Abstract
Supported metal single atom catalysts (SACs) present an emerging class of low-temperature catalysts with high reactivity and selectivity, which, however, face challenges on both durability and practicality. Herein, we report a single-atom Pt catalyst that is strongly anchored on a robust nanowire forest of mesoporous rutile titania grown on the channeled walls of full-size cordierite honeycombs. This Pt SAC exhibits remarkable activity for oxidation of CO and hydrocarbons with 90% conversion at temperatures as low as ~160 oC under simulated diesel exhaust conditions while using 5 times less Pt-group metals than a commercial oxidation catalyst. Such an excellent low-temperature performance is sustained over hydrothermal aging and sulfation as a result of highly dispersed and isolated active single Pt ions bonded at the Ti vacancy sites with 5 or 6 oxygen ions on titania nanowire surfaces.
Similar content being viewed by others
Introduction
Effective catalytic oxidation of CO, hydrocarbons (HCs), and nitrogen oxides (NOx) at low temperature is sought for ultra-clean and energy-efficient emission control over various energy systems, including both mobile and stationary sources. The exemplary catalytic devices range from diesel oxidation catalysts (DOCs)1,2, three-way catalysts (TWCs)3, selective catalytic reduction (SCR) of NOx catalysts4, to lean NOx traps (LNT)5. Developing low-temperature emission control solutions could enable advanced energy and environmental technologies that operate in a more energy-efficient and eco-friendly environment, such as the low-temperature combustion regime engine technology6.
In search of low-temperature oxidation catalysts, promising results have been shown using gold7,8,9, metal oxides, such as Co3O410,11, Co-Cu-Ce mixed oxides1, and La-based perovskites2. Unfortunately, these catalysts usually are poor in hydrothermal stability and susceptible to sulfur poisoning. Recently, supported platinum-group metal (PGM) single atom catalysts (SACs) have emerged as a new type of low-temperature catalysts with high reactivity and selectivity, due to their high metal dispersion, fewer types of active sites, low-coordination environments, quantum size effects, and enhanced metal-support interactions12,13,14,15,16,17,18,19. However, isolated metal atoms are usually mobile and tend to aggregate into clusters or particles during synthesis or reaction. Improving SACs’ stability and maintaining their high activity is one of the grand challenges20,21,22 currently. Recently, Nie et al. reported highly active and stable Pt SACs supported by CeO2 that were prepared using the atom trapping method23. The catalyst showed good CO oxidation performance with total CO conversion at 148 °C and stability up to 500 °C. However, deactivation was observed after exposure to 800 °C. Meanwhile, the studies on SACs up to now have been limited to model reactions, such as CO oxidation and water gas shift. Little success has been achieved in the supported SACs so far over hydrocarbon oxidation at low temperature. Furthermore, the reported SACs have been limited to lab-scale reactors with a small amount of powders. There has yet to be an evaluation of SACs in field-size catalytic reactors under realistic exhaust conditions, although such a real-world demonstration is necessary to translate the scientific advancement to technological applications.
In this work, durable single-atom Pt catalysts are successfully demonstrated to be anchored on titania nanowire array (nano-array, NA) forest integrated on full-size honeycomb substrates, as denoted as Pt1/TiO2 NA catalyst. As a SAC based monolithic catalyst, it displays a sustained and remarkably high oxidation activity over CO and hydrocarbons at low temperature under simulated diesel exhaust, the industry-defined conditions protocoled by USDRIVE24 and under heavy duty diesel (HDD) engine transient dynamic cycles, as illustrated and summarized in Fig. 1a. We demonstrate that the temperature of 90% conversion, T90, for CO and HCs in the clean diesel combustion (CDC) simulated exhaust evaluation25 approaches 160 °C, while using five times less PGM than a benchmark commercial DOC. Notably, the single-atom Pt active sites are highly robust after hydrothermal (HT) aging at 700 °C for 100 h as a result of their fixation at the five-fold coordinated Ti vacancy sites bonded with five or six oxygen ions on titania nanowire surfaces. Such a NA supported Pt SAC retains its nanowire array structure as well as abundant Pt single-atom species on the TiO2 nanowire surfaces after the hydrothermal aging and simulated exhaust test.
