Abstract
Inactivating mutations in SMARCA4 and concurrent epigenetic silencing of SMARCA2 characterize subsets of ovarian and lung cancers. Concomitant loss of these key subunits of SWI/SNF chromatin remodeling complexes in both cancers is associated with chemotherapy resistance and poor prognosis. Here, we discover that SMARCA4/2 loss inhibits chemotherapy-induced apoptosis through disrupting intracellular organelle calcium ion (Ca2+) release in these cancers. By restricting chromatin accessibility to ITPR3, encoding Ca2+ channel IP3R3, SMARCA4/2 deficiency causes reduced IP3R3 expression leading to impaired Ca2+ transfer from the endoplasmic reticulum to mitochondria required for apoptosis induction. Reactivation of SMARCA2 by a histone deacetylase inhibitor rescues IP3R3 expression and enhances cisplatin response in SMARCA4/2-deficient cancer cells both in vitro and in vivo. Our findings elucidate the contribution of SMARCA4/2 to Ca2+-dependent apoptosis induction, which may be exploited to enhance chemotherapy response in SMARCA4/2-deficient cancers.
Similar content being viewed by others
Introduction
The SWI/SNF family of ATP-dependent chromatin remodeling complexes control gene expression by regulating chromatin organization1,2. They also directly participate in DNA replication, repair, and recombination through modifying chromatin or recruiting relevant proteins3. Cancer genome-sequencing efforts have revealed mutations in SWI/SNF subunits in more than 20% of all human cancers, highlighting their critical roles in tumorigenesis4. However, identifying the driver mechanisms of SWI/SNF loss in promoting cancer remains a challenge.
SMARCA4 (BRG1) and SMARCA2 (BRM) are the two mutually exclusive ATPase subunits of SWI/SNF. SMARCA4 is inactivated by mutations or other mechanisms in ~10% of non-small cell lung cancer (NSCLC)5,6,7,8,9. Furthermore, concomitant loss of SMARCA4/2 protein expression occurs in a subset of NSCLC associated with a poor prognosis6,10. In addition to NSCLC, deleterious SMARCA4 mutations have been found to be the sole genetic driver in ~100% of small cell carcinoma of the ovary, hypercalcemic type (SCCOHT), a rare and aggressive ovarian cancer affecting young women11,12,13,14,15. SCCOHT is also characterized by concurrent loss of SMARCA4/2 protein expression, where SMARCA2 is epigenetically silenced and its reactivation strongly suppressed SCCOHT growth16,17. In contrast to other cancer types where experimental SMARCA2 inhibition is synthetic lethal with SMARCA4 loss18,19,20, SMARCA2 silencing may cooperate with SMARCA4 loss in SMARCA4/2-deficient SCCOHT and NSCLC for cancer development10,21. However, the underlying mechanisms are not understood.
In addition to regulating gene expression, SWI/SNF components, including SMARCA4, have also been implicated in DNA-damage repair (DDR)22,23,24. Thus, their inactivation may also lead to compromised DDR and genome instability which are widely recognized as driving events in cancer development25. However, SCCOHT has a simple genome and harbors few mutations or chromosomal alterations other than inactivating mutations in SMARCA415,26,27, suggesting that altered transcriptional regulation may be the predominant driver of tumorigenesis in this cancer28.
Platinum-based chemotherapies, such as cisplatin, induce DNA damage leading to cancer cell apoptosis and have been widely used in clinical practice for treating lung and ovarian cancers29,30. The involvement of SWI/SNF in DDR supports the use of these genotoxic agents for treating cancers with SMARCA4/2 deficiency, which does not often co-occur with other druggable oncogenic mutations. Indeed, previous studies have shown that experimental inhibition of SMARCA4 in SMARCA4-proficient cancer cells enhanced response to DNA damaging agents31,32,33. However, conventional chemotherapies are rarely effective for SCCOHT patients15,34 and compared to other ovarian cancer types, SCCOHT cell lines show substantial resistance to these drugs26,35. In line with this, NSCLC patients with concomitant loss of SMARCA4/2 have a poorer prognosis than others6,10 while adjuvant chemotherapy remains among primary treatment options for this cancer29. Thus, while SWI/SNF deficiencies have been widely associated to cancer progression, the mechanism by which SMARCA4/2-deficient cancer cells have adapted to resist chemotherapy is unknown.
In this study, we sought to examine the role of SMARCA4/2 in modulating chemotherapy responses in SCCOHT and NSCLC where SMARCA4/2 deficiency is frequently observed. Our results reveal a mechanism linking SMARCA4/2 loss to chemoresistance by inhibiting apoptosis induction and suggest a potential therapeutic strategy for improving treatment for SMARCA4/2-deficient cancers.
Results
SMARCA4/2 loss confers resistance to chemo-induced apoptosis in cancer cells
SCCOHT harbors few mutations or chromosomal alterations other than inactivating mutations in SMARCA4 but is typically resistant to conventional chemotherapy in patients15,34, suggesting a potential connection between SMARCA4 deficiency and chemotherapy resistance. Since SMARCA4 is also frequently inactivated in NSCLC, we investigated the association of SMARCA4 expression with chemotherapy response in this cancer type. We first analyzed the most comprehensive NSCLC microarray gene expression data set with clinical outcome from the Director’s Challenge data set of lung adenocarcinoma (LUAD, the most common NSCLC subtype) of diverse tumor staging36. For our analysis, we chose SMARCA4 “Jetset probe” unbiasedly identified by Kaplan–Meier (KM) plotter37,38, which is the optimal probe set for specificity, coverage, and degradation resistance without preassociation with patient outcome. We stratified the patients within each treatment group based on median of SMARCA4 expression and found that low SMARCA4 expression was significantly associated with worse survival with adjuvant therapies (chemotherapy and radiation) when compared to high SMARCA4 expression (Supplementary Fig. 1a). This was supported by similar results obtained from KM plotter analyzing multiple available LUAD data sets of diverse tumor staging using the same probe (Supplementary Fig. 1b). A similar trend in UT lung SPORE data set39 was also observed although not statistically significant (Supplementary Fig. 1c). Together, these patient outcome results suggest that SMARCA4 deficiency is associated with chemotherapy resistance in NSCLC, similar to that seen in SCCOHT.
Because patient outcomes from the data sets described above may be influenced by other variable factors such as treatment history, we next examined the role of SWI/SNF loss in mediating chemoresistance in more controlled experimental settings using cancer cell lines. First, we investigated the correlation between chemotherapy responses and mRNA expression levels of SMARCA4/2 in a large cohort of cell lines (n = 436) across different cancer types (Supplementary Fig. 2a), by integrating publicly available drug sensitivity data from Genomics of Drug Sensitivity in Cancer (GDSC)40 and RNA sequencing (RNA-seq) data from Cancer Cell Line Encyclopedia (CCLE)41,42. We stratified these pan cancer cell lines (n = 436) based on their SMARCA4/2 expression in tertiles (Supplementary Fig. 2b) and found that SMARCA4Low/SMARCA2Low (A4L/A2L, bottom tertile for both genes) group (n = 53) has the highest half maximal inhibitory concentration (IC50) among all four groups, for common chemotherapy drugs with different mechanisms of action, including cisplatin, cyclophosphamide, topotecan, paclitaxel, etoposide, and 5FU (Fig. 1a, Supplementary Fig. 2c). Notably, IC50 difference between A4L/A2L and the SMARCA4High/SMARCA2High (A4H/A2H top tertile for both genes) group (n = 50) was statistically significant for all of these drugs. The SMARCA4Low/SMARCA2High (A4L/A2H) group (n = 24) had the second highest IC50 which was significantly higher than that of the A4H/A2H group in three of the six drugs including cisplatin. We also observed a consistent trend of higher IC50 in the SMARCA4High/SMARCA2Low (A4H/A2L) group (n = 34) compared to A4H/A2H although it was not statistically significant. Similar results were also obtained when analyzing lung cancer cell lines only (Fig. 1b, Supplementary Fig. 2d), which represented the largest cancer type (n = 103) among the CCLE panel (Supplementary Fig. 2a). Together, these observations show that reduced SMARCA4/2 expression correlates with resistance to different chemotherapies, including cisplatin, and suggest that SMARCA4 may play a dominant role in regulating drug responses in cancer cells.
The half maximal inhibitory concentration (IC50) of cisplatin in pan cancer (a) and lung cancer (b) cell lines with differential mRNA expression for SMARCA4 and SMARCA2 (see Supplementary Fig. 2b for stratification). A4H: SMARCA4High; A4L: SMARCA4Low; A2H: SMARCA2High; A2L: SMARCA2Low. Cell line numbers are indicated in gray below each group. One-way ANOVA Kruskal–Wallis test followed by Dunn’s test for multiple comparisons to A4HA2H group, p values (p): a A4HA2L—0.5338, A4LA2H—0.0035, A4LA2L < 0.0001; b A4HA2L—0.4615, A4LA2H—0.0517, A4LA2L—0.0019. c Schematic outline of a pooled CRISPR screen with a sgRNA knockout library against epigenetic regulators to identify genes required for cisplatin response in OVCAR4 cells. d MAGeCK analysis43,44,81 for screen in c. Genes were ranked by robust rank aggregation (RRA). Immunoblots (e), annexin V+/PI− apoptotic cell population determined by flow cytometry (f), and representative phase-contrast images (g) of OVCAR4 cells with indicated SMARCA4/2 perturbations and cisplatin treatments (e, f 48 h). Immunoblots (h), annexin V+/PI− apoptotic cell population (i), and representative phase-contrast images (j) of H1703 cells with indicated SMARCA4/2 perturbations and cisplatin treatments (h, i 72 h). e–j Ctrl Control, A4KO SMARCA4 knockout, shA2 shRNA targeting SMARCA2, cl. PARP cleaved PARP, cl. caspase 3 cleaved caspase 3, A4 SMARCA4, A2 SMARCA2. Scale bar, 150 μm. Mean ± SD, n = 3 independent experiments, one-way ANOVA followed by Dunnett’s test for multiple comparisons, p values (p): f all (<0.0001); i A2 (0 μM)—0.0032, A4 (0 μM)—0.0023, A2 or A4 (3 μM) < 0.0001. **p < 0.01, ****p < 0.0001.
To help unbiasedly assess the potential roles of SWI/SNF genes in modulating cisplatin responses, we performed a pooled CRISPR knockout screen targeting 496 epigenetic modifiers in OVCAR4, a SMARCA4/2-proficient high-grade serous ovarian carcinoma (HGSC) cell line (Fig. 1c). Upon screen completion, we analyzed the data using the MAGeCK statistical software package43,44 to search for candidate genes whose knockout may confer cisplatin resistance. Validating the screen, we identified EP300 and CARM1 among the top candidates (ranked #1 and #5, respectively; Supplementary Table 1 and Fig. 1d), whose suppression is known to confer cisplatin resistance45,46,47. In keeping with our above findings in patient outcome and CCLE cell line responses to chemotherapies, SMARCA4 was also highly ranked (#11) in our screen suggesting that SMARCA4 loss confers cisplatin resistance (Fig. 1c, d and Supplementary Table 1). SMARCA2 was not significantly enriched (ranked #162), suggesting that SMARCA4 plays a dominant role in controlling cisplatin response, with SMARCA2 only compensating when SMARCA4 is lost.
