Abstract
Insertion of atoms into aromatic carbon-nitrogen bonds is an appealing method for the synthesis of nitrogen-containing molecules and it has the advantage of the availability and abundance of anilines. However, the direct cleavage of aromatic carbon-nitrogen bonds is challenging due to the particularly inert and stable nature of these bonds. Here we report a formal, enantioselective one-carbon insertion into an aromatic carbon-nitrogen bond via an aromaticity dissembly-reconstruction process to directly convert anilines to chiral α-branched benzylic amines. The process involves oxidative dearomatization of para-substituted anilines, chiral sulfur ylide-mediated asymmetric aziridination, and subsequent rearrangement. Chiral sulfur ylides serve as one-carbon insertion units.
Similar content being viewed by others
Introduction
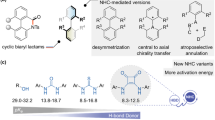
Insertion of atoms into chemical bonds is an attractive transformation of organic molecules because it leads to the simultaneous formation of two new chemical bonds. In recent decades, significant progress has been made in transition-metal-catalyzed insertion of atoms into unreactive chemical bonds such as carbon–carbon1,2,3, carbon–cyanide4,5, and aliphatic carbon–nitrogen bonds6,7,8,9,10. The insertion reaction normally involves transition-metal-catalyzed cleavage of the bond in question and subsequent insertion of unsaturated units, such as alkenes11,12,13,14,15, alkynes16,17,18, 1,3-dienes19,20, or carbenoids21 (Fig. 1a). These elegant reactions have emerged as an attractive approach to rapid building of complex structures from readily available starting materials. In this context, insertion of atoms into aromatic carbon–nitrogen bonds is an appealing method for the synthesis of nitrogen-containing molecules and has the advantage of the availability and the abundance of anilines. However, the direct cleavage of aromatic carbon–nitrogen bonds is challenging due to the particularly inert and stable nature of these bonds22,23. Although aromatic carbon–nitrogen bonds can be activated by converting anilines to more reactive intermediates such as aryldiazonium salts24,25,26,27, arylammonium salts28,29,30, amides31,32, or triazenes33, the nitrogen atom is usually discarded in byproducts (Fig. 1b).
Chiral α-branched benzylic amines are important structural motifs found in a wide range of natural products and biologically active compounds34,35,36,37. Driven by the value of active pharmaceutical ingredients, the asymmetric arylation of aldimines by arylmetallic reagents, including lithium38,39, zinc40, titanium41, tin42, and boron reagents43,44, has been established as an efficient method for the synthesis of enantiopure benzylic amines. Enantioselective one-carbon insertion into the aromatic carbon–nitrogen bonds is an appealing route with which to establish nitrogen-substituted benzylic stereocenters, and this reaction could satisfy an unmet need in reaction design (Fig. 1c).
Here, we report a formal, enantioselective aromatic carbon–nitrogen bond one-carbon insertion reaction that converts an aniline to a highly functionalized chiral α-branched benzylic amine via an aromaticity dissembly-reconstruction process. The process involves oxidative dearomatization of para-substituted anilines, chiral sulfur ylide-mediated asymmetric aziridination, and subsequent rearrangement. Chiral sulfur ylides serve as the one-carbon insertion unit.
Results
Initial tests
In connection with our recent works on the dearomatization conversion of anilines, oxidative dearomatization can transform the aromatic carbon–nitrogen bond in anilines to a carbon–nitrogen double bond by destroying the aromatic system45,46,47,48,49. As shown in Fig. 2, chiral sulfur ylides might serve as a one-carbon unit to be introduced through asymmetric aziridination50,51,52,53,54,55. Subsequent rearrangement might be promoted by a Brønsted or Lewis acid to redevelop the aromaticity and complete the formal enantioselective one-carbon insertion. This builds the nitrogen-substituted benzylic stereocenter and is accompanied by migration of the para-substituent to the meta position and concomitant para-substitution by a nucleophilic reagent. To implement this strategy, the rapid oxidative dearomatization of p-toluidine 1 was tested by examining various oxidants including mCPBA, AcOOH, t-BuOOH, H2O2, PhI(OAc)2, and PhIO. PhIO together with methanol as the solvent proved to be the best oxidation conditions for the dearomatization. After removing the methanol in vacuo, the crude dearomatized product was mixed with a solution of achiral sulfonium salt S1 in acetonitrile in the presence of 1.2 equivalents of NaH, followed by the treatment with 2 equivalents of CF3COOH (Fig. 3). To our delight, the aziridination and subsequent rearrangement proceeded smoothly and delivered benzylic amine 2 in good yield (71%).
