1 complex that was subjected to piperidino benzoate at 80 °C for 6 h successfully afforded the expected product 2a in 57% yield. These results demonstrated that the reaction could be initiated by transmetalation of arylboronic acid with Ni(II) precatalyst and subsequent alkene insertion occurred prior to engagement with an electrophile to give the observed regiochemical outcome. Although attempts to isolate and identify the intermediate was failed, the proposed nickel intermediate ligated with DMF was detected by HR-MS (ESI) analysis (m/z calcd for [M + H]+, 385.1300; found, 385.1308, Supplementary Fig. 2). On the other side, a control experiment of 1a with Ni(COD)2 followed by treatment with piperidino benzoate at 80 °C for 12 h was conducted (Fig. 9b). In contrast, the reaction mixture resulted in >98% recovery of 1a upon alcoholic quenching, without the formation of any hydroamination product. This result indicated that migratory insertion of the alkene did not occur into the Ni(II)-N bond, whereas oxidative addition of piperidino benzoate to Ni(0) should be easy. In a separate vessel, the same reaction mixture reacted with phenylboronic acid at 80 °C for 12 h afforded 2a in a low yield (23%), and no aminoarylation side product was detected. Overall, we preferred the reaction proceeded via a NiI/NiIII catalytic cycle (Pathway B) rather than a Ni0/NiII cycle.
a Control experiment using Ni(II) catalyst. b Control experiment using Ni(0) catalyst. See the Supplemental Information for detailed experimental procedures and HR-MS (high-resolution mass spectrometer) analysis. DMF dimethylformamide.
Discussion
In summary, we have disclosed a methodology for the regio- and diastereoselective intermolecular arylamination of unactivated alkenes with base-metal catalyst and readily available reagents under simple conditions. The removable bidentate PA auxiliary facilitated the formation of stabilized 4-, 5-, or 6-membered nickelacycles and enabled the difunctionalization of both terminal and internal alkenyl amines, leading to the concomitant introduction of important aryl groups and structurally diverse amino groups into the C=C bonds with good functional group compatibility. Interestingly, the reaction of α-substituted terminal alkenes led to the formation of trans-isomeric products with high levels of diastereoselectivity, in which two stereocenters were remote from each other. Our protocol was suitable for large-scale synthesis and the synthetic utility of this method was further demonstrated by the modification of pharmaceutical agents. The expansion of this strategy to the synthesis of drug and natural products and other electrophiles for alkene difunctionalization is currently underway in our laboratory.
Methods
General procedure for the Ni-catalyzed arylamination of alkenyl amines
In an argon-filled glovebox, NiBr2•DME (0.03 mmol, 15 mol%), K3PO4 (0.6 mmol, 3.0 eq), alkene substrate (0.2 mmol, 1.0 eq), appropriate amine benzoate electrophile (0.4 mmol, 2 eq), appropriate arylboronic nucleophile (0.6 mmol, 3.0 eq), t-BuOH (2 mL) were added to a 10 mL schlenk flask. The reaction mixture was stirred at 80 °C for 24 h and the resulting solution was concentrated in vacuum. The crude product was purified by column chromatography on alumina gel with a mixture of ethyl acetate and hexane as eluent. The conditions for flash chromatography and data for characterization of the products are listed below.
Data availability
X-ray crystallographic data for compounds 4b (CCDC 2054628) and 4i (CCDC 2054629) is freely available from the Cambridge Crystallographic Data Centre. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data in support of the findings of this study are available within the article and its Supplementary Information or from the corresponding author. Source data are provided with this paper.
References
McDonald, R. I., Liu, G. & Stahl, S. S. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 111, 2981–3019 (2011).
Schultz, D. M. & Wolfe, J. P. Recent developments in palladium-catalyzed alkene aminoarylation reactions for the synthesis of nitrogen heterocycles. Synthesis 44, 351–361 (2012).
Wolfe, J. P. Synthesis of heterocycles via metal-catalyzed alkene carboamination or carboalkoxylation. Top. Heterocycl. Chem. 32, 1–38 (2013).