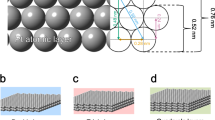
a Schematic illustration of integration process of Pt1/TiO2 nanowire array forest onto ceramic monoliths and physicochemical and catalytic characteristics of such DOC catalytic converters. b Cross-sectional and c top view SEM images of rutile TiO2 NA on a cordierite honeycomb; inset: low-magnification cross-sectional view of cordierite substrate interface with conformably distributed TiO2 nanowire forest. d HAADF STEM of a rutile nanowire bundle. The arrays of dark spots on the HAADF STEM image identified the mesoporosity of the TiO2 nano-arrays. e–g ac-HAADF STEM images of Pt1/TiO2 NA prepared by microwave-assisted dip-coating (0.71 gPt L−1) (e) before, (f) after hydrothermal aging at 700 °C for 100 h, and (g) after hydrothermal aging at 700 oC for 100 h followed by simulated CDC exhaust test treatment. The bright dots on the surface of TiO2 are Pt atoms, as pointed by red arrow-heads.
Results
Structure and stability of single-atom Pt on TiO2 nanowire arrays
It is noted that pure rutile phase, the most thermodynamically stable among TiO2 polymorphs as typical oxidation catalyst support materials26,27, is realized in the form of TiO2 NA forest in this study. Additionally, the hierarchically structured NA forest allows efficient mass transport and good catalytic performance in supported catalysts in a honeycomb monolithic device form, with advantageous attributes, such as low pressure drop and high surface area28. In this work, densely packed forest of mesoporous rutile TiO2 NA was successfully grown onto channel surfaces of cordierite honeycombs with various sizes ranging from a size of 0.2 L (63 mm × 63 mm × 50 mm), to a full-size core of 1.2 L (Ф 145 mm × 75 mm). The TiO2 loading, determined from inductively coupled plasma mass spectrometry (ICP-MS) measurement, is ~23 wt.% or ~69 g L−1. The rutile TiO2 NA was grown uniformly with a height of ~3 μm, and arranged in the form of individual nanowire-bundles ~50–100 nm wide (Fig. 1b). The comprised TiO2 nanowires are ~10–20 nm wide, as revealed in the high angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) image in Fig. 1d and high resolution transmission electron microscopy (HRTEM) image (Supplementary Fig. 1a). The bundled TiO2 nanowires are mesoporous, revealed by arrays of darker spots (3–20 nm in diameter) in Fig. 1d, consistent with the BJH pore size distribution (Supplementary Fig. 2b). The HRTEM lattice image identified an inter-planar lattice spacing of 0.326 nm, matching the {110} planes of rutile TiO2, corroborating with the XRD pattern in Supplementary Fig. 1b (JCPDS #88-1175). We note that a thin mesoporous layer of SiO2 was also formed during the TiO2 growth process. These mesoporous SiO2 are formed due to the leaching of Al, Fe, and Mg from cordierite by hydrochloric (HCl) acid at the initial stage of the solvothermal TiO2 growth process (Supplementary Fig. 3 and Supplement Note 1).
The rutile TiO2 NA integrated cordierite honeycomb has a high BET surface area of ~89.6 m2 g−1, equivalent to 25,984 m2 for a 1 L cordierite honeycomb core. Such a high surface area of the TiO2 NA integrated honeycomb is attributed to the unique mesoporous structures of nanowire arrays and partially etched cordierite substrate surface as discussed earlier (Fig. 1d and Supplementary Fig. 2). After Pt loading using a dip-coating method (Method section), the surface area reduces to 45.7 m2 g−1 (Supplementary Fig. 2b), comparable to the surface area of bench-mark commercial DOC sample (49.8 m2/g). After hydrothermal aging at 700 °C for 100 h, the surface area of the sample is further reduced to ~16 m2 g−1, however with the array structure well retained (Supplementary Fig. 2b). The loss of surface area after both dip-coating and HT aging processes are due to the closure of small pores within TiO2 nanowires (<7 nm), as revealed in the pore size distribution measured by N2 isotherms and by presence of fewer (dark-spot) pores revealed in the HAADF STEM image of the respective samples (Supplementary Fig. 2b). It is noted that, despite the over 60% decrease of surface area after hydrothermal aging on the Pt1/TiO2 NA, the superb catalytic oxidation performance has been largely retained. This is due to the remarkable activity and durability found on the well-dispersed and isolated single-atom Pt catalysts anchored on the mesoporous rutile TiO2 nanowire surfaces.