To validate the above screen results, we knocked out SMARCA4 in OVCAR4 cells using CRISPR/Cas9 genome editing system and investigated their apoptotic responses known to be induced by cisplatin treatment. Compared to the parental control, SMARCA4 knockout (A4KO) cells were more resistant to cisplatin-induced elevation of annexin V (cell death marker; Supplementary Fig. 3a), cleaved PARP, and cleaved caspase 3 (apoptosis markers; Fig. 1e). They also exhibited reduced annexin V+/propidium iodide (PI)− apoptotic cell population (Fig. 1f), and had fewer morphological defects, a characteristic of the apoptotic cell (Fig. 1g) in response to cisplatin treatment. Similarly, SMARCA4 knockout also protected OVCAR4 cells against paclitaxel-induced apoptosis (Supplementary Fig. 3b). Furthermore, knockdown of SMARCA2 using two independent short hairpin RNAs (shRNAs) in these A4KO cells led to increased resistance to the above-described apoptotic responses induced by cisplatin (Fig. 1e–g, Supplementary Fig. 3a). Similar results were obtained in the HEC116 ovarian endometrial cancer cell line (Supplementary Fig. 3c, d), further validating the above results in OVCAR4 cells. We also noted that high dose of cisplatin treatment in OVCAR4 control cells led to reduced SMARCA4/2 protein expression (Fig. 1e), suggesting a potential negative feedback regulation or a selection for cells expressing low SMARCA4/2. To corroborate our results, we sought to perform the reverse experiments by restoring SMARCA4 or SMARCA2 in SMARCA4/2-deficient cancer cells. SMARCA4/2 restoration in SCCOHT cells both strongly suppressed their growth16,17, which limited the experimental window to study apoptosis regulation upon subsequent cisplatin treatment. In contrast, SMARCA4/2-deficient NSCLC cells including H1703 cells can tolerate restoration of SMARCA4/248 and thus are better suited for this analysis. Ectopic expression of SMARCA4 or SMARCA2 sensitized H1703 cells to cisplatin treatment and led to strong induction of apoptosis, indicated by elevation of annexin V, cleaved PARP, and cleaved caspase 3, a marked increase of the annexin V+/PI− apoptotic cell population, acquisition of apoptotic cell morphology, and impaired growth (Fig. 1h–j, Supplementary Fig. 3e, f). Further supporting this, CRISPR/Cas9-mediated SMARCA4 knockout in SMARCA4/2-proficient H1437 NSCLC cancer cells conferred resistance to apoptosis induced by cisplatin treatment; knockdown of SMARCA2 in these A4KO cells led to further increased resistance to cisplatin, indicated by reduction of cleaved PARP and cleaved caspase 3 and increased cell viability (Supplementary Fig. 3g, h).
We further examined the effect of SMARCA4 loss in response to other common chemotherapeutics using above-described isogenic cell pairs of HEC116 and H1703 that differ only in SMARCA4 status. Consistent with cisplatin results, SMARCA4 knockout in HEC116 cells suppressed elevation of cleaved PARP and cleaved caspase 3 induced by cyclophosphamide, topotecan, and paclitaxel (Supplementary Fig. 4a) and led to increased cell viability in the presence of these agents (Supplementary Fig. 4b). Conversely, SMARCA4 restoration sensitized H1703 cells to the treatment with these drugs, as indicated by elevation of apoptosis and increased cell viability (Supplementary Fig. 4c, d). Together, our data indicate that SMARCA4/2 loss inhibits chemotherapy-induced apoptotic responses in ovarian and lung cancer cells.
SMARCA4/2 loss results in altered intracellular Ca2+ homeostasis in cancer cells
To understand how SMARCA4/2 regulate chemotherapy sensitivity and apoptosis induction, we analyzed the transcriptome regulated by SMARCA4 using SCCOHT cells, taking advantage of their simple genetic background. Gene set enrichment analysis (GSEA) of RNA-seq data generated in SCCOHT-1 and BIN-67 cells ± SMARCA4 restoration49 reveals top ten Gene Ontology (GO) terms regulated by SMARCA4 consistently shared by these two SCCOHT cell lines (Supplementary Fig. 5). Multiple terms associated with ion/calcium homeostasis were identified including “ion transmembrane transporter” and “calcium ion binding” (Fig. 2a, b). The established crucial role of calcium ion (Ca2+) homeostasis in apoptosis induction50 makes these GO terms particularly interesting. Transient Ca2+ release from the endoplasmic reticulum (ER), the major intracellular Ca2+ store, into the cytosol and subsequent transfer to mitochondria is important for cellular signal transductions as well as ATP production51. However, excessive ER-Ca2+ release leads to mitochondrial Ca2+ overload and cell death, which has recently been associated to the selective vulnerability of cancer cells52,53,54. Together, these transcriptome analyses in SCCOHT cell lines indicate that Ca2+ homeostasis may be a commonly altered cellular process by SMARCA4, contributing to their roles in apoptosis regulation and cancer cell survival.
Gene set enrichment analysis plots of indicated Gene Ontology terms in SCCOHT-1 (a) and BIN-67 (b) cells ± SMARCA4 (A4) restoration49. Ctrl control, FDR false discovery rate. c Immunoblots of indicated proteins in SCCOHT-1 cells ± A4 restoration. Changes of cytosolic (d) and mitochondrial (e) Ca2+ contents in SCCOHT-1 cells ± A4 restoration upon histamine stimulation. d 28 Ctrl and 21 A4 cells from n = 4 independent experiments were analyzed. e 44 Ctrl and 20 A4 cells from n = 4 independent experiments were analyzed. f Immunoblots of indicated proteins in H1703 cells ± A4 restoration. Changes of cytosolic (g) and mitochondrial (h) Ca2+ contents in H1703 cells ± A4 restoration upon histamine stimulation. g 41 Ctrl and 74 A4 cells from n = 3 independent experiments were analyzed. h 45 Ctrl and 63 A4 cells from n = 3 independent experiments were analyzed. i Immunoblots of indicated proteins in OVCAR4 cells ± SMARCA4 knockout (A4KO). Changes of cytosolic (j) and mitochondrial (k) Ca2+ contents in OVCAR4 cells with ± A4KO upon histamine stimulation. j 60 Ctrl and 53 A4KO cells from n = 3 independent experiments were analyzed. k 41 Ctrl and 40 A4KO cells from n = 3 independent experiments were analyzed. l Immunoblots of indicated proteins in H1437 cells ± A4KO. Changes of cytosolic (m) and mitochondrial (n) Ca2+ contents in H1437 cells ± A4KO upon histamine stimulation. m 39 Ctrl and 37 A4KO cells from n = 3 independent experiments were analyzed. n 38 Ctrl and 42 A4KO cells from n = 3 independent experiments were analyzed. d, e, g, h, j, k, m, n Left: traces of cytosolic and mitochondrial Ca2+ contents upon 100 μM histamine stimulation (mean ± SEM). Right: quantification of the maximal Ca2+ signal peaks induced by histamine stimulation (mean ± SD). The Ca2+ probes R-GECO (R-GECO F/F0) and CEPIA-2mt (CEPIA-2mt F/F0) were used to monitor cytosolic and mitochondrial Ca2+, respectively. Arb. units arbitrary units. Two-tailed t-test, p values (p): d 0.0003, e 0.0016, g 0.0012, h 0.0084, j 0.0088, k 0.0182, m 0.0004, n 0.0084. *p < 0.05, **p < 0.01, ***p < 0.001.
Given the crucial role of intracellular Ca2+ signaling in apoptosis induction, we reasoned that SMARCA4/2 may affect apoptosis by regulating intracellular Ca2+ flux. To validate the role of SMARCA4/2 in Ca2+ homeostasis and transfer to mitochondria, we measured the changes in cytosolic and mitochondrial Ca2+ content of SCCOHT-1 cells, ±SMARCA4 restoration, in response to histamine, an inositol trisphosphate (IP3) agonist activating ER-Ca2+ release via inositol trisphosphate receptor (IP3R)55. In order to monitor intracellular Ca2+ dynamics, we expressed genetically encoded Ca2+ indicators (GECI) targeted to the cytosol (R-GECO)56 or mitochondria (CEPIA-2mt)57 and monitored GECI fluorescence upon ER-Ca2+ release stimulation by spinning disk confocal microscopy (Supplementary Fig. 6). While histamine stimulation induced little changes in cytosolic or mitochondrial Ca2+ in SCCOHT-1 control cells, it strongly elevated Ca2+ content in both compartments in SMARCA4-restored cells (Fig. 2c–e). Consistent with this, restoration of SMARCA4 in H1703 cells also significantly increased ER-Ca2+ release to the cytosol and Ca2+ transfer to the mitochondria upon histamine stimulation, compared to control cells (Fig. 2f–h). These data indicate that SMARCA4 plays a causal role in regulating intracellular Ca2+ homeostasis by enabling ER-Ca2+ release to the cytosol and mitochondria.
The increased cytosolic and mitochondrial Ca2+ content observed upon SMARCA4 restoration could be due to either direct enhanced Ca2+ release from the ER or elevated capacity of the ER-Ca2+ content. To distinguish these possibilities, we measured the cytosolic Ca2+ changes in above isogenic cell pairs of SCCOHT-1 and H1703 in response to thapsigargin, an inhibitor of sarcoplasmic/ER Ca2+-ATPase, which can entirely deplete ER-Ca2+ stores55. Interestingly, restoration of SMARCA4 does not increase maximal cytosolic ER-Ca2+ release induced by thapsigargin treatment in SCCOHT-1 or H1703 cells (Supplementary Fig. 7a, b), suggesting that SMARCA4 promotes Ca2+ release from the ER rather than an increase in ER-Ca2+ storage capacity. Further supporting this, SMARCA4 knockout in OVCAR4 and H1437 cells significantly decreased the induction of cytosolic and mitochondrial Ca2+ upon histamine treatment (Fig. 2i–n), even though SMARCA4 knockout had increased ER-Ca2+ stores as indicated by an increase in cytosolic Ca2+ in OVCAR4 cells, but not in H1437 cells, following thapsigargin stimulation (Supplementary Fig. 7c, d). Finally, to rule out the potential contribution of the mitochondrial Ca2+ uptake machinery in this phenotype, we showed that protein levels of the mitochondrial calcium uniporter (MCU) and its regulators58,59 were unchanged in these cell lines, indicating that Ca2+ transfer defects were not due to defective mitochondrial Ca2+ import machinery (Supplementary Fig. 7e). Together, these results suggest that SMARCA4/2 regulate intracellular Ca2+ homeostasis and mitochondrial Ca2+ content likely by controlling Ca2+ release from the ER.