The transformation might proceed via a dearomatization of para-substituted anilines, an chiral sulfur ylides-mediated asymmetric aziridination, followed by a rearrangement to recover the aromaticity and complete the formal enantioselective one-carbon insertion accompanied by a migration of the para-substituent to the meta position and para-substitution by a nucleophilic reagent. PG protecting group, Nuc nucleophile.
Optimization of reaction conditions
Encouraged by this result, a chiral sulfonium salt S2 derived from the Metzner sulfide56,57 was employed, and this reaction gave rise to compound 2 with 51% yield and 17% ee. Various chiral sulfonium salts were examined58,59,60,61,62,63,64, and S8, derived from isothiocineole65,66,67,68, was observed to be the best chiral ylide precursor, leading to a 64% yield and 95% ee of 2. Changing the anion of sulfonium salts has an influence on the yield of 2 but leads to no change in the enantioselectivity. Reaction with sulfonium trifluoromethane-sulfonate S10 provided 2 in 78% yield and 94% ee. Changing the nature of protecting group on the aniline nitrogen atom has a major effect on the transformation. The reaction works well with various sulfamide groups but not with acetamide or benzamide. A set of reaction variables including bases, Lewis and Brønsted acids, solvents, temperatures, and the ratio of reagents were investigated to establish the optimum reaction conditions. NaH proved to be the best base. A variety of Brønsted or Lewis acids shown different activities to promote the rearrangement. When 20 mol% Cu(OTf)2 was used instead of 2 equivalents CF3COOH, the yield of 2 was improved to 79% yield and 96% ee (for details, see Supplementary Table 1 in the Supplementary Information).
Scope of anilines
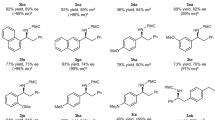
The scope of this transformation was investigated by systematically varying the anilines and the chiral sulfonium salts. As shown in Fig. 4, reactions of a range of anilines with the chiral benzyl sulfonium salt S10 proceeded smoothly. In addition to the methyl group, ethyl, n-butyl, isopropyl, cyclohexyl, or phenyl groups can be the para-substituent in the anilines and migrate to the meta position in the product. An electronic effect was observed for anilines with different meta-substituents. For example, anilines with a meta-phenyl group gave 9 in higher yield than the reaction with a meta-methyl group, and aniline bearing a meta-4-fluorophenyl group gave 12 in higher yield than that bearing a meta-4-methoxyphenyl group. The ortho-substituent of anilines have an effect on the reaction. When 2-fluoro-4-methylaniline was employed, the 4-methyl group migrated to the C-3 position, leading to the formation of 17 in 61% yield and 99% ee. When 2-bromo-4-methylanilines were used, the reaction gave rise to a mixture of the corresponding C-3 or C-5 migration products 18. Reaction of 4-methyl-2-(phenylethynyl)aniline also provided a mixture of the C-3 or C-5 migration products 19. The formation of the mixture of migration products might be caused by the combined influence of the electron-withdrawing and steric effects of the ortho-substituent. The electron-withdrawing effect makes the C-3 position more positively charged compared to the C-5 position, and the steric effect makes the C-3 position more hindered than the C-5 position. Due to the strong electron-withdrawing property and the small size of the fluorine atom, the C-3 position is the preferred site for the migration of the 4-methyl group. Therefore the reaction of 2-fluoro-4-methylaniline only produced the C-3 migration product 17. When the ortho-substituent is a bromine atom or a phenylethynyl group, the relatively weaker electron-withdrawing property and the bigger size led to the formation of mixture of the C-3 or C-5 migration products. Different functional groups can be introduced into the para-position by varying the solvent used in the dearomatization step. For example, the use of ethanol, isopropanol or trifluoroethanol instead of methanol led to the formation of the 4-ethoxy, the 4-isopropoxy, or the 4-trifluoroethoxy-substituted products 20–22.
