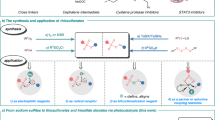
Abstract
Sulfonium salts bearing a positively charged sulfur atom with three organic substituents have intrigued chemists for more than a century for their unusual structures and high chemical reactivity. These compounds are known to undergo facile single-electron reduction to emerge as a valuable and alternative source of aryl radicals for organic synthesis. However, the generation of non-stabilized alkyl radicals from sulfonium salts has been a challenge for several decades. Here we report the treatment of S-(alkyl) thianthrenium salts to generate non-stabilized alkyl radicals as key intermediates granting the controlled and selective outcome of the ensuing reactions under mild photoredox conditions. The value of these reagents has been demonstrated through the efficient construction of alkylboronates and other transformations, including heteroarylation, alkylation, alkenylation, and alkynylation. The developed method is practical, and provides the opportunity to convert C–OH bond to C–B and C–C bonds.
Similar content being viewed by others
Introduction
Sulfonium salts are among the most versatile of all reactive intermediates in organic chemistry1,2,3. In general, the reactivity of sulfonium salts is determined by the positive charge that they contain, which is mainly located at the sulfur atom. In this context, many sulfonium salt-based reagents including Umemoto’s reagent have been designed for electrophilic substitution4,5,6,7,8,9,10. In nature, enzymes catalyzing the generation of alkyl radicals from sulfonium salts known to date all belong to the radical S-adenosylmethionine (SAM) superfamily (Fig. 1a)11,12,13,14,15. In a synthetic chemistry setting, general methods that utilize sulfonium salts as radical precursors have been investigated over the past decades16,17,18. Recently, the emerging field of photoredox catalysis has also offered new possibilities for these single electron transfer (SET)-induced transformations under mild conditions19,20,21. Ritter and coworkers just reported the site-selective C–H functionalization of arenes to build S-(aryl) thianthrenium salts, which could be utilized in radical-involved transformations by photoredox catalysis (Fig. 1b)22,23,24,25,26. The Procter group also explored a one-pot strategy for the facile construction of (hetero)biaryl motifs with intermediate S-(aryl) dibenzothiophenium salts enabled by organic photoredox catalysts27. Despite these advances, the vast majority of the sulfonium salts that undergo SET process have been limited to form aryl radicals; The generation of alkyl radicals, especially the non-stabilized ones from sulfonium salts is synthetically challenging.
As precursors for S-ylides, S-(alkyl) sulfonium salts have been widely applied in cyclization28,29 and rearrangement reactions30. The early studies from the Kellogg group found the reduction of S-(alkyl) sulfonium salts under photoredox catalytic conditions31,32. However, the reported protocols have been limited for the generation of stabilized alkyl radicals (benzyl or with α-electron-withdrawing groups) so far33,34,35,36,37. To mimic the radical-involved biosynthesis from SAM, here we show S-(alkyl) thianthrenium salts, which are ready to engage in diverse SET-induced fragmentations and transformations, especially by mild photoredox processes (Fig. 1c). Moreover, the formed thianthrene (TT) reagent could be recycled after the reactions. A significant observation of the reactivity of these compounds is that diverse non-stabilized alkyl radicals can be effectively generated as key intermediates in these transformations.
Results
Synthesis of S-(alkyl) thianthrenium salts
Instead of the known methods to access S-(alkyl) thianthrenium salts by using alkyl-mercurial and alkyl-tin reagents in the 1990s38,39, they were easily prepared by an alternative method from the corresponding alcohols and TT in one-pot on the gram scale (for an example of 1a, Fig. 2). A crystal of the substrate 1a was generated and subjected to X-ray crystallographic analysis (see Supplementary Data 1).