During operation, oxidation catalysts may endure mechanical vibrations and high velocity multi-phase exhaust flow such as the vehicular conditions. The associated mechanical stresses might cause the separation or delamination of washcoat layers from the monolithic substrates, resulting in loss of active materials, deactivation of catalysts, and even damage of downstream functional devices29. Since the TiO2 nano-array forest was “in situ grown” instead of “wash-coated” on the honeycomb monoliths, these nano-array integrated monoliths have an improved adherence over conventional washcoated samples. Evidently, after ultrasonicating at 40 kHz in water bath at 25 °C for 4 h, both fresh and HT aged Pt1/TiO2 NA integrated monoliths showed little morphology change (Supplementary Fig. 4). A negligible weight loss of ~1% was observed in both fresh and aged nano-array integrated monoliths, as compared to that of >10 % weight loss for the commercial DOC after similar ultra-sonication testing.
Pt was loaded on TiO2 nano-arrays, with a volumetric Pt loading of 0.53-1.73 g L−1 (0.18–0.58 wt.%, Supplementary Table 1) to prepare functional DOC devices, employing either microwave-assisted dip-coating or wet-incipient impregnation (WII)12. Aberration-corrected (ac) HAADF STEM, a powerful tool for discerning individual heavy atoms (e.g., Pt) from lighter supporting atoms (e.g., Ti, O), was employed to investigate the distribution of Pt on the TiO2 nano-array. Supplementary Fig. 5 shows ac-HAADF STEM images of Pt supported on TiO2 NA prepared using Na-promoted WII method, clearly revealing the well-dispersed Pt single atoms on TiO2 nanowire surface. High Pt dispersions of 80% and 74% were determined for the Pt loading of 0.71 and 1.73 gPt L−1, respectively, by H2 chemisorption measurement, further confirming the dominant atomically dispersed Pt sites. Interestingly, without any promoter, microwave-assisted dip-coating also results in atomic dispersion of Pt on TiO2 nano-arrays shown in Fig. 1e and Supplementary Fig. 6. However, the Pt dispersion of dip-coating samples is lower, 33% and 24% for the Pt loading of 0.71 and 1.73 gPt L−1, respectively, due to a higher amount of Pt nanoparticles dispersed on the adjacent mesoporous SiO2 (Supplementary Figs. 3 and 6). Meanwhile, TiO2 NA and NW samples with different mesoporosity were prepared under different calcination temperatures and used to look into the support porosity influence on the Pt SAC loading. It is noted that the Pt single-atom distribution is retained in all these different mesoporous TiO2 nanowire surfaces, where few to no Pt nanoparticles were observed (Supplementary Fig. 7). This may suggest that the Pt SAC loading is relatively insensitive to the mesoporosity of TiO2 NWs. In addition, the mesoporous NA samples maintained a similar specific surface area of 20–25 m2 g−1 despite the obvious evolution of mesopore size and distribution as the calcination temperature increased from 500 to 900 °C (Supplementary Fig. 8), indicating the excellent thermal stability of these rutile nano-arrays.