Substrate scope of sulfonium salts
A wide range of substituents, such as alkyl, methoxy, halogens, trifluoromethyl, phenyl, ester, or boron functional groups on the aryl group of chiral benzyl sulfonium salts, is tolerated under the reaction conditions (Fig. 5). The reaction proceeds smoothly and independently of the different electronic demands on the aryl substituents of chiral benzyl sulfonium salts. For example, the reaction involving a 4-methylphenyl or 4-(trifluoromethyl)phenyl gave rise to compound 25 in 79% yield with 95% ee and 35 in 76% yield with 92% ee. Changing the substituent position in benzyl sulfonium salts affected the reaction yield and enantioselectivity. For example, the 2-methoxy substituted benzyl sulfonium salt gave compound 26 in 91% yield with 96% ee, and the 4-methoxy substituted salt gave compound 28 in 58% yield with 92% ee. The reaction of a sulfonium salt bearing two meta-tert-butyl groups provided 41 in 77% yield with 95% ee. When furan-3-ylmethyl sulfonium salts were employed, the reaction provided α-furan substituted benzylic amine 43 in 59 yield with 80% ee. When α-unsubstituted allyl and propargyl sulfonium salts were employed, the corresponding α-branched benzylic amines 45 and 46 were formed in moderate yields but with lower enantioselectivity. As the steric hindrance of the allyl sulfonium salts is increased by incorporation of an α-methyl substitutent, the stereochemical control of the reaction increases markedly leading to the formation of 46 in 96% ee, but the yield decreased. When the steric hindrance of anilines is increased by incorporation of an ortho-methyl substitutent, product 47 was not formed because the corresponding aziridination reaction did not occur. The absolute configuration of 34 was confirmed by X-ray crystallography (see Supplementary Fig. 1 in the Supplementary Information).
Mechanistic studies
To gain more insight into the transformation, the corresponding dearomatized intermediate 48 and the azidination intermediate 49 were isolated. Both of them can be converted into product 2 under the standard conditions (Fig. 6a). When unsubstituted benzenamine was employed as the substrate, 2.1 equivalents of PhIO were required to facilitate the oxidative dearomatization to generate a quinone imine ketal 51 as the intermediate. However, the reaction of 51 under the standard conditions gave rise to N-(4-methoxyphenyl)-4-methylbenzenesulfonamide 52 instead of the insertion product (Fig. 6b). When two aziridination intermediates 53 and 54 were mixed and treated with Cu(OTf)2, the reaction gave rise to compounds 55 and 56, and the formation of 57 and 58 was not observed (Fig. 6c). This result indicated the migration of the para-alkyl group proceeds via an intramolecular manner.
A plausible pathway for this transformation was depicted in Fig. 7. PhIO mediates the oxidative dearomatization of para-substituted anilines in methanol to generate cyclohexadienimines. The nucleophilic addition of chiral sulfur ylides to cyclohexadienimines and subsequent cyclization lead to the generation of the spiro intermediates. Rearomatizing to release the tension of the spiro structure is a great driving force for the rearrangement. With the aid of a Brønsted or Lewis acid, the migration of the alkyl or the aryl group forms intermediate I (path a), while the migration of the methoxy group forms intermediate II (path b). Because the positive charge in intermediate I can be stabilized by the oxygen atom (intermediate III), rearrangement via path a is preferred. Final aromatization delivers the one-carbon insertion products. When the substituent at the para-position of amino group was a methoxy group, dearomatization occurred to give an acetal intermediate, but aziridination and C–C bond cleavage were not observed under standard conditions.