Reaction design
Alkylboronates are valuable building blocks in organic synthesis and can be transformed into various functional groups40,41,42,43,44,45. Traditional methods to prepare them include electrophilic borylation of organolithium or Grignard reagents and hydroboration of olefins46. The recent state-of-the-art synthesis of these compounds has evolved to utilize bench-stable alkyl radical precursors, especially redox-active alkyl halides47,48,49,50,51,52,53,54,55,56,57,58, N-hydroxyphthalimide (NHPI) esters59,60,61, Katritzky salts62,63,64,65, xanthates/oxalates66, thionocarbonates67, and even alkanes68. Given the importance of alkylboronic esters, we explored the desulfurative borylation of thianthrenium salts under photo- and thermoinduced conditions (Fig. 3). Treatment of 1a with 2.0 equiv of bis(catecholato)diboron (B2cat2) in DMA under irradiation with a blue LED gave the best result of intermediate 1b, which can be further converted into a stable boronic ester 1c in 82% total yield with pinacol and NEt3. Rigorous control experiments demonstrated that light irradiation greatly facilitated borylation. Other sulfonium triflates such as 1a’ derived from tetrahydrothiophene completely failed, and borylation of diphenyl sulfonium salt 1a” generated PhBpin as a major product. Dibenzothiophenium salt 1a”‘ was also a reliable alkyl radical precursor for this transformation, albeit with a lower reactivity (for details of optimization studies, see Supplementary Table 1). Our group recently developed the Lewis base-promoted deaminative borylation reaction of Katritzky salts65. When the reaction mixture was heated at 80 °C in the presence of 4,4’-dimethoxy-2,2’-bipyridine (B1), the desired product was also formed in a 75% yield. Other Lewis bases such as 2,2’-bipyridine (B2) and 4,4’-di-tert-butyl-2,2’-bipyridine (B3) exhibited lower reactivity, and the reaction in the absence of the Lewis base gave more than trace amounts of 1c (for details of optimization studies, see Supplementary Table 2). Such thermoinduced conditions provided an alternative approach for this transformation.
Scope of the methodology
With the optimized conditions in hand, the scope of the thianthrenium salts was explored under photo- and thermoinduced conditions (Fig. 4). Desulfurative borylation of primary alkyl-substituted thianthrenium salts with alkyl chains (2–4a), methoxy (5a), ester (6a), cyano (7a), halides (F, Cl, Br and I, 8–12a), heterocycles (thiophene, benzofuran, oxazole and carbazole, 13–16a), alkenes (17a) and alkynes (18–19a) were converted into the corresponding pinacol boronic esters 2–19c in good to high yields. Secondary alkyl-substituted substrates, either appended to the acyclic chain (20–21a) or to a variety of rings (cyclohexyl, cyclo cyclododecyl and adamantan-2-y, 22–24a), underwent smooth borylation under the two developed reaction conditions. Benzylic-substituted thianthrenium salt 25a was also compatible under photoinduced conditions, but only trace amount of the desired product 25c was observed under thermoinduced conditions. Borylation of thianthrenium salt 26a containing a perfluoroalkyl chain was facile to form desired product 26c. Thianthrenium salt 27a, which is derived from α-linolenic acid with three cis double bonds, was compatible with excellent configuration retention. The functional group tolerance of both reaction systems was further tested with complex molecules 28-30a. Among them, idebenone derivative 30a was an eligible substrate to provide 30c in a modest yield with visible light irradiation, but it did not work under the Lewis base system. In addition, substrate 31a containing two sulfonium salt motifs could undergo double borylation in both reaction systems. In most of the above examples, the photochemical process showed better reactivity than that using the Lewis base. In the case of a thianthrenium salt 32a with an aromatic C–I bond, the thermal reaction conditions showed excellent chemoselectivity for desulfurative borylation; however, the choice of the photochemical setup led to poor compatibility52.
Standard conditions I: thianthrenium salts 1–32a (0.40 mmol), B2cat2 (0.80 mmol) in 1.0 mL DMA, irradiation from a 40 W Kessil blue LED bulb (440 nm) for 12 h under Ar; then pinacol (1.60 mmol) in 1.0 mL Et3N was added to the mixture, 1 h, isolated yields; Standard conditions II: thianthrenium salts 1–32a (0.40 mmol), B2cat2 (0.80 mmol), 30 mol% of B1 in DMA (1.0 mL), 80 °C, 3 h, under Ar, isolated yields; The yields in standard conditions II were afforded in parentheses. aB2cat2 (1.60 mmol) in DMA (1.5 mL). bB2cat2 (1.60 mmol), 60 mol% of B1 in DMA (1.5 mL).