It was noted earlier that the single Pt atom catalysts are often not stable under the HT condition due to their tendency to agglomerate, thus hindering their practical applications30. Here on the mesoporous rutile TiO2 nanowire supports, the atomically dispersed Pt was found to retain its dispersion even after HT aging at 700 oC for 100 h and simulated exhaust treatment (Fig. 1f, g and Supplementary Fig. 9). On both dip-coating and Na-promoted WII samples, these single-atom Pt catalysts retained their dispersion after HT degreening at 700 °C for 4 h (Supplementary Figs. 10 and 11). On the contrary, Pt nanoparticles supported on adjacent SiO2 on both samples severely sintered after HT degreening (Supplementary Figs. 10 and 11). To understand the excellent durability displayed here, a series of in situ CO oxidation studies were conducted over the Pt1/TiO2 SAC catalysts using synchrotron X-ray adsorption spectroscopy (XAS), X-ray photoelectron spectroscopy (XPS), and density functional theory (DFT) computation (Figs. 2a–d). It is found that the strong electrostatic interactions between Pt and TiO2 support surface are responsible for the excellent durability in these single Pt atom catalysts21,22,30. Specifically, on the TiO2 nanowire surfaces, isolated and catalytically active Pt ions are pre-dominantly located at the surface Ti vacancies, and strongly anchored by five or six neighboring O ions, thus exhibiting outstanding hydrothermal durability. Additional studies using in situ diffuse reflectance infrared fourier-transform spectroscopy (DRIFTS) and temperature programmed reduction (TPR) under hydrogen have revealed an outstanding low-temperature catalytic activity of these TiO2 nanowire supported SAC catalysts toward CO oxidation (Figs. 2e, f).
a k2-weighted Fourier-transformed EXAFS spectra of Pt1/TiO2 NW. Pt NP/TiO2 NA, Pt foil, and PtO2 are employed as references. b Normalized XANES spectra at the Pt L3 edge of Pt1/TiO2 NW under in situ CO oxidation. c Core Pt 4 f XPS spectrum of the Pt1/TiO2 NW. d The proposed Eley-Rideal mechanism for CO oxidation on Pt1/TiO2 NA. The reaction cycle shows the structure of intermediates and transient state (TS) of the key elementary steps. The inset shows the calculated energy profile. e In situ DRIFT spectra of CO adsorption and oxidation at 30 and 100 °C of Pt1/TiO2 NA, and f, H2-TPR profile of Pt1/TiO2 NW.
Structure and reactivity of single-atom Pt on TiO2 nanowire surface
The in situ XAS studies for CO oxidation were performed on 0.2 wt.% Pt1/rutile TiO2 nanowires (NW) for understanding the structural characteristics and low-temperature reactivity of the Pt single-atom active sites. TiO2 NW powder, collected in the same batch with the synthesis of TiO2 NA monolith, was employed as the support to avoid the interference from the cordierite substrates. The fourier transformed-extended X-ray absorption fine structure (EXAFS) spectrum of 0.2 wt.% Pt1/TiO2 at the Pt L3 edge in r-space shows a dominant peak at 1.6 Å from the Pt–O contribution and very weak peaks at 2.6 and 3.0 Å from either the Pt–Pt or the Pt–Ti contribution (Fig. 2a), confirming that Pt is atomically distributed on TiO2 NW surface. Structural parameters extracted from the Pt L3-edge EXAFS fitting, summarized in Supplementary Table 2, indicates that on Pt1/TiO2 NW, the Pt site has 5.5 Pt–O coordinations, which have an interatomic distance of 1.99 ± 0.02 Å, almost the same as the Pt–O distance in PtO2 (2.01 ± 0.02 Å). As shown in Fig. 2b, the white line intensity at ~11570 eV of Pt1/TiO2 NW in the normalized X-ray Absorption Near-edge Structure (XANES) is between the intensities of Pt(NH3)4(NO3)2 and PtO2, suggesting that the Pt single atoms have oxidation states in between 2+ and 4+, corroborating with the XPS analysis that the sample only contains Pt4+ and Pt2+ (Fig. 2c). During the CO oxidation reaction in lean conditions (2000 ppm CO and 12% O2), the overall oxidation state of Pt decreases slightly, but remain in between 2+ and 4+, indicated by the decrease in the white line intensity of Pt1/TiO2 NW under the in situ XANES measurements.