Synthetic applications
The reaction magnifying 50 times can occur normally which can get product in 77% yield and 94% ee (Fig. 8a). Transformations of the one-carbon insertion products have been explored. For example, under reductive conditions, the N-tosyl protecting group of compound 2 can be removed, leading to the formation of N–H free chiral benzylic amine 48 (Fig. 8b). Compound 23 bearing an ortho-methyl group undergoes a radical sp3 C–H amination reaction to form a 1-substituted isoindoline 49 (Fig. 8c). Compound 33 with an ortho-iodo group is readily converted to the 1,3-disubstituted isoindoline 50 via a Pd-catalyzed cascade coupling/cyclization reaction (Fig. 8d). In these transformations, the enantiopurity of the substrates is preserved in the products.
Discussion
In summary, we report a formal enantioselective aromatic carbon–nitrogen bond one-carbon insertion reaction via an aromaticity dissembly-reconstruction process to directly convert anilines to chiral α-branched benzylic amines. The process involves three steps: oxidative dearomatization to activate the aromatic carbon–nitrogen bond in para-substituted anilines by breaking the aromatic system, chiral sulfur ylide-mediated asymmetric aziridination to introduce the one-carbon unit, and subsequent rearrangement to recover the aromaticity and complete the formal enantioselective one-carbon insertion accompanied by a migration of the para-substituent to the meta position and concomitant para-substitution by a nucleophilic reagent. Development of extensions of this group insertion strategy to other aromatic systems is currently in progress in this laboratory.
Methods
Representative procedure
PhIO (0.22 mmol) was added to a solution of N-Ts p-toluidine 1 (0.2 mmol) in MeOH (2.0 mL) at 25 °C. After 5 min, the reaction mixture was concentrated in vacuo. The resulting mixture was mixed with the sulfonium salt (0.24 mmol) and sodium hydride (0.24 mmol) in MeCN (2 mL). The reaction was stirred at rt for 3 h, then Cu(OTf)2 (0.02 mmol) was added. After the intermediate was completely consumed (monitored by TLC analysis), the reaction was quenched with saturated NaHCO3 (25 mL), and extracted with EtOAc (25 mL × 3). The organic layer was dried over Na2SO4, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (eluent: petroleum ether/EtOAc) to furnish the desired compound 2. White solid; mp: 105–106 °C; 1H NMR (400 MHz, CDCl3): δ 7.54 (d, J = 8.2 Hz, 2H), 7.22–7.17 (m, 3H), 7.15–7.10 (m, 4H), 6.84 (dd, J = 8.4, 1.9 Hz, 1H), 6.80–6.72 (m, J = 2.0 Hz, 1H), 6.62 (d, J = 8.4 Hz, 1H), 5.48 (d, J = 7.1 Hz, 1H), 5.23 (d, J = 7.1 Hz, 1H), 3.75 (s, 3H), 2.37 (s, 3H), 2.05 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 157.1, 143.0, 140.8, 137.4, 132.1, 129.7, 129.2, 128.4, 127.3, 127.2, 126.7, 125.8, 109.6, 60.9, 55.3, 21.4, 16.1; HRMS (m/z): [M + Na]+ calcd. for C22H23NO3S, 404.1291; found, 404.1291. The ee value was determined by HPLC analysis: Chiralcel OD-H Column, hexane/2-propanol = 90/10, 25 °C, 1.0 mL/min, 220 nm, retention time: 15.8 min (minor) and 21.3 min (major).
Data availability
All data that support the findings of this study are available within this article and its Supplementary Information (including experimental procedures and compound characterization data). The X-ray crystallographic coordinates for structure 34 reported in this study has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1966589. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Data are also available from the corresponding author upon reasonable request.
References
Chen, P., Billett, B. A., Tsukamoto, T. & Dong, G. “Cut and Sew” transformations via transition-metal-catalyzed carbon–carbon bond activation. ACS Catal. 7, 1340–1360 (2017).