Synthetic applications
We next turned our attention to improve the practicability and operability of this transformation (Fig. 5). Although using a catalytic amount of TT in our reaction remains challenging at this stage, this compound could, nevertheless, be recycled (Fig. 5a). After the reaction of 1a, TT reagent was formed accompanying with the main product 1c. The reaction mixture was then separated by flash column chromatography on silica gel affording the product 1c in a good yield and regenerating TT reagent in nearly quantitative recovery. To further demonstrate the potential of this transformation to simplify synthesis, one-pot reactions were conducted. For example, the one-pot sulfonation and thianthrenation with alcohol I followed by borylation under irradiation conditions led to the generation of alkylboronate 1c in 67% yield (Fig. 5b). The secondary alkyl-substituted thianthrenium salt 33 bearing a chloride group was not stable and difficult to prepare. Notably, one-pot transformation of formyloxy 34 could provide a convenient way toward the corresponding product 35 (Fig. 5c).
Discussion
Several mechanistic experiments were then performed to gain insight into the mechanism of this transformation (Fig. 6). We tested the CV of phenethyl-containing thianthrenium salt 1a, Katritzky salt II and NHPI ester III (Fig. 6a). Thianthrenium salt 1a showed irreversible reduction profiles, with Ered = −1.28 V (versus Ag/AgNO3) in DMA, which is more prone to be reduced than Katritzky salt II (Ered = −1.33 V, versus Ag/AgNO3) and NHPI ester III (Ered = −1.30 V, versus Ag/AgNO3). Then, the absorption spectra of 1a, B2cat2, and a mixture of 1a and B2cat2 were obtained (Fig. 6b). A bathochromic shift of the mixture proves the strong evidence for the existence of an electron-donor-acceptor (EDA) complex60,63,64,65,68,69,70,71. Furthermore, using TEMPO or 1,1-diphenylethylene as radical scavengers in the reactions with visible light irradiation, trapped products 36 and 37 were detected and isolated (Fig. 6c). In addition, desulfurative borylation of 38 could form major product 39 accompanied by cyclization product 40 (Fig. 6d)72. These results indicate that alkyl radicals are formed during the reaction. In addition, “light/dark” experiments further showed that visible light was a necessary component of the transformation. Finally, the quantum yield of the transformation to form 1c was determined to be Ф = 46, indicating that a radical chain process was operative (for details, see Supplementary Information)73.
a Cyclic voltammograms (CV) of thianthrenium salt 1a, Katritzky salt II and NHPI ester III. b UV/visible absorption spectra of DMA solutions of 1a (0.1 M), B2Cat2 (0.2 M), and a mixture of 1a (0.1 M) and B2cat2 (0.2 M) in DMA. c Radical trapping experiments of thianthrenium salt 1a. d Radical clock experiment of thianthrenium salt 38. TEMPO 2,2,6,6-tetramethylpiperidinooxy.
Based on the above observations, a proposed mechanism is shown in Fig. 7. Photoexcitation of EDA complex A derived from S-(alkyl) thianthrenium salts and B2cat2·DMA adduct initiates the radical chain reaction, forming alkyl radical B, DMA·Bcat adduct C, DMA-stabilized boron-centered radical D, and TT. Then, propagation of the desulfurative borylation probably proceeds through reaction of alkyl radical B with the B2cat2·DMA adduct to produce radical complex E. Cleavage of the B-B bond generates borylated products b and boryl radical D, which can further reduce substrates a to form DMA·Bcat adduct C and regenerate alkyl radical B (for the proposed mechanism of thermoinduced borylation, see the Supplementary Information).
In addition to desulfurative borylation under metal-free conditions, the reaction of thianthrenium salts with several different coupling partners can also be carried out by photoredox catalysis at ambient temperature (Fig. 8). For example, Giese radical addition of thianthrenium salt 1a with electron-deficient olefins 41a–b can be accessed to provide products 42-43 using Hantzsch ester as a reductant with a catalytic amount of Ir[(ppy)2dtbpy]PF6 under irradiation with a blue LED74. With the same photocatalyst, an efficient and mild defluorinative alkylation of α-trifluoromethyl alkene 41c using thianthrenium salt 1a as a radical precursor could produce gem-difluoroalkene 44 in a 65% yield75. Under the same reaction conditions, we also developed alkyl radical olefination (45) and alkynylation (46) reactions with secondary thianthrenium salt 23a with sulfone reagents 41d-e76. The photoredox Minisci-type functionalization of isoquinoline (41 f) and 9-methyl-9H-purine (41 g) with 1a efficiently led to alkylation products 47–48 with catalytic amounts of Ir[dF(CF3)ppy]2(dtbpy)PF6 and triflate acid in DMA under a blue LED77,78. Treatment of 1,1-diphenylethylene with substrate 23a with visible light irradiation could provide the trapped products 49 in 67% yield. Moreover, direct cross-coupling of C(sp3)−H bonds in N-aryl tetrahydroisoquinoline 41 h with thianthrenium salt 1a by visible-light photoredox system could generate product 50 in an excellent yield79. In addition, S-(alkyl) thianthrenium salt can also serve as an electrophile reagent to provide an alkyl source with nucleophiles80. For example, when a complex molecule atorvastatin (51) with several possible reactive sites was examined with thianthrenium salt 1a, the reaction showed excellent selectivity for esterification product 52 in a good yield.