SMARCA4/2 directly regulate ITPR3 expression
To dissect the detailed mechanism by which SMARCA4/2 regulate Ca2+ homeostasis, we further investigated Ca2+-related genes in ion/calcium associated GO terms identified from the above transcriptome analysis in SCCOHT cells (Fig. 2a, b). Overlapping the two datasets yielded 198 common genes affected by SMARCA4 restoration in both SCCOHT-1 and BIN-67 cells (Fig. 3a; Supplementary Table 2). To help identify direct targets of SMARCA4, we examined these 198 commonly regulated genes in a chromatin immunoprecipitation sequencing (ChIP-seq) data set profiling SMARCA4 occupancy in BIN-67 cells ± SMARCA4 restoration60. This analysis revealed 69 of the 198 genes showing SMARCA4 occupancy in their loci (Fig. 3a; Supplementary Table 2). Considering that SMARCA4 and SMARCA2 may regulate the same target genes and that SMARCA4 also modulates Ca2+ homeostasis in NSCLC cells (Fig. 2f–h), we then examined the regulation of these 69 genes in an independent RNA-seq data set of BIN-67 cells ± SMARCA4/2 restoration60 and a microarray data set of NSCLC cell line H1299 ± SMARCA4 restoration61. Notably, all of the 69 SMARCA4-affected genes were also regulated by SMARCA2 in BIN-67 cells, indicating that SMARCA4/2 may have redundant function in controlling Ca2+ homeostasis (Fig. 3b). In keeping with the fact that lung cancer cells have more complex genetic landscapes than SCCOHT15,62, only four genes, namely ITPR3, MATN2, EHD4, and ATP2B4, were consistently upregulated by SMARCA4 in both cancer types (Fig. 3b).
a Venn diagram of Ca2+-related genes from Fig. 2a, b that are enriched in SCCOHT-1 and BIN-67 cells with SMARCA4 restoration. b Heatmap of Ca2+-related genes bound by SMARCA4 (n = 69) in indicated SCCOHT (SCCOHT-1 and BIN-67) and NSCLC (H1299) cell lines with SMARCA4/2 restoration. Left: normalized reads from RNA-seq data of BIN-67 and SCCOHT-1 cells with SMARCA4 restoration49. Middle: normalized reads from RNA-seq data of BIN-67 cells with SMARCA4/2 restoration60. Right: normalized signal from microarray data of H1299 cells with SMARCA4 restoration61. Row scaling was used to generate the heatmap. The last column represents changes of genes in H1299 cells ± SMARCA4 restoration: ns not significant, up upregulated, down downregulated. c RT-qPCR measurements of ITPR3 mRNA expression in indicated SCCOHT and NSCLC cell lines with SMARCA4/2 restoration. GAPDH was used for normalization. Mean ± SD, n = 3 (BIN-67, SCCOHT-1, H1299) or 4 (H1703) independent experiments, one-way ANOVA followed by Dunnett’s test for multiple comparisons to the control group (BIN-67, SCCOHT-1, H1703) or two-tailed t-test (H1299), p values (p): BIN-67, all <0.0001; SCCOHT-1, A2—0.0004, A4—0.0001; H1299—0.0318; H1703, A2—0.0097, A4—0.0013. d Immunoblots of indicated proteins in indicated SCCOHT and NSCLC cell lines ± SMARCA4/2 restoration. e SMARCA4 occupancy in vicinity of the ITPR3 locus assessed by chromatin immunoprecipitation sequencing (ChIP-seq) in indicated SCCOHT and lung cancer cell lines ± SMARCA4 restoration. SMARCA4 in H1299 cells was induced by doxycycline (Dox)61. Track height is normalized to relative number of mapped reads. f Chromatin structure changes in vicinity of the ITPR3 locus assessed by H3K27Ac ChIP-seq and assay for transposase-accessible chromatin sequencing (ATAC-seq) in indicated SCCOHT and lung cancer cell lines ± SMARCA4/2 restoration. Track height is normalized to relative number of mapped reads. a–f Ctrl control, A4 SMARCA4, A2 SMARCA2. *p < 0.05, **p < 0.01, ****p < 0.0001.
Among these four common genes, ITPR3 encodes inositol 1,4,5-trisphosphate receptor type 3 (IP3R3), one of the IP3R family members that forms Ca2+ channels on the ER and plays critical roles in intracellular Ca2+ homeostasis and cell apoptosis52,63. IP3R3 localizes at the mitochondria-associated membranes, a signaling platform allowing the generation of microdomains of high Ca2+ concentration required for efficient mitochondrial Ca2+ uptake64, and preferentially transmits apoptotic Ca2+ signals into mitochondria over other IP3Rs65. Tumor suppressors such as PTEN, BAP1, and PML have been shown to induce apoptosis in cancer cell by promoting IP3R3-mediated Ca2+ flux from the ER to mitochondria66,67,68. Thus, we hypothesized that SMARCA4/2 may promote Ca2+ flux to the mitochondria and apoptosis induction by directly regulating ITPR3 gene expression. Corroborating our transcriptome data above (Fig. 3b), ectopic expression of SMARCA4 or SMARCA2 in both SCCOHT (BIN-67, SCCOHT-1) and NSCLC (H1299, H1703) cells resulted in elevated mRNA and protein expression of IP3R3 (Fig. 3c, d). Conversely, SMARCA4 knockout in OVCAR4, HEC116, and H1437 cells suppressed IP3R3 expression which was further downregulated upon subsequent SMARCA2 knockdown (Supplementary Fig. 8). These data established that SMARCA4/2 promote IP3R3 expression in both ovarian and lung cancer cells, likely through direct regulation of transcription.
Given the chromatin remodeling role of SWI/SNF, we then focused on the chromatin architecture of the ITPR3 locus and its potential regulation by SMARCA4/2. Indeed, SMARCA4 occupancy was observed at the ITPR3 promoter in ChIP-seq data of the BIN-67 cells upon SMARCA4 restoration (Fig. 3e)60. We also detected this SMARCA4 occupancy in H1703 cells with SMARCA4 restoration48 and in H1299 cells expressing inducible SMARCA461 (Fig. 3e). These data suggest that SMARCA4/2 may directly regulate ITPR3 expression. Consistent with this, we found that ChIP-seq signals of H3K27Ac, a chromatin mark associated with active promoter and enhancer, were elevated at the upstream and gene body regions of ITPR3 in BIN-67 cells after SMARCA4 restoration60 and in H1703 cells after restoration of SMARCA4 or SMARCA248 (Fig. 3f, upper panel). Furthermore, the assay for transposase-accessible chromatin using sequencing (ATAC-seq) peaks at these ITPR3 genomic regions were also elevated upon SMARCA4/2 restoration in H1703 cells (Fig. 3f, lower panel), indicating an enhanced chromatin accessibility at the ITPR3 locus when SMARCA4/2 were present. Together, these data suggest that SMARCA4/2 promote ITPR3 transcription by directly remodeling chromatin structure at its gene locus.
SMARCA4/2 loss inhibits apoptosis by restricting IP3R3-mediated Ca2+ flux to mitochondria
Next, we investigated whether reduced IP3R3 expression accounts for compromised Ca2+ flux in SMARCA4/2-deficient SCCOHT and NSCLC cells (Fig. 2c–n). To this end, we performed rescue experiments by suppressing SMARCA4-mediated IP3R3 induction in SCCOHT-1 and H1703 cells. Accompanied by an increase of IP3R3 levels (Fig. 4a), ectopic SMARCA4 expression in SCCOHT-1 cells strongly elevated cytosolic (Fig. 4b) and mitochondrial (Fig. 4c) Ca2+ contents in response to histamine stimulation. Notably, in these SMARCA4-restored cells, shRNA-mediated knockdown of IP3R3 to levels similar to control cells prevented ER-Ca2+ release, characterized by a significant decrease of cytosolic and mitochondrial Ca2+ contents (Fig. 4a–c). These results were confirmed in H1703 cells where suppression of IP3R3 was achieved by small-interfering RNA (siRNA) (Fig. 4d–f). Furthermore, cytosolic Ca2+ measurement upon thapsigargin stimulation in the above SCCOHT-1 and H1703 cells indicated that ER-Ca2+ storage capacity was not significantly altered upon ITPR3 knockdown (Supplementary Fig. 9). Together, these data indicate that reduced IP3R3 expression is the critical contributor to the compromised Ca2+ flux in SMARCA4/2-deficient cells.
a Immunoblots of SCCOHT-1 cells ± SMARCA4 (A4) and ITPR3 (R3) perturbations. shR3: shRNA targeting ITPR3. Changes of cytosolic (b) and mitochondrial (c) Ca2+ contents in SCCOHT-1 cells ± A4 and R3 perturbations upon histamine stimulation. b 43 control (Ctrl), 30 A4, 51 A4 ShR3♯1, and 50 A4 ShR3♯2 cells from n = 4 independent experiments were analyzed. c 31 Ctrl, 30 A4, 50 A4 ShR3♯1, and 50 A4 ShR3♯2 cells from n = 4 independent experiments were analyzed. d Immunoblots of H1703 cells ± A4 and R3 perturbations. siR3 siRNA targeting ITPR3. Changes of cytosolic (e) and mitochondrial (f) Ca2+ contents in H1703 cells ± A4 and R3 perturbations upon histamine stimulation. e 64 Ctrl, 70 A4, and 64 A4 siR3 cells from n = 3 independent experiments were analyzed. f 53 Ctrl, 53 A4, and 50 A4 siR3 cells from n = 3 independent experiments were analyzed. g Immunoblots of OVCAR4 cells with ITPR3 knockdown 48 h post cisplatin treatment. cl cleaved. h Annexin V+/PI− apoptotic cell population determined by flow cytometry in OVCAR4 cells described in g. Immunoblots of H1703 cells ± R3 overexpression (i) or ±A4 and R3 perturbations (j). Cells were collected 72 h after the treatment. k Annexin V+/PI− apoptotic cell population determined by flow cytometer in H1703 cells described in j. b, c, e, f Left: traces of cytosolic or mitochondrial Ca2+ contents upon 100 μM histamine stimulation (mean ± SEM). Right: quantification of the maximal Ca2+ signal peaks induced by histamine (mean ± SD). The Ca2+ probes R-GECO (R-GECO F/F0) and CEPIA-2mt (CEPIA-2mt F/F0) were used to monitor cytosolic and mitochondrial Ca2+, respectively. Arb. units arbitrary units. One-way ANOVA followed by Dunnett’s test for multiple comparisons to Ctrl, p values (p): b A4—0.0003, shR3#1—0.6223, shR3#2—0.9866; c A4 < 0.0001, shR3#1—0.9109, shR3#2—0.9845; e A4—0.0038, siR3—0.4232; f A4—0.0007, siR3—0.1620. h, k Mean ± SD, n = 3 independent experiments, one-way ANOVA followed by Dunnett’s test for multiple comparisons, p values (p): all <0.0001. *p < 0.01, ***p < 0.001, ****p < 0.0001; ns not significant.
In line with the established role of IP3R3 in Ca2+-mediated apoptosis, suppression of IP3R3 in OVCAR4 cells prevented cisplatin-induced apoptosis as indicated by reduced levels of cleaved PARP and cleaved caspase 3 (Fig. 4g) and the annexin V+/PI− apoptotic cell population (Fig. 4h). Conversely, ectopic expression of IP3R3 in H1703 cells enhanced apoptotic induction and growth suppression after cisplatin treatment (Fig. 4i, Supplementary Fig. 10a, b). Similarly, ectopic IP3R3 expression also sensitized BIN-67 cells to cisplatin treatment (Supplementary Fig. 10c, d). Thus, IP3R3 seems to be necessary and sufficient to mediate cisplatin-induced apoptosis in these models. Given that SMARCA4/2 directly activates ITPR3 expression (Fig. 3), we then investigated whether reduced IP3R3 expression in SMARCA4/2-deficient cells drives resistance to chemotherapy-induced apoptosis. As shown in Fig. 4j, k, while SMARCA4 restoration in H1703 cells led to increased IP3R3 expression with concomitant elevation of cleaved PARP and cleaved caspase 3 as well as the annexin V+/PI− apoptotic cell population after cisplatin treatment, knockdown of IP3R3 markedly suppressed the induction of these apoptosis markers in these SMARCA4-expressing cells, corroborating Ca2+ signaling defects in these cells (Fig. 4d–f). Together, these data suggest that SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by constricting IP3R3-mediated Ca2+ flux to mitochondria.