Fumagalli, G., Stanton, S. & Bower, J. F. Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 117, 9404–9432 (2017).
Souillart, L. & Cramer, N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9464 (2015).
Chen, F., Wang, T. & Jiao, N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev. 114, 8613–8661 (2014).
Wen, Q., Lu, P. & Wang, Y. Recent advances in transition-metal-catalyzed C–CN bond activations. RSC Adv. 4, 47806–47826 (2014).
Yu, H., Gao, B., Hu, B. & Huang, H. Charge-transfer complex promoted C–N bond activation for Ni-catalyzed carbonylation. Org. Lett. 19, 3520–3523 (2017).
Liu, Y., Xie, Y., Wang, H. & Huang, H. Enantioselective aminomethylamination of conjugated dienes with aminals enabled by chiral palladium complex-catalyzed C–N bond activation. J. Am. Chem. Soc. 138, 4314–4317 (2016).
Herzon, S. B. & Hartwig, J. F. Direct, catalytic hydroaminoalkylation of unactivated olefins with N-alkyl arylamines. J. Am. Chem. Soc. 129, 6690–6691 (2007).
Yang, P., Qi, L., Liu, Z., Yang, G. & Chai, Z. Lewis acid catalyzed dynamic kinetic asymmetric transformation of racemic N-sulfonylaziridines. J. Am. Chem. Soc. 140, 17211–17217 (2018).
Zhu, C., Feng, J. & Zhang, J. Divergent synthesis of functionalized pyrrolidines and γ-amino ketones via rhodium-catalyzed switchable reactions of vinyl aziridines and silyl enol ethers. Chem. Commun. 54, 2401–2404 (2018).
Saya, L. et al. Nickel‐catalyzed [3+2+2] cycloadditions between alkynylidenecyclopropanes and activated alkenes. Angew. Chem. Int. Ed. 49, 9886–9890 (2010).
Murakami, M., Itahashi, T. & Ito, Y. Catalyzed intramolecular olefin insertion into a carbon–carbon single bond. J. Am. Chem. Soc. 124, 13976–13977 (2002).
Ko, H. M. & Dong, G. Cooperative activation of cyclobutanones and olefins leads to bridged ring systems by a catalytic [4+2] coupling. Nat. Chem. 6, 739–744 (2014).
Zhou, X. & Dong, G. (4+1) vs (4+2): catalytic intramolecular coupling between cyclobutanones and trisubstituted allenes via C–C activation. J. Am. Chem. Soc. 137, 13715–13721 (2015).
Souillart, L. & Cramer, N. Highly enantioselective rhodium(I)‐catalyzed carbonyl carboacylations initiated by C–C bond activation. Angew. Chem. Int. Ed. 53, 9640–9644 (2014).
Komagawa, S. & Saito, S. Nickel‐catalyzed three‐component [3+2+2] cocyclization of ethyl cyclopropylideneacetate and alkynes-selective synthesis of multisubstituted cycloheptadienes. Angew. Chem. Int. Ed. 45, 2446–2449 (2006).
Lin, M., Kang, G., Guo, Y. & Yu, Z. Asymmetric Rh(I)-catalyzed intramolecular [3 + 2] cycloaddition of 1-Yne-vinylcyclopropanes for bicyclo[3.3.0] compounds with a chiral quaternary carbon stereocenter and density functional theory study of the origins of enantioselectivity. J. Am. Chem. Soc. 134, 398–405 (2012).
Chen, P.-H., Xu, T. & Dong, G. Divergent syntheses of fused β‐naphthol and indene scaffolds by rhodium‐catalyzed direct and decarbonylative alkyne–benzocyclobutenone couplings. Angew. Chem. Int. Ed. 53, 1674–1678 (2014).
Juliá-Hernández, F., Ziadi, A., Nishimura, A. & Martin, R. Nickel‐catalyzed chemo‐, regio‐ and diastereoselective bond formation through proximal C–C cleavage of benzocyclobutenones. Angew. Chem. Int. Ed. 54, 9537–9541 (2015).