a 1a (1.0 equiv), 41a-b (3.0 equiv), Ir[(ppy)2dtbpy]PF6 (2 mol%), Hantzsch ester (2.0 equiv), DMA, rt, blue LED (440 nm); (b) 1a or 23a (1.0 equiv), 41c–e (1.5 equiv), Ir[(ppy)2dtbpy]PF6 (2 mol%), DIPEA (3.0 equiv), CH3CN, rt, blue LED (440 nm); (c), for 46: 41f-g (1.0 equiv), 1a (1.5–2.0 equiv), Ir[(dFCF3ppy)2dtbpy]PF6 (2 mol%), TfOH, DMA, rt, blue LED (440 nm); (d) 23a (1.0 equiv), 1,1-diphenylethylene (1.5 equiv), Ir[(ppy)2dtbpy]PF6 (2 mol%), DABCO (1.0 equiv), CH3CN, rt, blue LED (440 nm); (e) 41 h (1.0 equiv), 1a (2.0 equiv), Ir[(ppy)2dtbpy]PF6 (2 mol%), KHCO3 (3.0 equiv), CH3CN, rt, blue LED (440 nm); (f) 51 (1.0 equiv), 1a (1.0 equiv), K2CO3 (3.0 equiv), THF, rt.
In conclusion, the challenges associated with sulfonium salts forming nonstabilized alkyl radicals could be efficiently solved by using S-(alkyl) thianthrenium salts in SET-induced fragmentations and transformations, especially under mild photoredox systems. These thianthrenium salts bearing a large range of functional groups are suitable electrophiles for borylation, heteroarylation, alkylation, alkenylation, and alkynylation under photochemical conditions. Given the ready availability of alcohols, our approach provides an efficient way to stepwise conversion of OH group to other functional groups, and especially, one-pot manners can be performed with the almost complete recovery of the TT reagent. Extending the developed sulfonium salts to other challenging and useful transformations is currently being explored in our laboratory.
Methods
General procedures I
A 10.0 mL Schlenk tube with a stirring bar was added with alkyl sulfonium 1a (0.4 mmol, 1.0 equiv), B2cat2 (190.3 mg, 0.8 mmol, 2.0 equiv) and DMA (1.0 mL) under argon. The resulting mixture was stirred at room temperature under blue LED irradiation for 12 h. Then pinacol (189.1 mg, 1.6 mmol, 4.0 equiv) was dissolved in Et3N (1.0 mL), added to the reaction mixture and stirred for 1 h. The mixture was cooled to 0 °C to precipitate thianthrene, which was removed by filtration. Then water was added, and the reaction mixture was extracted with EtOAc, dried over MgSO4, and concentrated under reduced pressure. The crude product 1c was purified by flash column chromatography.
General procedures II
A 10.0 mL Schlenk tube with a stirring bar was added with alkyl sulfonium 1a (0.4 mmol, 1.0 equiv), B2cat2 (190.3 mg, 0.8 mmol, 2.0 equiv), B1 (26.0 mg, 30.0 mol %) and DMA (1.0 mL) under argon. The resulting mixture was stirred at 80 °C under Ar for 3 h. The mixture was cooled to room temperature. Pinacol (189.1 mg, 1.6 mmol, 4.0 equiv) was dissolved in Et3N (1.0 mL), added to the reaction mixture, and stirred for 1 h. The mixture was cooled to 0 °C to precipitate thianthrene, which was removed by filtration. Then water was added, and the reaction mixture was extracted with EtOAc, dried over MgSO4, and concentrated under reduced pressure. The crude product 1c was purified by flash column chromatography.