IP3R3 expression is reduced in SMARCA4/2-deficient cancers
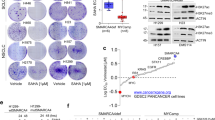
To further validate our findings of ITPR3 regulation by SMARCA4/2 in cell models with genetic perturbation, we analyzed mRNA expression of ITPR3 and SMARCA4/2 in RNA-seq data sets of ovarian (n = 47) and lung cancer (n = 192) cell lines available from CCLE41,42. For both cancer types, cell lines with low SMARCA4 expression (bottom quartile) also expressed lower levels of ITPR3 compared to the rest of cell lines with high SMARCA4 expression (Supplementary Fig. 11a). Furthermore, we observed a significant positive correlation between ITPR3 and SMARCA2 in these ovarian (n = 11, r = 0.825) and lung (n = 48, r = 0.584) cancer cell lines with low SMARCA4 expression (Fig. 5a). Moreover, in a panel of 20 NSCLC cell lines, reduced IP3R3 protein was observed in SMARCA4-deficient cells compared to SMARCA4-proficient cells; overall SMARCA4/2 dual deficient cell lines expressed the lowest levels of IP3R3 (Fig. 5b). These results are in line with our above functional data, supporting that IP3R3 expression is reduced in SMARCA4/2-deficient ovarian and lung cancer cells.
a Correlation of ITPR3 and SMARCA2 (A2) mRNA in ovarian (n = 11) and lung (n = 48) cancer cell lines with low expression of SMARCA4 (A4). Expression data were obtained from Cancer Cell Line Encyclopedia (CCLE) in reads per kilobase million (RPKM)42. A4Low, bottom quartile. r: Pearson correlation; p: p value (two-tailed). b Immunoblots of lung cancer cell lines with indicated SMARCA4/2 (A4/2) status. Pro proficient, def deficient; * KRAS mutant. c Correlation of ITPR3 and SMARCA2 mRNA in ovarian cancer (n = 89) and lung adenocarcinoma (LUAD, n = 128) patient tumors with low expression of SMARCA4. Gene expression data were obtained from UCSC Xena in fragments per kilobase million (FPKM). A4Low, bottom quartile. r, Pearson correlation; p, p value (two-tailed). d ITPR3 mRNA expression in SCCOHT and ovarian cancer patient tumors. TCGA ovarian cancers (OV) (n = 379) were stratified based on SMARCA4/2 expression as indicated in Supplementary Fig. 2b. ITPR3 expression in FPKM was normalized to ACTB. H: high; L: low. One-way ANOVA Brown–Forsythe and Welch tests followed by Dunnett’s test for multiple comparisons to A4HA2H, p values (p): A4HA2L—0.3830, A4LA2H—0.0009, A4LA2L < 0.0001, SCCOHT—0.0108, or two-tailed t-test between A4LA2L group and SCCOHT, p = 0.4953. Representative images (e, g) and H-score (f, h) of immunohistochemistry analysis for IP3R3 and SMARCA4 expression in patient tumors. e, f HGSC (n = 49) and SCCOHT (n = 45). g, h NSCLC (n = 59). Scale bar, 100 μm. Mann–Whitney test (two-tailed), p values (p): f 0.0066, h 0.0270. i Tumor growth in H1703 xenograft models ± exogenous SMARCA4 expression. Doxycycline (Dox) was given daily starting on day 21 to induce SMARCA4. Upper, tumor size; lower, endpoint tumor weight. Mean ± SEM, − Dox (n = 4 animals), + Dox (n = 6 animals), two-way ANOVA (upper), two-tailed t-test (lower), p values (p): upper—0.0014, lower—0.0008. Representative images (j) and digital quantification (k) of immunohistochemistry analysis in endpoint tumors described in i. Scale bar, 100 μm. Mean ± SD, − Dox (n = 4), + Dox (n = 6), two-tailed t-test (lower), p values (p): upper, middle <0.0001; lower—0.0041. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns not significant.
Next, we investigated the relationship between IP3R3 and SMARCA4/2 expression in patient tumors. We analyzed the available TCGA RNA-seq data sets of ovarian serous cystadenocarcinoma (OV)69, LUAD, and lung squamous cell carcinoma tumors7,70. Similar to the above observations in cell lines, ITPR3 mRNA in patient tumors with the bottom quartile of SMARCA4 expression is significantly reduced compared the other tumors in all three data sets (Supplementary Fig. 11b). Confirming the cell line results (Fig. 5a), ITPR3 was also significantly correlated with SMARCA2 mRNA in these tumors with low SMARCA4 expression (Fig. 5c, Supplementary Fig. 11c). Furthermore, we analyzed ITPR3 mRNA expression in SCCOHT patient tumors (n = 13) characterized by concomitant loss of SMARCA4/2 protein expression. In keeping with above analysis, ITPR3 mRNA in SCCOHT tumors is similar to OV tumors with low expression of SMARCA4/2 (n = 42) while significantly lower than OV tumors with high expression of SMARCA4/2 (n = 50)69 (Fig. 5d). Using immunohistochemistry (IHC), we also examined IP3R3 protein expression in patient tumors of SCCOHT and HGSC with an IP3R3 antibody whose IHC specificity was verified by RNAi (Supplementary Fig. 12). As shown in Fig. 5e, f, SCCOHT tumors (n = 45) expressed significantly lower levels of IP3R3 than HGSCs (n = 45). Consistently, NSCLC tumors with low SMARCA4 expression (n = 9, H-score ≤ 100) expressed significantly lower IP3R3 protein than those with higher SMARCA4 expression (n = 50, H-score > 200) (Fig. 5g, h). Together, these results from multiple cohorts of cell lines and patient tumor samples support the cooperative roles of SMARCA4/2 in regulating ITPR3 and confirm reduced IP3R3 expression in SMARCA4/2-deficient cancers.
Given that suppressed IP3R3-mediated Ca2+ flux and apoptosis has been linked to other major tumor suppressors PTEN, BAP1, and PML, in driving tumorigenesis66,67,68, our above analyses suggest that this may also play a role in SMARCA4/2-deficient cancers. We examined this possibility in vivo using a xenograft model of H1703 cells with exogenous SMARCA4 expression, using a validated doxycycline-controlled expression system49. Upon tumor establishment, we induced SMARCA4 expression with doxycycline treatment, which indeed resulted in suppression of tumor growth (Fig. 5i). Furthermore, IHC analysis of endpoint tumors showed that induced-SMARCA4 expression led to elevated expression of IP3R3 and cleaved caspase 3 (Fig. 5j, k). While this requires further studies, these data support that reduced IP3R3 expression in SMARCA4/2-deficient cancers may directly contribute to the tumorigenesis through suppression of apoptosis.
Histone deacetylase inhibitor (HDACi) rescues IP3R3 expression and enhances cisplatin response in SMARCA4/2-deficient cancer cells
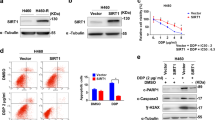
Our data show that SMARCA4/2-deficient cancer cells are resistant to cisplatin in part through suppression of IP3R3 and that ectopic IP3R3 expression can sensitize these cancer cells to cisplatin-induced apoptosis (Fig. 4g–k, Supplementary Fig. 10). Although IP3R3 is not targetable, its expression is directly activated by SMARCA4/2 (Fig. 3). In contrast to deleterious mutations in SMARCA4, SMARCA2 loss is caused by epigenetic silencing in SCCOHT and NSCLC17,71,72,73. Furthermore, HDACi, a class of anti-cancer drugs that blocks the deacetylation of chromatin and other cellular substrates involved in cancer initiation and progression74,75, has also been shown to reactivate SMARCA2 expression in SCCOHT and lung cancer cells17,76,77. Indeed, treatments with quisinostat78, a second-generation HDACi, resulted in strong activation of SMARCA2 with concomitant elevation of IP3R3 at both mRNA and protein levels in SCCOHT (Supplementary Fig. 13) and SMARCA4/2-deficient NSCLC cancer cells (Fig. 6a, b). Consistent with this, quisinostat treatment strongly elevated cytosolic (Supplementary Fig. 14a) and mitochondrial (Supplementary Fig. 14b) Ca2+ contents in response to histamine stimulation, similar to the levels induced by ectopic SMARCA4 expression in H1703 cells (Supplementary Fig. 14a–c). Notably, siRNA-mediated knockdown of IP3R3 in these quisinostat-treated cells prevented ER-Ca2+ release, characterized by a significant decrease of cytosolic and mitochondrial Ca2+ contents (Supplementary Fig. 14a–c). Together, these data indicate that quisinostat treatment can indirectly restore IP3R3 expression and rescue Ca2+ flux in SMARCA4/2-deficient cancer cells.
a SMARCA2 and ITPR3 mRNA expression in H1703 cells treated with quisinostat for 48 h. Mean ± SD, n = 3 independent experiments, one-way ANOVA followed by Dunnett’s tests for multiple comparisons to untreated, p values (p): left, 10 nM—0.0003, 40 nM < 0.0001; right, 10 nM—0.0027, 40 nM—0.0002. Immunoblots (b) and annexin V+/PI− apoptotic population (c) in H1703 cells treated with cisplatin (3 μM) and quisinostat (10 nM) for 72 h. cl cleaved. Mean ± SD, n = 3 independent experiments, one-way ANOVA followed by Dunnett’s test for multiple comparisons, p values: all <0.0001. d Colony formation assay for H1703 cells treated with cisplatin and quisinostat for 12 days. Immunoblots of H1703 cells ± SMARCA2 knockout (e) or ±IP3R3 knockdown (f) treated with cisplatin (3 μM) and quisinostat (10 nM) for 72 h. g Representative images from confocal live-cell imaging of H1703 cells overexpressing the mitochondrial Ca2+ probe CEPIA-2mt treated with quisinostat or/and cisplatin and stained with the mitochondrial marker Mitotracker deep red. Cisplatin: 2 μM, 24 h; quisinostat: 40 nM, 72 h. Scale bar, 25 μm. h Quantification of basal mitochondrial Ca2+ levels from g, showing the ratio of CEPIA-2mt/Mitotracker fluorescence intensities compared to control. 43 control (ctrl), 42 cisplatin, 38 quisinostat, and 46 quisinostat/cisplatin cells from n = 4 independent experiments were analyzed. Mean ± SD, one-way ANOVA followed by Dunnett’s test for multiple comparisons to ctrl, p values: cisplatin—0.2105, quisinostat—0.2985, combination—0.0032. i Tumor growth in H1703 xenograft models treated with cisplatin (4 mg kg−1) and quisinostat (10 mg kg−1). Vehicle (n = 6 animals), all other groups (n = 5 animals); upper, tumor volume, mean ± SEM, two-way ANOVA; lower, endpoint tumor weight, one-way ANOVA followed by Dunnett’s tests for multiple comparisons to the combination, p values: upper, cisplatin < 0.0001, quisinostat—0.0054; lower, cisplatin—0.0008, quisinostat—0.0330. Representative images (j) and digital quantification (k) of immunohistochemistry analysis in endpoint tumors described in i. Scale bar, 100 μm. Mean ± SD, all groups (n = 5), one-way ANOVA followed by Dunnett’s test for multiple comparisons, p values: all <0.0001. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns not significant.
Next, we explored the possibility of using HDACi to restore chemotherapy sensitivity in SMARCA4/2-deficient cancer cells. Indeed, the combination treatment of cisplatin and quisinostat in H1703 cells resulted in strong elevation of cleaved PARP and cleaved caspase 3 (Fig. 6b), the annexin V+/PI− apoptotic cell population (Fig. 6c), and growth suppression (Fig. 6d). Given that HDACi is expected to activate expression of genes other than SMARCA2, it was important to verify the essential contribution of SMARCA2 reactivation to apoptosis induction by this drug combination. Supporting this, CRISPR/Cas9-mediated SMARCA2 knockout in H1703 cells blunted the elevation of IP3R3 and cell apoptosis markers induced by combination treatment of quisinostat and cisplatin (Fig. 6e). Furthermore, siRNA-mediated knockdown of IP3R3 also prevented the elevation of apoptosis markers in H1703 cells induced by this treatment combination (Fig. 6f). Finally, confocal live-cell imaging demonstrated that the combination of cisplatin and quisinostat strongly induced an increase of basal mitochondrial Ca2+ levels in these cells (Fig. 6g, h). These results demonstrate that quisinostat can stimulate SMARCA2-dependent IP3R3 expression to restore ER-Ca2+ release-induced mitochondrial Ca2+ flux and chemotherapy sensitivity in SMARCA4/2-deficient cancer cells.