Garlets, Z. J., White, D. R. & Wolfe, J. P. Recent developments in Pd0-catalyzed alkene-carboheterofunctionalization reactions. Asian J. Org. Chem. 6, 636–653 (2017).
Zeng, W. S. & Chemler, R. Copper(II)-catalyzed enantioselective intramolecular carboamination of alkenes. J. Am. Chem. Soc. 129, 12948–12949 (2007).
Miao, L., Haque, I., Manzoni, M. R., Tham, W. S. & Chemler, S. R. Diastereo- and enantioselective copper-catalyzed intramolecular carboamination of alkenes for the synthesis of hexahydro-1H-benz[f]indoles. Org. Lett. 12, 4739–4741 (2010).
Wolfe, J. P. & Mai, D. N. Asymmetric palladium-catalyzed carboamination reactions for the synthesis of enantiomerically enriched 2-(Arylmethyl)- and 2-(Alkenylmethyl)pyrrolidines. J. Am. Chem. Soc. 132, 12157–12159 (2010).
Hopkins, B. A. & Wolfe, J. P. Synthesis of enantiomerically enriched imidazolidin-2-ones through asymmetric palladium-catalyzed alkene carboamination reactions. Angew. Chem. Int. Ed. 51, 9886–9890 (2012).
Babij, N. R. & Wolfe, J. P. Desymmetrization of meso-2,5-diallylpyrrolidinyl ureas through asymmetric palladium-catalyzed carboamination: stereocontrolled synthesis of bicyclic ureas. Angew. Chem., Int. Ed. 52, 9247–9250 (2013).
Casavant, B. J., Hosseini, A. S. & Chemler, S. R. 6-Azabicyclo [3.2.1]octanes via copper-catalyzed enantioselective alkene carboamination. Adv. Synth. Catal. 356, 2697–2702 (2014).
Faulkner, A., Scott, J. S. & Bower, J. F. An umpolung approach to alkene carboamination: palladium catalyzed 1,2-amino-acylation, -carboxylation, -arylation, -vinylation, and -alkynylation. J. Am. Chem. Soc. 137, 7224–7230 (2015).
Garlets, Z. J., Parenti, K. R. & Wolfe, J. P. Asymmetric palladium-catalyzed alkene carboamination reactions for the synthesis of cyclic sulfamides. Chem. Eur. J. 22, 5919–5922 (2016).
Yang, H.-B., Pathipati, S. R. & Selander, N. Nickel-catalyzed 1,2-aminoarylation of oxime ester-tethered alkenes with boronic acids. ACS Catal. 7, 8441–8445 (2017).
He, J. et al. Nickel-catalyzed asymmetric reductive 1,2-carboamination of unactivated alkenes. Angew. Chem. Int. Ed. 59, 2328–2332 (2020).
Wang, D. et al. Asymmetric copper-catalyzed intermolecular aminoarylation of styrenes: efficient access to optical 2,2-diarylethylamines. J. Am. Chem. Soc. 139, 6811–6814 (2017).
Monos, T. M., McAtee, R. C. & Stephenson, C. R. J. Arylsulfonylacetamides as bifunctional reagents for alkene aminoarylation. Science 361, 1369–1373 (2018).
Li, Z. et al. Multicomponent cyclopropane synthesis enabled by cu-catalyzed cyclopropene carbometalation with organoboron reagent: enantioselective modular access to polysubstituted 2-arylcyclopropylamines. Org. Lett. 21, 5432–5437 (2019).
Liu, Z. et al. Catalytic intermolecular carboamination of unactivated alkenes via directed aminopalladation. J. Am. Chem. Soc. 139, 11261–11270 (2017).
Puyl, V. A., Derosa, J. & Engle, K. M. Directed, nickel–catalyzed umpolung 1,2–carboamination of alkenyl carbonyl compounds. ACS Catal. 9, 224–229 (2019).
Han, C. et al. Palladium-catalyzed remote 1,n-arylamination of unactivated terminal alkenes. ACS Catal. 9, 4196–4202 (2019).
Zhang, A., Neumeyer, J. L. & Baldessarini, R. J. Recent progress in development of dopamine receptor subtype-selective agents: potential therapeutics for neurological and psychiatric disorders. Chem. Rev. 107, 274–302 (2007).