Data availability
All data supporting the findings of this study are available within the article and Supplementary Information file or from the corresponding author upon reasonable request. The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 2047564 (1a) and can be obtained free of charge from www.ccdc.cam.ac.uk/data_request/cif.
References
Kaiser, D. et al. Bond-forming and -breaking reactions at sulfur (IV): sulfoxides, sulfonium salts, sulfur ylides, and sulfinate salts. Chem. Rev. 119, 8701–8780 (2019).
Kozhushkov, S. I. & Alcarazo, M. Synthetic applications of sulfonium salts. Eur. J. Inorg. Chem. 2020, 2486–2500 (2020).
Fan, R. et al. A leap forward in sulfonium salt and sulfur ylide chemistry. Chin. Chem. Lett. 32, 299–312 (2021).
Umemoto, T. & Ishihara, S. Power-variable Electrophilic Trifluoromethylating Agents. S-, Se-, and Te-(trifluoromethyl)dibenzothio-, -seleno-, and -tellurophenium Salt System. J. Am. Chem. Soc. 115, 2156–2164 (1993).
Tian, Z.-Y. & Zhang, C.-P. Ullmann-type N-arylation of anilines with alkyl(aryl)sulfonium salts. Chem. Commun. 55, 11936–11939 (2019).
Altundas, B., Kumar, C. V. S. & Fleming, F. F. Acetonitrile–hexane extraction route to pure sulfonium salts. ACS Omega 5, 13384–13388 (2020).
Zhu, J., Liu, Y. & Shen, Q. Direct difluoromethylation of alcohols with an electrophilic difluoromethylated sulfonium ylide. Angew. Chem. Int. Ed. 55, 9050–9054 (2016).
Waldecker, B., Kraft, F., Golz, C. & Alcarazo, M. 5-(Alkynyl)dibenzothiophenium triflates: sulfur-based reagents for electrophilic alkynylation. Angew. Chem. Int. Ed. 57, 12538–12542 (2018).
Li, X., Golz, C. & Alcarazo, M. 5-(Cyano)dibenzothiophenium triflate: a sulfur-based reagent for electrophilic cyanation and cyanocyclizations. Angew. Chem. Int. Ed. 131, 9496–9500 (2019).
Wang, D. et al. Trifluoromethyl sulfoxides: reagents for metal-free C-H trifluoromethylthiolation. Angew. Chem. Int. Ed. 59, 15918–15922 (2020).
O’Hagan, D. & Schmidberger, J. W. Enzymes that catalyse SN2 reaction mechanisms. Nat. Prod. Rep. 27, 900–918 (2010).
Broderick, J. B. A radically different enzyme. Nature 465, 877–878 (2010).
Vey, J. L. & Drennan, C. L. Structural insights into radical generation by the radical SAM superfamily. Chem. Rev. 111, 2487–2506 (2011).
Zhang, Q., der Donk, W. A. V. & Liu, W. Radical-mediated enzymatic methylation: a tale of two SAMS. Acc. Chem. Res. 45, 555–564 (2012).
Broderick, J. B., Duffus, B. R., Duschene, K. S. & Shepard, E. M. Radical S-adenosylmethionine enzymes. Chem. Rev. 114, 4229–4317 (2014).
Beak, P. & Sullivan, T. A. One-electron chemical reductions of phenylalkylsulfonium salts. J. Am. Chem. Soc. 104, 4450–4457 (1982).
Saeva, F. D., Breslin, D. T. & Luss, H. R. Intramolecular photoinduced rearrangements via electron-transfer-induced, concerted bond cleavage and cation radical/radical coupling. J. Am. Chem. Soc. 113, 5333–5337 (1991).
Andrieux, C. P., Robert, M., Saeva, F. D. & Saveant, J.-M. Passage from concerted to stepwise dissociative electron transfer as a function of the molecular structure and of the energy of the incoming electron. electrochemical reduction of aryldialkyl sulfonium cations. J. Am. Chem. Soc. 116, 7864–7871 (1994).
Donck, S. et al. Visible-light photocatalytic reduction of sulfonium salts as a source of aryl radicals. Adv. Synth. Catal. 355, 1477–1482 (2013).