Finally, we validated the antitumor effect of this cisplatin and quisinostat drug combination in vivo using a xenograft model of H1703 cells. After tumor establishment, animals were treated with cisplatin (4 mg kg−1), quisinostat (10 mg kg−1), or their combination. Consistent with our in vitro results, the combination more effectively suppressed tumor growth than each single drug alone, as indicated by a significant reduction of both tumor volume and weight (Fig. 6i). We noted that some animals treated with cisplatin or the combination, but not quisinostat alone, showed body weight loss (Supplementary Fig. 15a), likely associated with chemotherapy-induced side effects. Nevertheless, when normalized to the animal body weight, the drug combination still showed significant reduction of both tumor volume and weight compared to single treatments (Supplementary Fig. 15b, c). Furthermore, IHC analysis of endpoint tumors revealed that quisinostat treatment was able to induce protein expression of SMARCA2 and IP3R3 (Fig. 6j, k) and, when combined with cisplatin, synergistically elicited a strong apoptotic response as indicated by a marked increase of cleaved caspase 3 levels (Fig. 6j, k). Taken together, our data provide a proof-of-concept treatment strategy for enhancing chemotherapy response in patients affected by SMARCA4/2-deficient cancers.
Discussion
We show that SMARCA4/2 deficiency impairs chemotherapy-induced apoptotic responses in ovarian and lung cancers at least in part by altering ER to mitochondria Ca2+ flux. By directly restricting ITPR3 expression, SMARCA4/2 loss inhibits Ca2+ transfer from the ER to mitochondria required for apoptosis induction. Consequently, stimulation of ITPR3 expression through SMARCA2 reactivation by HDACi enhanced chemotherapy response in SMARCA4/2-deficient cancer cells.
SWI/SNF subunits are frequently mutated in human cancers4, which has been connected to hallmarks of cancers including aberrant cell proliferation, linage differentiation, and altered metabolism1,28. Our findings establish a functional link between SMARCA4/2 loss and dampened IP3R3-mediated Ca2+ flux in resisting programmed cell death. While our current study mostly focuses on chemoresistance, we also found that SMARCA4 restoration alone suppressed tumor growth of H1703 xenografts associated with increased expression of IP3R3 and cleaved caspase 3. This suggests that altered Ca2+ homeostasis may also directly contribute to the tumorigenesis of SMARCA4/2 loss through suppression of apoptosis, as previously shown for other major tumor suppressors PTEN, BAP1, and PML66,67,68. Additional investigations are warranted to further confirm these results. Given the cooperative roles of SMARCA4/2 in regulating ER to mitochondria Ca2+ flux and apoptosis, it is likely that they exert these functions in a SWI/SNF-dependent manner. Therefore, exploring the potential role of other SWI/SNF subunits frequently altered in cancers, such as ARID1A4, in Ca2+ homeostasis and apoptosis may help understand the oncogenic mechanisms underlying other SWI/SNF-deficient cancers.
Our study examined the roles of SMARCA4/2 in regulating chemotherapy response and apoptosis induction using cancer cell lines that naturally harbor SMARAC4/2 alterations. This is different from previous studies employing RNAi-mediated SMARCA4 knockdown in SMARCA4-proficient cancer cells which led to enhanced response to DNA damaging agents31,32,33. We found that naturally occurred SMARCA4/2-deficient cancer cells are more resistant to chemotherapy, which is in line with previous reports showing that SCCOHT is typically more resistant to conventional chemotherapy in both cell models and patients15,34. Similarly, experimental suppression of SMARCA2 has been shown to be selective lethal to SMARCA4-deficient/SMARCA2 proficient cancer cells18,19,20. However, concomitant loss of SMARCA4/2 occurs in almost all SCCOHTs and a subset of NSCLCs associated with poorer prognosis in patients6,10,16,17. Therefore, naturally occurred SMARC4A/2-deficient cancers may represent a unique group with distinct properties such as altered Ca2+ homeostasis leading to chemotherapy resistance.
Similar to SCCOHT, our analysis in multiple NSCLC datasets of diverse tumor staging including the most comprehensive Director’s Challenge data set suggests that reduced SMARCA4 expression is associated with chemoresistance in NSCLC. A previous report79 analyzing the JBR.10 data set of NSCLCs from early stages80 showed that patients whose tumors expressed low SMARCA4, but not high SMARCA4, benefited from the adjuvant therapy of cisplatin and vinorelbine (a microtubule inhibitor). This discrepancy is likely due to differences in SMARCA4 microarray probe sets chosen, patient cohort compositions, and data analysis methods. While we used the optimal “Jetset probe” unbiasedly identified by the KM plotter without preassociation with patient outcome, microarray technology has limited sensitivity and specificity in quantifying gene expression. Thus, these results require further confirmation using better tools such RNA-seq. In addition, we recognize that patient outcome is often influenced by multiple factors such as treatment history, which was not uniform among all patients analyzed. Therefore, additional clinical studies are needed to better control these variants and evaluate roles of SMARCA4/2 expression in predicting chemotherapy responses in NSCLC patients.
HDACi have been clinically approved for the treatment of several hematological malignancies but their activity in solid tumors has been limited as single agents74,75. Thus, identifying genetic vulnerability of HDACi and effective drug combinations may enhance their clinical utility. SCCOHT cells have been shown to be more sensitive to HDACi than SMARCA4/2-deficient NSCLC cells76. This may be because NSCLC have a more complex genetic make-up than SCCOHT15,62. Our study provided proof-of-principle data supporting that HDACi may be a potential therapeutic strategy to stimulate ITPR3 transcription through SMARCA2 reactivation and sensitize SMARCA4/2-deficient cancers to chemotherapy. Other strategies may also be explored. For example, GGTi-2418, a geranylgeranyl transferase inhibitor, sensitizes A549 cells to apoptosis induction by photodynamic therapy both in vitro and in xenograft models via stabilizing the IP3R3 protein66. Of note, A549 is also a SMARCA4-deficient NSCLC cell line and this independent study does further support the notion of elevating IP3R3 expression to enhance chemotherapy response in SMARCA4/2-deficient cancers. However, both HDACi and GGTi-2418 intervene IP3R3 expression indirectly and may cause unexpected toxicity. Therefore, other agents that directly facilitate Ca2+ flux from the ER to mitochondria need to be investigated in the future. In addition to IP3R3, other common targets of SMARCA4/2 may also play a role in altered Ca2+ homeostasis impacting apoptosis, which could serve as potential drug targets in SMARCA4/2-deficient cancers and will require further studies.
In summary, we have uncovered that SMARCA4/2 loss restricts IP3R3-mediated Ca2+ flux from the ER to mitochondria, leading to resistance to chemotherapy-induced apoptosis in ovarian and lung cancers. Our study provides insights into the molecular mechanisms of SWI/SNF loss in promoting drug resistance and suggests a potential therapeutic strategy to enhance chemotherapy response in patients affected by SMARCA4/2-deficient cancers.
Methods
Cell culture
All cell lines were cultured in Roswell Park Memorial Institute 1640 Medium (Thermo Fisher Scientific, Cat# 11875-093) with 7% fetal bovine serum (Sigma, Cat# F1051), 1% penicillin/streptomycin (Thermo Fisher Scientific, Cat# 15140-122), and 2 mM L-glutamine (Thermo Fisher Scientific, Cat# 25030-081), except for 293T with Dulbecco’s modified Eagle medium (Thermo Fisher Scientific, Cat# 11995-065) and HEC116 with Eagle’s minimum essential medium (Wisent, Cat# 320-005-CL). Cells were maintained at 37 °C and 5% CO2 and regularly tested for Mycoplasma using Mycoalert Detection Kit (Lonza, Cat # LT07-318). All cell line origins are listed in Reporting Summary and have been validated by short tandem repeat analysis.
Lentivirus production and infection
All experiments with ectopic expression, shRNA knockdown, and CRISPR single guide RNA (sgRNA) knockout were performed using lentiviral constructs. For lentivirus production, 2.5 × 106 293T cells were plated in 2 mL of DMEM medium per well in a six-well plate and transfected after ~8 h with lentiviral constructs, the packaging (psPAX2), and envelope plasmid (pMD2.G) by CaCl2. Virus containing medium were harvested at 24 and 36 h after transfection before use or stored at −80 °C. For infection, ~5 × 105 target cells were plated the day before and infected with virus for ~8 h. After ~20 h recovery, cells were selected in medium containing 2 μg/mL puromycin or 5 μg/mL blasticidin for 2–3 days and harvested for the experiments.
Compounds and antibodies
Cisplatin (S1166), quisinostat (S1096), paclitaxel (S1150), and topotecan (S9321) were purchased from Selleck Chemicals. Cyclophosphamide (CA80500-080), histamine (H7125), and thapsigargin (T9033) were from Sigma-Aldrich. Mitotracker deep red FM was from Thermo Fisher Scientific (M22426). Antibodies against calregulin (Cat# sc-166837), HSP90 (Cat# sc-13119), and β-Actin (Cat# sc-47778) were from Santa Cruz Biotechnology; antibodies against SMARCA2 (Cat# 11996), cleaved PARP (Cat# 5625), and cleaved caspase 3 (Cat# 9664) were from Cell Signaling; antibodies against MICU2 (Cat# ab-101465), VDAC1 (Cat# ab-14734), and GRP75 (Cat# ab-2799) were from Abcam; antibody against SMARCA4 (Cat# A300-813A) was from Bethyl Laboratories (A300-813A); antibody against IP3R3 (Cat# 610312) was from BD Pharmingen; antibody against vinculin (Cat# V4505) was from Sigma-Aldrich; antibody against MCU (Cat# HPA0168480) was from Atlas; and antibody against MICU1 (Cat# orb-323178) was from Biorbyt. Antibody against SMARCA4 was used with 1:5000 dilution and all others with 1:1000 dilution. Antibodies for IHC are described in the Immunohistochemistry method section below. Secondary antibodies goat anti-mouse IgG (Cat#1706516) and goat anti-rabbit IgG (Cat#1706515) were purchased from Bio-Rad.
Plasmids
Individual shRNA vectors used were from the Mission TRC library (Sigma) provided by McGill Platform for Cellular Perturbation (MPCP) of Rosalind and Morris Goodman Cancer Research Centre and Biochemistry at McGill University: shSMARCA2#1 (TRCN0000358828); shSMARCA2#2 (TRCN0000020333); shITPR3#1 (TRCN0000061324); and shITPR3#2 (TRCN0000061326). For shRNA experiments, pLKO vector control was used. Scramble control sgRNA (SCR_6) and dual-sgRNAs targeting SMARCA4 (TEDH-1074701) or SMARCA2 (TEDH-1074696) were from the transEDIT-dual CRISPR Library (Transomic) provided by MPCP. Additional sgRNA (GCTGGCCGAGGAGTTCCGCCC) targeting SMARCA4 was cloned into pLentiCRISPRv2. pReceiver control vector, pReceiver-SMARCA4, and pReceiver-SMARCA2 were purchased from GeneCopoeia. pLX304-ITPR3 was generated by gateway cloning with pENTR223.1-ITPR3 (BC172406). pENTR223.1-ITPR3 (BC172406) and pLX304-GFP control (ccsbBroad304_07515) were from Transomic provided by MPCP. transEDITTM pCLIP-All-EFS-Puro Epigenetics CRISPR Screening library was from Transomic (Cat# CAHD9001). pLentiCas9-Blast (Cat# 52962), pLentiCRISPRv2 (Cat# 52961), pCMV-R-GECO1 (Cat# 32444), and pCMV-CEPIA2mt (Cat# 58218) were from Addgene. pIN20 and pIN20-SMARCA482 were provided by Dr. Jannik N. Andersen (The University of Texas, MD Anderson Cancer Center).