Zhang, Y., Yu, B., Gao, B., Zhang, T. & Huang, H. Triple-bond insertion triggers highly regioselective 1,4-aminomethylamination of 1,3-enynes with aminals enabled by Pd-catalyzed C–N bond activation. Org. Lett. 21, 535–539 (2019).
Qin, G., Li, L., Li, J. & Huang, H. Palladium-catalyzed formal insertion of carbenoids into aminals via C–N bond activation. J. Am. Chem. Soc. 137, 12490–12493 (2015).
Wang, Q., Su, Y., Lia, L. & Huang, H. Transition-metal catalysed C–N bond activation. Chem. Soc. Rev. 45, 1257–1272 (2016).
Ouyang, K., Hao, W., Zhang, W. & Xi, Z. Transition-metal-catalyzed cleavage of C–N single bonds. Chem. Rev. 115, 12045–12090 (2015).
Kim, D. H., Lee, J. & Lee, A. Visible-light-driven silver-catalyzed one-pot approach: a selective synthesis of diaryl sulfoxides and diaryl sulfones. Org. Lett. 20, 764–767 (2018).
Liu, J., Shen, X., Wang, Y., Wang, X. & Bi, X. [3 + 2] cycloaddition of isocyanides with aryl diazonium salts: catalyst-dependent regioselective synthesis of 1,3- and 1,5-disubstituted 1,2,4-triazoles. Org. Lett. 20, 6930–6933 (2018).
Liu, Q. et al. A general electrochemical strategy for the Sandmeyer reaction. Chem. Sci. 9, 8731–8737 (2018).
Khan, R. K. M., Zhao, Y., Scully, T. D. & Buchwald, S. L. Catalytic arylhydroxylation of dehydroalanine in continuous flow for simple access to unnatural aminoacids. Chem. Eur. J. 24, 15215–15218 (2018).
Rand, A. W. & Montgomery, J. Catalytic reduction of aryl trialkylammonium salts to aryl silanes and arenes. Chem. Sci. 10, 5338–5344 (2019).
He, R. et al. Reductive coupling between C–N and C–O electrophiles. J. Am. Chem. Soc. 141, 12481–12486 (2019).
Yang, Z. et al. Cross-coupling polycondensation via C–O or C–N bond cleavage. Nat. Commun. 9, 1587 (2018).
Mao, S. et al. Synthesis of aryl trimethylstannane via BF3·OEt2-mediated cross-coupling of hexaalkyl distannane reagent with aryl triazene at room temperature. J. Org. Chem. 84, 463–471 (2019).
Tan, J. et al. Divergent synthesis of densely substituted arenes and pyridines via cyclotrimerization reactions of alkynyl triazenes. J. Am. Chem. Soc. 141, 10372–10383 (2019).
Tobisu, M., Nakamura, K. & Chatani, N. Nickel-catalyzed reductive and borylative cleavage of aromatic carbon–nitrogen bonds in N-aryl amides and carbamates. J. Am. Chem. Soc. 136, 5587–5590 (2014).
Elbein, A. & Molyneux, R. I. Alkaloids, Chemical and Biological Perspectives (John Wiley, New York, 1990).
Nugent, T. C. Chiral Amine Synthesis: Methods, Developments and Applications (Wiley-VCH, Weinheim, 2010).
Fotie, J. et al. Trypanocidal and antileishmanial dihydrochelerythrine derivatives from Garcinia lucida. J. Nat. Prod. 70, 1650–1653 (2007).
Plobeck, N. et al. New diarylmethylpiperazines as potent and selective nonpeptidic δ opioid receptor agonists with increased in vitro metabolic stability. J. Med. Chem. 43, 3878–3894 (2000).
Schmidt, F., Stemmler, R. T., Rudolph, J. & Bolm, C. Catalytic asymmetric approaches towards enantiomerically enriched diarylmethanols and diarylmethylamines. Chem. Soc. Rev. 35, 454–470 (2006).