Peter, A., Perry, G. J. P. & Procter, D. J. Radical C–C bond formation using sulfonium salts and light. Adv. Synth. Catal. 362, 2135–2142 (2020).
Huang, C. et al. Redox-neutral borylation of aryl sulfonium salts via C–S activation enabled by light. Org. Lett. 21, 9688–9692 (2019).
Berger, F. et al. Site-selective and versatile aromatic C–H functionalization by Thianthrenation. Nature 567, 223–228 (2019).
Engl, P. S. et al. C–N cross-couplings for site-selective late-stage diversification via aryl sulfonium salts. J. Am. Chem. Soc. 141, 13346–13351 (2019).
Sang, R. et al. Site-selective C–H oxygenation via aryl sulfonium salts. Angew. Chem. Int. Ed. 58, 16161–16166 (2019).
Ye, F. et al. Aryl sulfonium salts for site-selective late-stage trifluoromethylation. Angew. Chem. Int. Ed. 58, 14615–14619 (2019).
Li, J. et al. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 12, 56–62 (2020).
Aukland, M. H. et al. Metal-free photoredox-catalysed formal C–H/C–H coupling of arenes enabled by interrupted pummerer activation. Nat. Catal. 3, 163–169 (2020).
Li, A. H., Dai, L. X. & Aggarwal, V. K. Asymmetric ylide reactions: epoxidation, cyclopropanation, aziridination, olefination, and rearrangement. Chem. Rev. 97, 2341–2372 (1997).
Lu, L.-Q., Li, T.-R., Wang, Q. & Xiao, W.-J. Beyond sulfide-centric catalysis: recent advances in the catalytic cyclization reactions of sulfur ylides. Chem. Soc. Rev. 46, 4135–4149 (2017).
Jana, S., Guo, Y. & Koenigs, R. M. Recent perspectives on rearrangement reactions of ylides via carbene transfer reactions. Chem. Eur. J. https://doi.org/10.1002/chem.202002556 (2020).
Vanbergen, T. J., Hedstrand, D. M., Kruizinga, W. H. & Kellogg, R. M. Chemistry of dihydropyridines. 9. hydrides transfer from 1,4-Dihydropyridines to sp3-hybridized carbon in sulfonium salts and activated halides - studies with NAD(P)H models. J. Org. Chem. 44, 4953–4962 (1979).
Hedstrand, D. M., Kruizinga, W. H. & Kellogg, R. M. Light-induced and dye accelerated reductions of phenacyl onium salts by 1,4-Dihydropyridines. Tetrahedron Lett. 19, 1255–1258 (1978).
Kampmeier, J. A. et al. Regioselectivity in the reductive bond cleavage of diarylalkylsulfonium salts: variation with driving force and structure of sulfuranyl radical intermediates. J. Am. Chem. Soc. 131, 10015–10022 (2009).
Koike, T. & Akita, M. Fine design of photoredox systems for catalytic fluoromethylation of carbon−carbon multiple bonds. Acc. Chem. Res. 49, 1937–1945 (2016).
Liu, Y.-Y. et al. Visible-light-driven Aza-ortho-quinone methide generation for the synthesis of indoles in a multicomponent reaction. Angew. Chem. Int. Ed. 56, 9527–9531 (2017).
Otsuka, S., Nogi, K., Rovis, T. & Yorimitsu, H. Photoredox-catalyzed alkenylation of benzylsulfonium salts. Chem. Asian J. 14, 532–536 (2019).
Varga, B., Gonda, Z., Toth, B. L., Kotschy, A. & Novak, Z. A Ni-Ir dual photocatalytic liebeskind coupling of sulfonium salts for the synthesis of 2-Benzylpyrrolidines. Eur. J. Org. Chem. 2020, 1466–1471 (2020).
Lochynski, S., Shine, H. J., Soroka, M. & Venkatachalam, T. K. Evidence for electron-transfer in reactions of thianthrene cation radical with dialkylmercurials. J. Org. Chem. 55, 2702–2713 (1990).
Lochynski, S., Boduszek, B. & Shine, H. J. Oxidation of organotins (R4Sn, RSnMe3, and R3SnSnR3) by the thianthrene cation radical. J. Org. Chem. 56, 914–920 (1991).
Hall, D. G., Boronic Acids, Wiley, New York (2005).
Hall, D. G., Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim (2005).