CRISPR/Cas9 editing
Plasmid-based CRISPR/Cas9 editing was used to generate SMARCA4 knockout in OVCAR4 and H1437 cells using standard lentiviral delivery followed by single-cell cloning. For HEC116 cells, ribonucleoprotein (RNP) delivery was used. cRNA targeting SMARCA4 (sequence = GCGGTGGCATCACGGGCG) and tracrRNA duplexes (1 µM) were formed by heating at 95 °C and gradual cool down to room temperature (RT). RNP complexes were formed by combining the 1 µM guide RNA oligos with 1 µM Alt-R S. pyogenes Cas9 endonucleases (IDT) in Gibco Opti-MEM media (Thermo Fisher Scientific) for 5 min at RT. Transfection complexes containing the RNP complex and Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific) were diluted in Opti-MEM media and incubated at RT for 20 min. HEC116 endometrial cancer cells were added to transfection complexes in the wells of a 24-well tissue culture plate to achieve a final concentration of 40,000 cells/well and final concentration of RNP of 10 nM. Flow cytometry (University of Alberta, Faculty of Medicine and Dentistry, Flow Cytometry Facility) was utilized to enrich for CRISPR transfected cells positive for tracrRNA-ATTOTM 550 fluorescence. Single clones were either generated by flow cytometry plating a single cell per well into a 96-well plate or manually plating of 0.5 cells/well into a 96-well plate upon filtration through a cell strainer. Single-cell-derived clones were validated by Sanger sequencing over the guided nuclease target region.
CRISPR sgRNA screen
The transEDITTM pCLIP-All-EFS-Puro Epigenetics CRISPR Screening library (Transomic) containing 5080 sgRNAs targeting 496 epigenetic genes was used in this study. Library virus was generated in 293T cells as described above. OVCAR4 cells were infected with library virus at low multiplicity of infection achieving single sgRNA integration. After selection, ~5 × 106 cells (1000-time coverage) were plated in 15 cm dishes and treated with ±100 nM cisplatin for 14 days before harvesting. Genomic DNA was isolated with High Pure PCR Template Preparation Kit (Roche) by following the manufacturer’s instruction. Library preparation for next-generation sequencing was done as described before83. Briefly, the gRNA sequences were amplified from 20 µg genomic DNA by PCR using Phusion HF DNA polymerase (Thermo Fisher Scientific) using a two-step amplification adding a unique 6-bp index per sample and sequencing adapter sequences. PCR products were purified using the High Pure PCR Product Purification Kit (Roche) and quantified using the Quant-iT™ PicoGreen™ dsDNA Assay Kit (Thermo Fisher Scientific) before sequencing on a HiSeq2500 System (Illumina). Sequencing reads were mapped to the library using xcalibr and counts were then analyzed with MAGeCK (version 0.5.8) using the robust rank aggregation algorithm. Primers used are as follows: PCR1: PTRC_index (forward), IllSeqR_CR_r (reverse); PCR2: P5_Illuseq (forward), P7_index_IR_r (reverse). Please see Supplementary Table 3 for primer sequences. Index sequences: Control: ACATCG, cisplatin: GCCTAA. Please see Supplementary Table 3 for sequence details.
Colony formation assays
Since different cell lines have variable proliferation rates and sizes, plating densities for each line were first optimized to allow about two weeks of drug treatment, before cells reach 90% confluency in six-well plates. Single-cell suspensions of all cell lines were then counted and seeded into six-well plates with the densities predetermined (2–8 × 104 cells/well). Cells were treated with vehicle control or drugs on the next day and culture medium was refreshed every 3 days for 10–14 days in total. At the endpoints of colony formation assays, cells were fixed with 3.75% formaldehyde, stained with crystal violet (0.1%w/v), and photographed. All relevant assays were performed independently at least three times.
Cell viability assays
Cultured cells were seeded into 96-well plates (1,000–6,000 cells per well). Twenty-four hours after seeding, different dilutions of compounds were added to cells. Cells were then incubated for 4 days and cell viability was measured using the CellTiter-Blue Viability Assay (Promega) by measuring the fluorescence (560/590 nm) in a microplate reader. Relative survival in the presence of drugs was normalized to the untreated controls after background subtraction.
Protein lysate preparation and immunoblots
Cells were first seeded in normal medium without inhibitors. After 24 h, the medium was replaced with fresh medium containing the inhibitors as indicated in the text. After the drug stimulation, cells were washed with cold PBS, lysed with protein sample buffer, and processed with Novex® NuPAGE® Gel Electrophoresis Systems (Thermo Fisher Scientific). HSP90, actin, vinculin, or calreticulin were used as loading controls. The quantification of immunoblots was performed on two independent experiments using Image J and normalized to loading controls are displayed in Supplementary Fig. 16. Uncropped immunoblots presented in main figures are displayed in Supplementary Fig. 17. Uncropped immunoblots presented in Supplementary Figures are included in Source Data.
Annexin V staining and IncuCyte imaging
Cells in 96-well plates were treated with cisplatin at different concentrations in medium containing IncuCyte® annexin V for apoptosis (Essen Bioscience, Cat# 4641). IncuCyte® live-cell analysis imaging system was used to record four images every 2–4 h. Images were analyzed by IncuCyte® Zoom (2016B) software and annexin V positive cells were normalized to phase-contrast confluency for each well.
Annexin V and PI flow cytometry
For apoptosis assays, negative controls were prepared by incubating cells in the absence of inducing agent and positive controls for apoptosis were prepared using 10 μM H2O2. Cells were harvested after treatment and washed in cold phosphate buffered saline and resuspended in 1X annexin binding buffer (BMS500BB) to 106 cells/mL. One hundred microliters of cell suspension was used per assay and 5 μL of FITC annexin V (A13199) and 1 μL of PI (P1304MP) diluted to 100 μg/mL in annexin V binding buffer was added to each suspension. Cells were incubated following addition of fluorescent reagents for 15 min at RT. Four hundred microliters of 1X annexin V binding buffer were added to each suspension following incubation and the samples were mixed gently and kept in the dark and on ice prior to analysis.
Flow cytometry was performed using Guava easyCyte HT (Sigma) with the guavaSoft 2.5 software (Sigma) based on the manufacturer’s instructions. Fluorescence emission was measured at 530 and >575 nm to separate between the annexin V+ and PI+ populations in green and red. Technical controls for gating were prepared with uninduced cells with both PI and annexin V stains, with either PI or annexin V only, or in the absence of both. Apoptotic cell population (annexin V+/PI−) showed green fluorescence only. Gating strategy is exemplified in Supplementary Fig. 18.
RNA isolation and real-time quantitative reverse transcription PCR (qRT-PCR)
After indicated drug treatment or genetic modifications, cells were harvested for RNA isolation using Trizol (Thermo Fisher Scientific, Cat # 15596018) the next day. Synthesis of complementary DNAs (cDNAs) using Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Cat# K1642) and qRT-PCR assays using PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific, Cat# A25742) were carried out according to the manufacturer’s protocols. Relative mRNA levels of each gene shown were normalized to the expression of the housekeeping gene GAPDH. The sequences of the primers are listed in Supplementary Table 3.
Survival analysis
Survival analyses were performed on LUAD patients from the following datasets with available information on adjuvant chemotherapy status: Director’s Challenge Consortium for the Molecular Classification of Lung Adenocarcinoma36, KM plotter37, and The UT Lung SPORE (GSE42127)39. For all Affymetrix microarray datasets, 213720_s_at was the probe used to assess SMARCA4 expression. Li et al. defined this probe as the “JetSet” probe—the most suitable gene probe based on its specificity, coverage, and degradation resistance characteristics38. Director’s Challenge and The UT Lung SPORE (GSE42127) datasets were analyzed by stratifying patients into SMARCA4 high and low groups, separated by median SMARCA4 level. The survival data were analyzed by one-tailed Mantel–Cox analysis in GraphPad Prism. Parameters for kmplot.com query were: gene symbol—SMARCA4; probe set options—use JetSet best probe set; split patients by—auto select best cutoff; survival—censor at threshold; histology—adenocarcinoma; and all other default settings. Patients with and without adjuvant chemotherapy were analyzed separately in all datasets.
Transcriptome analysis
Cell lines
There were three sets of transcriptome data used in this study, namely SMARCA4 restoration in BIN-67 and SCCOHT-1 cells (GSE120297, RNA-seq), SMARCA4/2 restoration in BIN-67 cells (GSE117735, RNA-seq), and SMARCA4 restoration in H1299 cells (GSE109010, microarray). Processed gene expression data were retrieved from original study for GSE120297 and by GEOquery (2.56.0)84 for GSE109010. For GSE117735, sequencing files were downloaded from sequence read archive and mapped to reference human genome sequence (hg19) with STAR (2.6.1c)85. Gene expression counts were calculated by featureCounts (v1.6.4)86 with UCSC hg19 gene annotation GTF file. Heatmaps for gene expression were generated with pheatmap (1.0.12) after normalization. Differential expression genes were identified with DESeq2 (version 1.19.38) for GSE120297, with GEO2R analysis for GSE109010 and from original study60 for GSE117735.
Patient tumors
Total RNA from three SCCOHT patient tumors was subjected for RNA-seq at Genome Quebec. Briefly, quantification was performed using a NanoDrop Spectrophotometer ND-1000 (NanoDrop Technologies, Inc.) and its integrity was assessed using a 2100 Bioanalyzer at Genome Quebec. Libraries were generated from 250 ng of total RNA using the TruSeq stranded mRNA Sample Preparation Kit (Illumina, Cat# RS-122-2101), as per the manufacturer’s recommendations. Libraries were quantified using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Thermo Fisher Scientific, Cat# P7589) and the Kapa Illumina GA with Revised Primers-SYBR Fast Universal kit (Kapa Biosystems). Average size fragment was determined using a LabChip GX (PerkinElmer) instrument. RNA-seq data of another ten SCCOHT patient tumors were obtained from a previous study87. Sequencing results were processed by following mRNA quantification analysis pipeline of Genomic Data Commons (https://docs.gdc.cancer.gov/Data/Bioinformatics_Pipelines/Expression_mRNA_Pipeline/): first aligning reads to the GRCh38 reference genome with STAR-2.6.0c and then by quantifying the mapped reads with HTSeq-0.6.188. RNA-seq read counts of 379 ovarian cancer tumors were obtained from UCSC Xena (http://xena.ucsc.edu/) which followed the exact same pipeline. The fragments per kilobase of transcript per million mapped reads (FPKM) for each gene was calculated as
where RCg is the number of reads mapped to the gene; RCpc is the number of reads mapped to all protein-coding genes; and L is mean of lengths of isoforms of a gene.
Gene set enrichment analysis
Preranked gene listed were generated on the log2 transformed fold change for significantly changed genes (adjusted p value smaller than 0.05). The R package clusterProfiler (v3.12.0)89 was used to perform GSEA with the following parameters: ont = “MF”, nPerm = 10,000, minGSSize = 3, maxGSSize = 800, and pvalueCutoff = 0.05.
siRNA and plasmids transfection
For siRNA experiments, cells were transfected using Lipofectamine RNAimax (Thermo Fisher Scientific, Cat# 13778150) with 20 nM SMARTPool ON-TARGETplus HUMAN ITPR3 siRNA (Horizon Discovery, cat# L-006209-00-0005) for 3 days according to the manufacturer’s recommendations. Plasmids were transfected for 24 h using FuGENE HD (Promega, Cat# E2311) following manufacturer’s recommendations.