Reddy, L. R. et al. Diastereoselective addition of anisoles to N-tert-butanesulfinyl imines via four-membered lithium cycles. Chem. Commun. 54, 7007–7009 (2018).
Fu, P., Snapper, M. L. & Hoveyda, A. H. Catalytic asymmetric alkylations of ketoimines. Enantioselective synthesis of N-substituted quaternary carbon stereogenic centers by Zr-catalyzed additions of dialkylzinc reagents to aryl-, alkyl-, and trifluoroalkyl-substituted ketoimines. J. Am. Chem. Soc. 130, 5530–5541 (2008).
Hayashi, T., Kawai, M. & Tokunaga, N. Asymmetric synthesis of diarylmethyl amines by rhodium‐catalyzed asymmetric addition of aryl titanium reagents to imines. Angew. Chem. Int. Ed. 43, 6125–6128 (2004).
Hayashi, T. & Ishigedani, M. Rhodium-catalyzed asymmetric arylation of imines with organostannanes. asymmetric synthesis of diarylmethylamines. J. Am. Chem. Soc. 122, 976–977 (2000).
Kuriyama, M., Soeta, T., Hao, X., Chen, Q. & Tomioka, K. N-Boc-l-valine-connected amidomonophosphane rhodium(I) catalyst for asymmetric arylation of N-tosylarylimines with arylboroxines. J. Am. Chem. Soc. 126, 8128–8129 (2004).
Schrapel, C. & Peters, R. Exogenous-base-free palladacycle‐catalyzed highly enantioselective arylation of imines with arylboroxines. Angew. Chem. Int. Ed. 54, 10289–10293 (2015).
Ma, C. et al. Design and catalytic asymmetric construction of axially chiral 3,3′‐bisindole skeletons. Angew. Chem. Int. Ed. 58, 3014–3020 (2019).
Ma, C., Zhang, T., Zhou, J., Mei, G. & Shi, F. Catalytic asymmetric chemodivergent arylative dearomatization of tryptophols. Chem. Commun. 53, 12124–12127 (2017).
Liu, L. et al. Organocatalytic Para-selective amination of phenols with iminoquinone monoacetals. Org. Lett. 19, 3823–3826 (2017).
Wang, S., Wang, L., He, Q. & Fan, R. Destruction and construction: application of dearomatization strategy in aromatic carbon–nitrogen bond functionalization. Angew. Chem. Int. Ed. 54, 13655–13658 (2015).
Han, D., He, Q. & Fan, R. Formal group insertion into aryl C‒N bonds through an aromaticity destruction-reconstruction process. Nat. Commun. 9, 3423 (2018).
Lu, L., Chen, J. & Xiao, W. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 45, 1278–1293 (2012).
Sun, X. & Tang, Y. Ylide-initiated michael addition−cyclization reactions beyond cyclopropanes. Acc. Chem. Res. 41, 937–948 (2008).
McGarrigle, E. M. et al. Chalcogenides as organocatalysts. Chem. Rev. 107, 5841–5883 (2007).
Li, A., Dai, L. & Aggarwal, V. K. Asymmetric ylide reactions: epoxidation, cyclopropanation, aziridination, olefination, and rearrangement. Chem. Rev. 97, 2341–2372 (1997).
Zhurakovskyi, O. et al. Enantioselective synthesis of the cyclopiazonic acid family using sulfur ylides. Angew. Chem. Int. Ed. 57, 1346–1350 (2018).
Li, A. et al. Asymmetric aziridination over ylides: highly stereoselective synthesis of acetylenyl‐N‐sulfonylaziridines. Angew. Chem. Int. Ed. 36, 1317–1319 (1997).
Zanardi, J., Lamazure, D., Minière, S., Reboul, V. & Metzner, P. First enantioselective synthesis of vinyl oxiranes from aldehydes and ylides generated from allyl halides and chiral sulfides. J. Org. Chem. 67, 9083–9086 (2002).