Mkhalid, I. A. I. et al. C−H Activation for the Construction of C−B Bonds. Chem. Rev. 110, 890–931 (2010).
Hartwig, J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 40, 1992–2002 (2011).
Hartwig, J. F. Borylation and silylation of C–H bonds: a platform for diverse C–H bond functionalizations. Acc. Chem. Res. 45, 864–873 (2012).
Collins, B. S. L., Wilson, C. M., Myers, E. L. & Aggarwal, V. K. Asymmetric synthesis of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 56, 11700–11733 (2017).
Wang, M. & Shi, Z. Methodologies and strategies for selective borylation of C–Het and C–C bonds. Chem. Rev. 120, 7348–7398 (2020).
Dudnik, A. S. & Fu, G. C. Nickel-catalyzed coupling reactions of alkyl electrophiles, including unactivated tertiary halides, to generate carbon–boron bonds. J. Am. Chem. Soc. 134, 10693–10697 (2012).
Yang, C.-T. et al. Alkylboronic esters from copper-catalyzed borylation of primary and secondary alkyl halides and pseudohalides. Angew. Chem. Int. Ed. 51, 528–532 (2012).
Bose, S. K. et al. Zinc-catalyzed borylation of primary, secondary and tertiary alkyl halides with alkoxy diboron reagents at room temperature. Angew. Chem. Int. Ed. 53, 1799–1803 (2014).
Atack, T. C., Lecker, R. M. & Cook, S. P. Iron-catalyzed borylation of alkyl electrophiles. J. Am. Chem. Soc. 136, 9521–9523 (2014).
Atack, T. C. & Cook, S. P. Manganese-catalyzed borylation of unactivated alkyl chlorides. J. Am. Chem. Soc. 138, 6139–6142 (2016).
Cheng, Y., Mueck-Lichtenfeld, C. & Studer, A. Metal-free radical borylation of alkyl and aryl iodides. Angew. Chem. Int. Ed. 57, 16832–16836 (2018).
Tian, Y.-M. et al. Selective photocatalytic C–F borylation of polyfluoroarenes by Rh/Ni dual catalysis providing valuable fluorinated arylboronate esters. J. Am. Chem. Soc. 140, 17612–17623 (2018).
Paul, S. et al. Ir-catalysed functionalization of 2-substituted indoles at the 7-position: nitrogen-directed aromatic borylation. J. Am. Chem. Soc. 128, 15552–15553 (2006).
Mazzarella, D., Magagnano, G., Schweitzer-Chaput, B. & Melchiorre, P. Photochemical organocatalytic borylation of alkyl chlorides, bromides, and sulfonates. ACS Catal. 9, 5876–5880 (2019).
Iwamoto, H. et al. Copper(I)-catalyzed enantioconvergent borylation of racemic benzyl chlorides enabled by quadrant-by-quadrant structure modification of chiral bisphosphine ligands. Angew. Chem. Int. Ed. 58, 11112–11117 (2019).
Xu, W. et al. Visible‐light‐induced selective defluoroborylation of polyfluoroarenes, gem‐difluoroalkenes, and trifluoromethylalkenes. Angew. Chem. Int. Ed. 59, 4009–4016 (2020).
Zhang, L., Wu, Z.-Q. & Jiao, L. Photoinduced radical borylation of alkyl bromides catalyzed by 4-phenylpyridine. Angew. Chem. Int. Ed. 59, 2095–2099 (2020).
Li, C. et al. Decarboxylative borylation. Science 356, eaam7355 (2017).
Fawcett, A. et al. Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017).
Hu, D., Wang, L. & Li, P. Decarboxylative borylation of aliphatic esters under visible-light photoredox conditions. Org. Lett. 19, 2770–2773 (2017).
Rössler, S. L. et al. Pyridinium salts as redox-active functional group transfer reagents. Angew. Chem. Int. Ed. 59, 9264–9280 (2020).
Wu, J., He, L., Noble, A. & Aggarwal, V. K. Photoinduced deaminative borylation of alkylamines. J. Am. Chem. Soc. 140, 10700–10704 (2018).
Sandfort, F. et al. Deaminative borylation of aliphatic amines enabled by visible light excitation of an electron donor-acceptor complex. Chem. Eur. J. 24, 17210–17214 (2018).