Intracellular Ca2+ measurements
To measure cytosolic or mitochondrial Ca2+, OVCAR4, H1703, SCCOHT-1, and H1437 cells were cultured on Nunc Lab-Tek chambered eight-well cover glass (Thermo Fisher Scientific, cat# 154534) and transiently transfected with the cytosolic R-GECO1 (Addgene, cat# 32444)56 or mitochondrial CEPIA-2mt (Addgene, cat# 58218)57 Ca2+ reporter probes. Cells were washed three times in a balanced salt solution buffer + Ca2+ (BSS) (120 mM NaCl, 5.4 mM KCl, 0,8 mM MgCl2, 6 mM NaHCO3, 5.6 mM D-glucose, 2 mM CaCl2, and 25 mM HEPES [pH 7.3]). Fluorescence values were then collected every 2 s for 3 min. ER-Ca2+ release was stimulated by injection of 100 µM histamine final in BSS + Ca2+ at 10 s. Images were acquired using a 40× objective of the Nikon Eclipse Ti-E microscope, coupled to an Andor Dragonfly spinning disk confocal system equipped with an Andor Ixon camera, exciting with 488 nm or 561 nm laser for CEPIA-2mt or R-GECO1, respectively.
To measure total ER-Ca2+ content, OVCAR4, H1703, SCCOHT-1, and H1437 cells were cultured on Nunc Lab-Tek chambered eight-well cover glass and transiently transfected with the cytosolic R-GECO Ca2+ reporter probe. Cells were washed three times in a BSS-Ca2+ (120 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 6 mM NaHCO3, 5.6 mM D-glucose and 25 mM HEPES [pH 7.3]). Fluorescence values were then collected every 2 s for 5 min. ER-Ca2+ release was stimulated by injection of 10 µM thapsigargin final in BSS-Ca2+ at 10 s. Images were acquired using microscope and laser described above.
To measure basal mitochondrial Ca2+ pools, H1703 cells were cultured on Nunc Lab-Tek chambered eight-well over glass, treated with appropriate drugs, and transiently transfected with the mitochondrial CEPIA-2mt Ca2+ reporter probe. Cells were stained with 100 nM Mitotracker deep red (Thermo Fisher Scientific, cat# M22426) for 20 min followed by three washes in complete culture media prior to imaging. Fluorescence values were then collected every 2 s for 30 s. Images were acquired using a 40× objective of the Nikon Eclipse Ti-E microscope, coupled to an Andor Dragonfly spinning disk confocal system equipped with an Andor Ixon camera, exciting with 488 and 647 nm lasers for CEPIA-2mt and Mitotracker deep red, respectively.
Immunohistochemistry
Tissue microarrays (TMAs) of tumor samples of HGSC and NSCLC patients used in this study were previously described48,49. A TMA of 52 SCCOHT patient tumors was constructed for this study. Studies on SCCOHT patient tumors were approved by the Institutional Review Board (IRB) at McGill University, McGill IRB # A08-M61-09B. Studies on 59 pathologist-confirmed ovarian HGSC samples were approved by the ethics boards at the University Hospitals Network and the Jewish General Hospital respectively. Informed consent was obtained from all participants in accordance with the relevant IRB approvals. Hematoxylin and eosin-stained sections of the 50 SCCOHTs (confirmed by DNA mutation analysis or/and SMARCA4 IHC) and 52 HGSC cases were reviewed and areas containing tumor only were demarcated and cored to construct TMAs using duplicate 0.6-mm cores from the demarcated areas. A panel of 100 resected LUAD patient tumors were analyzed. This study was approved by the ethics boards at the McGill University Health Centre (F11HRR, 17212). The NSCLC TMA was comprised of 4 mm cores from the selected paraffin-embedded tissue blocks. For all IHC analysis, cores with low tumor cellularity and artifacts were not included in the analysis.
The 4 µm thick sections from these TMAs were cut, deparaffinized, and stained using the BenchMark Ultra system (Ventana Medical Systems Inc). Heat-induced epitope retrieval (HIER) was performed with Ultra Cell Conditioning Solution (CC1) for 64 min at 95 °C, followed by 16 min of incubation at 36 °C with the rabbit monoclonal antibody against SMARCA4 (clone EPNCIR111A; dilution, 1:100; Abcam). For IP3R3, HIER was performed in CC1 for 48 min at 100 °C followed by incubation for 48 min at 36 °C with the mouse anti-IP3R3 (BD Transduction Laboratories). After primary antibody incubation, detection was performed using the default OptiView DAB protocol as per the manufacturer’s directions (Ventana). The slides were digitalized using an Aperio scanner and the Lumenera INFINITY X CMOS camera.
For patient tumors, assessment of SMARCA4, unequivocally absent staining in the nuclei of viable tumor cells as opposed to strong staining in background stromal cells was considered IHC negative. Expression in the tumor cells that is equivalent to the staining of nonneoplastic cells in the background was considered IHC positive. Positive cells were analyzed according to the staining intensity on a scale of 0–3 (0 = negative, 1 = weak, 2 = moderate, 3 = strong). H-scores were calculated as the sum of the percent of cells at each intensity (Pi) multiplied by the intensity score (i). H-score = Σ (Pi(i)) × 100. Score values range between 0 and 300. Cores with low tumor cellularity and artifacts were not included in the analysis.
For xenograft tumor sections, quantification of percentage positive staining for SMARCA4, SMARCA2, and cleaved caspase 3 was performed unbiasedly using the Aperio nuclear algorithm on Aperio ImageScope. Quantification of percentage positive staining for IP3R3 was performed unbiasedly using the Aperio cytoplasm algorithm on Aperio ImageScope. Weak IP3R3 (+) staining resulting from the background was not considered in the analysis.
Mouse xenografts and in vivo drug studies
Animal experiments were performed according to standards outlined in the Canadian Council on Animal Care Standards (CCAC) and the Animals for Research Act, R.S.O. 1990, Chapter c. A.22, and by following internationally recognized guidelines on animal welfare. All animal procedures (Animal Use Protocol) were approved by the Institutional Animal Care Committee according to guidelines of the CCAC. All animal experiments were carried out at the Goodman Cancer Research Center of McGill University, using 8–12-week-old in house bred male NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice.
For in vivo drug studies, Quisinostat (SelleckChem) was resuspended in 20% hydroxypropyl-β-cyclodextrin (Sigma-Aldrich) buffer (pH = 8.70) at a concentration of 50 mg mL−1 (administrated intraperitoneally at 10 mg kg−1 dose for a 25–28 g mouse in a volume of 100 µL). Cisplatin (SelleckChem) was resuspended in 0.9% sodium chloride solution (administrated intraperitoneally at 4 mg kg−1 dose for a 25–28 g mouse in a volume of 200 µL). These two reagents were stored at −80 °C. Tubes were thawed overnight at 4 °C.
For the tumor model, single-cell suspension was created by dissociating a sufficient number of subconfluent flasks of cells to produce 4 × 106 cells (H1703 or H1703 expressing pIN20-SMARCA4) in 200 µL of Matrigel HC in a 50:50 ratio (Corning Matrigel HC, VWR). The tumor cell suspension was subcutaneously injected into the left flank of each NSG mouse. For the doxycycline inducible model using H1703 cells expressing pIN20-SMARCA4, as tumor volumes (V = (H × W2)/2) reached ~150 mm3 (21 days post inoculation), experimental mice (n = 6) were injected with 2.5 mg/mL doxycycline (Millipore Sigma) intraperitoneally followed by 2 mg/mL in sucralose (MediDrop, ClearH20) solution ad libitum. Experimental mice were again injected intraperitoneally with doxycycline at day 32 to ensure they were acquiring adequate drug. Control mice (n = 4) received intraperitoneal injections of saline (diluent) and received sucralose ad libitum. All mice were placed on sucralose a week prior to the experiment to acclimatize mice to the taste. For the chemo drug treatment experiment, when tumor volumes reached ~150 mm3 (20 days post inoculation), which was assigned as day 0, the mice were entered into the treatment regimen (21 days). Mice were randomly allocated to control (vehicle, n = 6), quisinostat (10 mg kg−1 quisinostat76, three times per week, n = 5), cisplatin (4 mg kg−1 cisplatin90, once per week, n = 5), or combination (10 mg kg−1 quisinostat and 4 mg kg−1 cisplatin, n = 5) group. Mice were housed in groups of 4–5, with each group consisting of both vehicle control and treatment animals matched for tumor size on day 0 of treatment. Tumor progression was monitored and measurements using digital calipers (VWR) were recorded twice weekly. The persons who performed all the tumor measurements and the IHC analysis for the endpoint tumor samples were blinded to the treatment information.
Statistics and reproducibility
GraphPad Prism 8 software was used to generate graphs and statistical analyses. Methods for statistical tests, exact value of n, and definition of error bars are indicated in figure legends, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
All experiments have been reproduced in at least two independent experiments, unless otherwise specified in the figure legends. All immunoblots and images shown are the representative of these independent experiments.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Original data for IC50 of chemotherapy drugs are available from GDSC (https://www.cancerrxgene.org/). mRNA expression data of SMARCA4/2 and ITPR3 are available from the Cancer Cell Line Encyclopedia (https://portals.broadinstitute.org/ccle) for cell lines and from UCSC Xena (https://xenabrowser.net/datapages/) for TCGA tumors of lung and ovarian cancer patients. Out of 13 SCCOHT patient tumors, RNA-seq data of ten cases were obtained from a previous study87 and that of the other three cases can be found using the accession number EGAS00001005448. Source data for RNA-seq, microarray, ChIP-seq, and ATAC-seq can be found using the accession number GSE12029749, GSE11773560, GSE12175548, GSE109010, and GSE10902061. All unique materials generated are readily available from the authors. All other data supporting the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Change history
21 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41467-023-37144-1
References
Kadoch, C. & Crabtree, G. R. Mammalian SWI/SNF chromatin remodeling complexes and cancer: mechanistic insights gained from human genomics. Sci. Adv. 1, e1500447 (2015).
Wilson, B. G. & Roberts, C. W. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 11, 481–492 (2011).
Hodges, C., Kirkland, J. G., Crabtree, G. R. The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb. Perspect. Med. 6, a026930 (2016).
Kadoch, C. et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 45, 592–601 (2013).
Campbell, J. D. et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 48, 607–616 (2016).
Reisman, D. N., Sciarrotta, J., Wang, W., Funkhouser, W. K. & Weissman, B. E. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer Res. 63, 560–566 (2003).
Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014).
Rodriguez-Nieto, S. et al. Massive parallel DNA pyrosequencing analysis of the tumor suppressor BRG1/SMARCA4 in lung primary tumors. Hum. Mutat. 32, E1999–E2017 (2011).
Marquez, S. B., Thompson, K. W., Lu, L. & Reisman, D. Beyond mutations: additional mechanisms and implications of SWI/SNF complex inactivation. Front. Oncol. 4, 372 (2014).
Matsubara, D. et al. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci. 104, 266–273 (2013).
Ramos, P. et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat. Genet. 46, 427–429 (2014).
Witkowski, L. et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat. Genet. 46, 438–443 (2014).
Kupryjanczyk, J. et al. Ovarian small cell carcinoma of hypercalcemic type—evidence of germline origin and SMARCA4 gene inactivation. a pilot study. Pol. J. Pathol. 64, 238–246 (2013).