Zhang, J., Xiong, B., Zhen, X. & Zhang, A. Dopamine D1 receptor ligands: where are we now and where are we going. Med. Res. Rev. 29, 272–294 (2009).
Gao, P., Chen, L.-A. & Brown, M. K. Nickel-catalyzed stereoselective diarylation of alkenylarenes. J. Am. Chem. Soc. 140, 10653–10657 (2018).
Li, W., Boon, J. K. & Zhao, Y. Nickel-catalyzed difunctionalization of allyl moieties using organoboronic acids and halides with divergent regioselectivities. Chem. Sci. 9, 600–607 (2018).
Derosa, J. et al. Nickel–catalyzed 1,2–diarylation of simple alkenyl amides. J. Am. Chem. Soc. 140, 17878–17883 (2018).
Apolinar, O., Tran, V. T., Schmidt, M. A., Derosa, J. & Engle, K. M. Sulfonamide directivity enables Ni-catalyzed 1,2-diarylation of diverse alkenyl amines. ACS Catal. 10, 14234–14239 (2020).
Gu, J.-W., Min, Q.-Q., Yu, L.-C. & Zhang, X. Tandem difluoroalkylation-arylation of enamides catalyzed by nickel. Angew. Chem., Int. Ed. 55, 12270–12274 (2016).
Yang, Z.-F., Xu, C., Zheng, X. & Zhang, X. Nickel-catalyzed carbodifunctionalization of N-vinylamides enables access to γ-amino acids. Chem. Commun. 56, 2642–2645 (2020).
Matsuda, N., Hirano, K., Satoh, T. & Miura, M. Copper-catalyzed amination of arylboronates with N,N-dialkylhydroxylamines. Angew. Chem. Int. Ed. 51, 3642–3645 (2012).
Rucker, R. P., Whittaker, A. M., Dang, H. & Lalic, G. Synthesis of hindered anilines: copper-catalyzed electrophilic amination of aryl boronic esters. Angew. Chem. Int. Ed. 51, 3953–3956 (2012).
Vilches-Herrera, M., Domke, L. & Brner, A. Isomerization-hydroformylation tandem reactions. ACS Catal. 4, 1706–1724 (2014).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Sommer, H., Julia-Hernández, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Dhungana, R. K., KC, S., Basnet, P. & Giri, R. Transition metal-catalyzed dicarbofunctionalization of unactivated olefins. Chem. Rec. 18, 1314–1340 (2018).
Giri, R. & KC, S. Strategies towards dicarbofunctionalization of unactivated olefins by combined heck carbometalation and cross-coupling. J. Org. Chem. 83, 3013–3022 (2018).
Derosa, J., Apolinar, O., Kang, T., Tran, V. T. & Engle, K. M. Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes. Chem. Sci. 11, 4287–4296 (2020).
Tu, H.-Y., Zhu, S., Qing, F.-L. & Chu, L. Recent advances in nickel-catalyzed three-component difunctionalization of unactivated alkenes. Synthesis 52, 1346–1356 (2020).
Qi, X. & Diao, T. Nickel-catalyzed dicarbofunctionalization of alkenes. ACS Catal. 10, 8542–8556 (2020).
Luo, Y.-C., Xu, C. & Zhang, X. Nickel–catalyzed dicarbofunctionalization of alkenes. Chin. J. Chem. 38, 1371–1394 (2020).
Liu, Z. et al. Palladium(0)-catalyzed directed syn-1,2-carboboration and -silylation: alkene scope, applications in dearomatization, and stereocontrol by a chiral auxiliary. Angew. Chem. Int. Ed. 58, 17068–17073 (2019).
Schreib, B. S. & Carrieira, E. M. Palladium-catalyzed regioselective C–H iodination of unactivated alkenes. J. Am. Chem. Soc. 141, 8758–8763 (2019).
Schreib, B. S., Fadel, M. & Carrieira, E. M. Palladium-catalyzed C-H alkynylation of unactivated alkenes. Angew. Chem., Int. Ed. 59, 7818–7822 (2020).