Hu, J., Wang, G., Li, S. & Shi, Z. Selective C-N borylation of alkyl amines promoted by lewis base. Angew. Chem. Int. Ed. 57, 15227–15231 (2018).
Friese, F. W. & Studer, A. Deoxygenative borylation of secondary and tertiary alcohols. Angew. Chem. Int. Ed. 58, 9561–9564 (2019).
Wu, J. et al. Photoinduced deoxygenative borylations of aliphatic alcohols. Angew. Chem. Int. Ed. 58, 18830–18834 (2019).
Shu, C., Noble, A. & Aggarwal, V. K. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature 586, 714–719 (2020).
Li, J. et al. Metal-free direct deoxygenative borylation of aldehydes and ketones. J. Am. Chem. Soc. 142, 13011–13020 (2020).
Lima, C. G. S. et al. Organic synthesis enabled by light-irradiation of EDA complexes: theoretical background and synthetic applications. ACS Catal. 6, 1389–1407 (2016).
Crisenza, G. E. M., Mazzarella, D. & Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor–acceptor complexes. J. Am. Chem. Soc. 142, 5461–5476 (2020).
Griller, D. & Ingold, K. U. Free-radical clocks. Acc. Chem. Res. 13, 317–323 (1980).
Cismesia, M. A. & Yoon, T. P. Characterizing chain processes in visible light photoredox catalysis. Chem. Sci. 6, 5426–5434 (2015).
Wu, J. et al. Catalyst-free deaminative functionalizations of primary amines by photoinduced single-electron transfer. Angew. Chem. Int. Ed. 58, 5697–5701 (2019).
Lang, S. B., Wiles, R. J., Kelly, C. B. & Molander, G. A. Photoredox generation of carbon-centered radicals enables the construction of 1,1-difluoroalkene carbonyl mimics. Angew. Chem. Int. Ed. 56, 15073–15077 (2017).
Huang, H. et al. Visible-light-induced chemoselective deboronative alkynylation under biomolecule-compatible conditions. J. Am. Chem. Soc. 136, 2280–2283 (2014).
Jin, J. & MacMillan, D. W. C. Alcohols as alkylating agents in heteroarene C–H functionalization. Nature 525, 87–90 (2015).
Klauck, F. J. R., James, M. J. & Glorius, F. Deaminative strategy for the visible-light-mediated generation of alkyl radicals. Angew. Chem. Int. Ed. 56, 2336–12339 (2017).
Zhou, W.-J. et al. Visible-light-driven palladium-catalyzed radical alkylation of C–H bonds with unactivated alkyl bromides. Angew. Chem. Int. Ed. 56, 15683–15687 (2017).
Wang, M., Zhao, Y., Zhao, Y. & Shi, Z. Bioinspired design of a robust D3-methylating agent. Sci. Adv. 6, eaba0946 (2020).
Acknowledgements
We would like to thank the National Natural Science Foundation of China (grant 22025104, 21871131, and 22071100), the National Natural Science Foundation of Jiangsu Province (Grant BK20170632), the Excellent Youth Foundation of Jiangsu Scientific Committee (Grant BK20180007), and the “Innovation & Entrepreneurship Talents Plan” of Jiangsu Province for their financial support. The project was also supported by Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University.
Author information
Authors and Affiliations
Contributions
Z.S. & H.L. conceived and designed the study, and wrote the paper. C.C. performed the experiments. Z.J.W. conducted additional experiments during the revision. Y.Z. performed the crystallographic studies.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, C., Wang, ZJ., Lu, H. et al. Generation of non-stabilized alkyl radicals from thianthrenium salts for C–B and C–C bond formation. Nat Commun 12, 4526 (2021). https://doi.org/10.1038/s41467-021-24716-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-24716-2
This article is cited by
-
Metallaphotoredox deuteroalkylation utilizing thianthrenium salts
Nature Communications (2024)
-
Unified metal-free intermolecular Heck-type sulfonylation, cyanation, amination, amidation of alkenes by thianthrenation
Nature Communications (2024)
-
Orthogonal bioconjugation targeting cysteine-containing peptides and proteins using alkyl thianthrenium salts
Nature Communications (2024)
-
Dinuclear gold-catalyzed divergent dechlorinative radical borylation of gem-dichloroalkanes
Nature Communications (2024)
-
A general arene C–H functionalization strategy via electron donor–acceptor complex photoactivation
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.