Jelinic, P. et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat. Genet. 46, 424–426 (2014).
Tischkowitz, M. et al. Small-cell carcinoma of the ovary, hypercalcemic type-genetics, new treatment targets, and current management guidelines. Clin. Cancer Res. 26, 3908–3917 (2020).
Jelinic, P. et al. Concomitant loss of SMARCA2 and SMARCA4 expression in small cell carcinoma of the ovary, hypercalcemic type. Mod. Pathol. 29, 60–66 (2016).
Karnezis, A. N. et al. Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type. J. Pathol. 238, 389–400 (2016).
Hoffman, G. R. et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc. Natl Acad. Sci. USA 111, 3128–3133 (2014).
Oike, T. et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer Res. 73, 5508–5518 (2013).
Wilson, B. G. et al. Residual complexes containing SMARCA2 (BRM) underlie the oncogenic drive of SMARCA4 (BRG1) mutation. Mol. Cell Biol. 34, 1136–1144 (2014).
Karnezis, A. N., Cho, K. R., Gilks, C. B., Pearce, C. L. & Huntsman, D. G. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat. Rev. Cancer 17, 65–74 (2017).
Chen, Y. et al. A PARP1-BRG1-SIRT1 axis promotes HR repair by reducing nucleosome density at DNA damage sites. Nucleic Acids Res. 47, 8563–8580 (2019).
Park, J. H. et al. Mammalian SWI/SNF complexes facilitate DNA double-strand break repair by promoting gamma-H2AX induction. EMBO J. 25, 3986–3997 (2006).
Kwon, S. J. et al. ATM-mediated phosphorylation of the chromatin remodeling enzyme BRG1 modulates DNA double-strand break repair. Oncogene 34, 303–313 (2015).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Gamwell, L. F. et al. Small cell ovarian carcinoma: genomic stability and responsiveness to therapeutics. Orphanet J. Rare Dis. 8, 33 (2013).
Fahiminiya, S. et al. Molecular analyses reveal close similarities between small cell carcinoma of the ovary, hypercalcemic type and atypical teratoid/rhabdoid tumor. Oncotarget 7, 1732–1740 (2015).
Mittal, P. & Roberts, C. W. M. The SWI/SNF complex in cancer—biology, biomarkers and therapy. Nat. Rev. 17, 435–448 (2020).
Zappa, C. & Mousa, S. A. Non-small cell lung cancer: current treatment and future advances. Transl. Lung Cancer Res. 5, 288–300 (2016).
Dasari, S. & Tchounwou, P. B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharm. 740, 364–378 (2014).
Kothandapani, A., Gopalakrishnan, K., Kahali, B., Reisman, D. & Patrick, S. M. Downregulation of SWI/SNF chromatin remodeling factor subunits modulates cisplatin cytotoxicity. Exp. Cell Res. 318, 1973–1986 (2012).
Smith-Roe, S. L. et al. SWI/SNF complexes are required for full activation of the DNA-damage response. Oncotarget 6, 732–745 (2015).
Park, J. H., Park, E. J., Hur, S. K., Kim, S. & Kwon, J. Mammalian SWI/SNF chromatin remodeling complexes are required to prevent apoptosis after DNA damage. DNA Repair 8, 29–39 (2009).
Witkowski, L. et al. The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecol. Oncol. 141, 454–460 (2016).
Otte, A., Rauprich, F., Hillemanns, P., Park-Simon, T. W., von der Ohe, J. & Hass, R. In vitro and in vivo therapeutic approach for a small cell carcinoma of the ovary hypercalcaemic type using a SCCOHT-1 cellular model. Orphanet J. Rare Dis. 9, 126 (2014).
Director’s Challenge Consortium for the Molecular Classification of Lung A. et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat. Med. 14, 822–827 (2008).
Gyorffy, B., Surowiak, P., Budczies, J. & Lanczky, A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 8, e82241 (2013).
Li, Q., Birkbak, N. J., Gyorffy, B., Szallasi, Z. & Eklund, A. C. Jetset: selecting the optimal microarray probe set to represent a gene. BMC Bioinform. 12, 474 (2011).
Tang, H. et al. A 12-gene set predicts survival benefits from adjuvant chemotherapy in non-small cell lung cancer patients. Clin. Cancer Res. 19, 1577–1586 (2013).
Yang, W. et al. Genomics of drug sensitivity in cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 41, D955–961 (2013).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Ghandi, M. et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 569, 503–508 (2019).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Wang, B. et al. Integrative analysis of pooled CRISPR genetic screens using MAGeCKFlute. Nat. Protoc. 14, 756–780 (2019).
Wang, L. et al. MED12 methylation by CARM1 sensitizes human breast cancer cells to chemotherapy drugs. Sci. Adv. 1, e1500463 (2015).
Hu, Y. et al. MiR-106b~25 cluster regulates multidrug resistance in an ABC transporter-independent manner via downregulation of EP300. Oncol. Rep. 35, 1170–1178 (2016).
Asaduzzaman, M. et al. Tumour suppressor EP300, a modulator of paclitaxel resistance and stemness, is downregulated in metaplastic breast cancer. Breast Cancer Res. Treat. 163, 461–474 (2017).
Xue, Y. et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat. Commun. 10, 557 (2019).
Xue, Y. et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat. Commun. 10, 558 (2019).
Naon, D. & Scorrano, L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim. Biophys. Acta 1843, 2184–2194 (2014).
Cardenas, C. et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283 (2010).
Pinton, P., Giorgi, C., Siviero, R., Zecchini, E. & Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27, 6407–6418 (2008).
Cardenas, C. et al. Selective vulnerability of cancer cells by inhibition of Ca(2+) transfer from endoplasmic reticulum to mitochondria. Cell Rep. 14, 2313–2324 (2016).
Clapham, D. E. Calcium signaling. Cell 131, 1047–1058 (2007).
Palmer, A. E. & Tsien, R. Y. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat. Protoc. 1, 1057–1065 (2006).
Zhao, Y. et al. An expanded palette of genetically encoded Ca(2)(+) indicators. Science 333, 1888–1891 (2011).
Suzuki, J., Kanemaru, K., Ishii, K., Ohkura, M., Okubo, Y. & Iino, M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 5, 4153 (2014).
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011).
Baughman, J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011).
Pan, J. et al. The ATPase module of mammalian SWI/SNF family complexes mediates subcomplex identity and catalytic activity-independent genomic targeting. Nat. Genet. 51, 618–626 (2019).
Lissanu Deribe, Y. et al. Mutations in the SWI/SNF complex induce a targetable dependence on oxidative phosphorylation in lung cancer. Nat. Med. 24, 1047–1057 (2018).
Bailey, M. H. et al. Comprehensive characterization of cancer driver genes and mutations. Cell 173, 371–385 e318 (2018).
Bartok, A. et al. IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat. Commun. 10, 3726 (2019).
Rizzuto, R., De Stefani, D., Raffaello, A. & Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 (2012).
Mendes, C. C. et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J. Biol. Chem. 280, 40892–40900 (2005).
Kuchay, S. et al. PTEN counteracts FBXL2 to promote IP3R3- and Ca(2+)-mediated apoptosis limiting tumour growth. Nature 546, 554–558 (2017).
Giorgi, C. et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science 330, 1247–1251 (2010).
Bononi, A. et al. BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature 546, 549–553 (2017).
Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012).
Glaros, S., Cirrincione, G. M., Muchardt, C., Kleer, C. G., Michael, C. W. & Reisman, D. The reversible epigenetic silencing of BRM: implications for clinical targeted therapy. Oncogene 26, 7058–7066 (2007).
Reisman, D., Glaros, S. & Thompson, E. A. The SWI/SNF complex and cancer. Oncogene 28, 1653–1668 (2009).
Liu, G. et al. BRM promoter polymorphisms and survival of advanced non-small cell lung cancer patients in the Princess Margaret Cohort and CCTG BR.24 trial. Clin. Cancer Res. 23, 2460–2470 (2017).
Li, Y. & Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 6, a026831 (2016).
Bolden, J. E., Peart, M. J. & Johnstone, R. W. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5, 769–784 (2006).
Wang, Y. et al. Histone deacetylase inhibitors synergize with catalytic inhibitors of EZH2 to exhibit antitumor activity in small cell carcinoma of the ovary, hypercalcemic type. Mol. Cancer Ther. 17, 2767–2779 (2018).
Yamamichi, N. et al. The Brm gene suppressed at the post-transcriptional level in various human cell lines is inducible by transient HDAC inhibitor treatment, which exhibits antioncogenic potential. Oncogene 24, 5471–5481 (2005).
Arts, J. et al. JNJ-26481585, a novel “second-generation” oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin. Cancer Res. 15, 6841–6851 (2009).
Bell, E. H. et al. SMARCA4/BRG1 is a novel prognostic biomarker predictive of cisplatin-based chemotherapy outcomes in resected non-small cell lung cancer. Clin. Cancer Res. 22, 2396–2404 (2016).
Zhu, C. Q. et al. Prognostic and predictive gene signature for adjuvant chemotherapy in resected non-small-cell lung cancer. J. Clin. Oncol. 28, 4417–4424 (2010).
Li, W. et al. Quality control, modeling, and visualization of CRISPR screens with MAGeCK-VISPR. Genome Biol. 16, 281 (2015).
Vangamudi, B. et al. The SMARCA2/4 ATPase domain surpasses the bromodomain as a drug target in SWI/SNF-mutant cancers: insights from cDNA rescue and PFI-3 inhibitor studies. Cancer Res. 75, 3865–3878 (2015).
Huang, S. et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-beta receptor signaling. Cell 151, 937–950 (2012).
Davis, S. & Meltzer, P. S. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 23, 1846–1847 (2007).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Le Loarer, F. et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat. Genet. 47, 1200–1205 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Weeden, C. E. et al. Cisplatin increases sensitivity to FGFR inhibition in patient-derived xenograft models of lung squamous cell carcinoma. Mol. Cancer Ther. 16, 1610–1622 (2017).
Acknowledgements
The authors thank A. Leary for sharing RNA-seq data and R. Hass for sharing the SCCOHT-1 cells. The authors thank members of the Pelletier Laboratory for assistance with flow cytometry studies. This work was supported by Canadian Institutes of Health Research (CIHR) grants MOP-130540, PJT-156233, and PJT-438303 to S.H. and grant FDN-148390 to W.D.F. and a core grant from Medical Research Council (MRC) MC_UU_00015/7 to J.P. Author Y.X. was supported by CIHR Fellowship (MFE-171249), J.L.M. was supported by a MRC-funded graduate student fellowship, and S.H. was supported by a CRC Chair in Functional Genomics.
Author information
Authors and Affiliations
Contributions
Y.X., J.L.M., K.Y., Z.F., X.Z., B.M., L.W., G.M., A.M., and V.P. carried out experiments. Y.X. and J.L.M. performed statistical analyses. Y.X., F.J., and W.L. conducted bioinformatic analysis. A.Y., T.G., M.C., S.J., A.V.G., L.-M.P., J.S., and W.H.G. contributed samples and provided advice. P.O.F., S.C.-B., L.F., and M.-C.G. provided pathology expertise. Y.X., J.L.M., J.P., and S.H. wrote the manuscript with inputs from all authors. J.R., M.P., W.D.F., J.P., and S.H. supervised the experiments. J.P. and S.H. conceived and oversaw the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Geert Bultynck and other, anonymous, reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, Y., Morris, J.L., Yang, K. et al. SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by restricting IP3R3-mediated Ca2+ flux to mitochondria. Nat Commun 12, 5404 (2021). https://doi.org/10.1038/s41467-021-25260-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-25260-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.