Julienne, K. & Metzner, P. A simple C2 symmetrical sulfide for a one-pot asymmetric conversion of aldehydes into oxiranes. J. Org. Chem. 63, 4532–4534 (1998).
Bellenie, B. R. & Goodman, J. M. Sulfonium ylide epoxidation reactions: methylene transfer. Chem. Commun. 9, 1076–1077 (2004).
Foubelo, F., Moreno, B., Soler, T. & Yus, M. Reductive ring opening of dihydrodibenzothiepine and dihydrodinaphtho-oxepine and -thiepine. Tetrahedron 61, 9082–9096 (2005).
Deng, X.-M. et al. Enantioselective synthesis of vinylcyclopropanes and vinylepoxides mediated by camphor-derived sulfur ylides: rationale of enantioselectivity, scope, and limitation. J. Am. Chem. Soc. 128, 9730–9740 (2006).
Aggarwal, V. K. et al. Highly enantioselective synthesis of glycidic amides using camphor-derived sulfonium salts. mechanism and applications in synthesis. J. Am. Chem. Soc. 128, 2105–2114 (2006).
Li, A.-H., Dai, L.-X., Hou, X.-L., Huang, Y.-Z. & Li, F.-W. Preparation of enantiomerically enriched (2R,3R)- or (2S,3S)-trans-2,3-diaryloxiranes via camphor-derived sulfonium ylides. J. Org. Chem. 61, 489–493 (1996).
Aggarwal, V. K. et al. Catalytic asymmetric epoxidation of aldehydes. Optimization, mechanism, and discovery of stereoelectronic control involving a combination of anomeric and Cieplak effects in sulfur ylide epoxidations with chiral 1,3-oxathianes. J. Am. Chem. Soc. 120, 8328–8339 (1998).
Saito, T., Akiba, D., Sakairi, M. & Kanazawa, S. Preparation of a novel, camphor-derived sulfide and its evaluation as a chiral auxiliary mediator in asymmetric epoxidation via the Corey-Chaykovsky reaction. Tetrahedron Lett. 42, 57–59 (2001).
Weitkamp, A. W. I. The action of sulfur on terpenes. The limonene sulfides. J. Am. Chem. Soc. 81, 3430–3434 (1959).
Illa, O. et al. Practical and highly selective sulfur ylide-mediated asymmetric epoxidations and aziridinations using a cheap and readily available chiral sulfide: extensive studies to map out scope, limitations, and rationalization of dastereo- and enantioselectivities. J. Am. Chem. Soc. 135, 11951–11966 (2013).
Illa, O., Arshad, M., Ros, A., McGarrigle, E. M. & Aggarwal, V. K. Practical and highly selective sulfur ylide mediated asymmetric epoxidations and aziridinations using an inexpensive, readily available chiral sulfide. Applications to the synthesis of quinine and quinidine. J. Am. Chem. Soc. 132, 1828–1830 (2010).
Fearraigh, M. P. O., Matlock, JohnathanV., Illa, O., McGarrigle, E. M. & Aggarwal, V. K. Synthesis of isothiocineole and application in multigram-scale sulfur ylide mediated asymmetric epoxidation and aziridination. Synthesis 132, 3337–3343 (2018).
Acknowledgements
We are grateful to the National Natural Science Foundation of China (21572033 and 21971043) and the Science and Technology Commission of Shanghai Municipality (18XD1400800 and 19ZR1403400) for support of this research.
Author information
Authors and Affiliations
Contributions
R.F. directed the research and developed the concept of the reaction with L.L. and M.Y., who also performed the experiments and prepared the Supplementary Methods. Q.H. checked the experimental data. R.F. wrote the manuscript with contributions from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, L., Yang, M., He, Q. et al. Conversion of anilines to chiral benzylic amines via formal one-carbon insertion into aromatic C–N bonds. Nat Commun 11, 4805 (2020). https://doi.org/10.1038/s41467-020-18593-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-18593-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.