Wang, H. et al. Palladium–catalyzed regiocontrollable reductive heck reaction of unactivated aliphatic alkenes. J. Am. Chem. Soc. 140, 3542–3546 (2018).
Wang, C.-D. et al. Regiocontrollable reductive heck reaction of unactivated aliphatic alkenes. J. Am. Chem. Soc. 140, 9332–9336 (2018).
Tang, C.-L. et al. Directed copper-catalyzed intermolecular heck-type reaction of unactivated olefins and alkyl halides. J. Am. Chem. Soc. 140, 16929–16935 (2018).
Li, Y. et al. Copper-catalyzed intermolecular aminative difunctionalization of unactivated alkenes. J. Am. Chem. Soc. 141, 18475–18485 (2019).
Yang, D. et al. Directed cobalt-catalyzed anti-markovnikov hydroalkylation of unactivated alkenes enabled by “Co-H” catalysis. Org. Lett. 22, 4333–4338 (2020).
Liu, R. Y. & Buchwald, S. L. CuH-catalyzed olefin functionalization: from hydroamination to carbonyl addition. Acc. Chem. Res. 53, 1229–1243 (2020).
Niu, D. & Buchwald, S. L. Design of modified amine transfer reagents allows the synthesis of α-chiral secondary amines via CuH-catalyzed hydroamination. J. Am. Chem. Soc. 137, 9716–9721 (2015).
Jeon, J., Lee, C., Seo, H. & Hong, S. NiH-catalyzed proximal-selective hydroamination of unactivated alkenes. J. Am. Chem. Soc. 142, 20470–20480 (2020).
Xiao, Y.-L., Guo, W.-H., He, G.-Z., Pan, Q. & Zhang, X. Nickel-catalyzed cross-coupling of functionalized difluoromethyl bromides and chlorides with aryl boronic acids: a general method for difluoroalkylated arenes. Angew. Chem. Int. Ed. 53, 9909–9913 (2014).
Yang, Y. et al. Nickel-catalyzed fluoroethylation of arylboronic acids via suzuki-type coupling. Org. Chem. Front. 6, 1463–1470 (2019).
Diccianni, J. B., Katigbak, J., Hu, C. & Diao, T. Mechanistic characterization of (xantphos)ni(i)-mediated alkyl bromide activation: oxidative addition, electron transfer, or halogen-atom abstraction. J. Am. Chem. Soc. 141, 1788–1796 (2019).
Zarate, C., Yang, H., Bezdek, M. J., Hesk, D. & Chirik, P. J. Ni(I)–X complexes bearing a bulky α-diimine ligand: synthesis, structure, and superior catalytic performance in the hydrogen isotope exchange in pharmaceuticals. J. Am. Chem. Soc. 141, 5034–5044 (2019).
Ilies, L., Matsubara, T. & Nakamura, E. Nickel-catalyzed synthesis of diarylamines via oxidatively induced C–N bond formation at room temperature. Org. Lett. 14, 5570–5573 (2012).
Tasker, S. Z. & Jamison, T. F. Highly regioselective indoline synthesis under nickel/photoredox dual catalysis. J. Am. Chem. Soc. 137, 9531–9534 (2015).
Corcoran, E. B. et al. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 353, 279–283 (2016).
Oderinde, M. S. et al. Highly chemoselective iridium photoredox and nickel catalysis for the cross-coupling of primary aryl amines with aryl halides. Angew. Chem. Int. Ed. 55, 13219–13223 (2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21901185) and funds provided by Tianjin Normal University.
Author information
Authors and Affiliations
Contributions
L.X., S.W. and L.Z. planned and conducted most of the experiments; L.Z., C.L. and L.M. prepared substrates for the reaction scope evaluation; X.W. conducted the X-ray crystallographic analysis; C.W. directed the projects and wrote the manuscript. All authors contributed to the discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, L., Wang, S., Zhang, L. et al. Directed nickel-catalyzed regio- and diastereoselective arylamination of unactivated alkenes. Nat Commun 12, 6280 (2021). https://doi.org/10.1038/s41467-021-26527-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-26527-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.