DFT calculations were performed to find the most stable configurations of single-atom Pt on TiO2 NW surfaces. The binding energy, defined as the energy needed to move a Pt atom from the TiO2 surface to vacuum (>10 Å), is used to assess the stability of the Pt atom on the surface. We only considered configurations on rutile TiO2 (110) with and without Ti vacancies since the {110} is the dominant surfaces and no O vacancies were detected on TiO2 NW (Supplement Note 2). The structures and the corresponding binding energy of Pt are shown in Supplementary Fig. 12. The calculation predicts that the configuration where Pt stays in the five-fold coordinated Ti vacancy (Pt–TiV) is the most stable, with 2.3 eV higher in binding energy compared to that with Pt substituting six-fold coordinated Ti (Pt–TiVI) and at least 1.8 eV higher than other configurations where Pt adsorbed on the TiO2 (110). This prediction is in good agreement with XAS fine structure studies. We note that the Pt–TiV configuration has two models, with (Model A) and without (Model B) O on top (Otop) of Pt. In model A, Pt is fully oxidized (Pt4+) and has six Pt–O coordinations including four in-plane Pt–Oi, one Pt–Osub, and one Pt–Otop where Oi is in-plane O, and Osub is O from the sub-surface layer. The Pt–Otop is a weak bond and can be easily activated, thus this bond is absent in the partially oxidized form (Pt2+) in model B, leaving 5 coordinations on the Pt sites. The binding energies of Pt in the Models A and B are close to each other (10.44 eV vs. 11.09 eV), suggesting the likely co-existence of both Pt4+ and Pt2+on the TiO2 NW surface, therefore, the average coordination number of Pt is between 5 and 6, in agreement with the experimental result from both fitting EXAFS curve (Supplementary Table 2) and XPS analyses (Fig. 2c and Supplementary Table 3).
It is noted that the white line intensity of Pt1/TiO2 NW decreases in the CO/O2 mixture even at room temperature, suggesting isolated Pt atoms supported on TiO2 NW may catalyze room-temperature CO oxidation. We employed DRIFTS to obtain mechanistic insights into low-temperature CO oxidation reactivity of Pt1/TiO2 NA. Fig. 2e shows the in situ DRIFT spectra of CO adsorption and oxidation at 30 and 100 °C of Pt1/TiO2 NA. After CO is adsorbed on Pt1/TiO2 NA, quasi-symmetrical IR bands at 2085 and 2081 cm−1 are observed for the spectra collected at room temperature and 100 °C, respectively, and ascribed to CO linearly adsorbed on isolated ionic Ptδ+ sites19,21,22. The IR band at 2085 cm−1 shows a reduction of 80% in peak area after O2 was introduced for 10 min at room temperature, suggesting CO is readily oxidized at room temperature on Pt1/TiO2 NA. All adsorbed CO is oxidized when the temperature increases to 100 °C as the IR band at 2081 cm−1 vanishes with the introduction of O2. These evidences clearly demonstrate low-temperature reactivity of Pt1/TiO2 NA.
DFT calculations were also performed to elucidate the high catalytic activity for CO oxidation of Pt1/TiO2 NA and NW. CO can be oxidized over supported Pt catalysts in three ways, namely Langmuir-Hindshelwood (LH), Mars-van Krevelen (MvK), and Eley-Rideal (ER) mechanisms31,32,33. On Pt1/TiO2 NA and NW, the ER mechanism was found to be favored due to a very low DFT derived activation energy of ~ 0.2 eV (~19 kJ mol−1), in good agreement with the experimentally measured activation energy of 22.7 kJ mol−1 (Supplementary Fig. 13). The ER catalytic cycle and the corresponding calculated energy profile of CO oxidation on Pt1/TiO2 NA is shown in Fig. 2d. Model A with a Pt4+ reactive center was employed as the starting configuration (Intermediate I). CO reacts with the O on top of Pt via the ER mechanism with an activation energy of 0.2 eV and exothermicity (∆H) of –3.6 eV, leaving one free coordination on Pt (Intermediate II). This free coordination is then filled by adsorption of an O2 molecule (Intermediate III). The O–O bond of O2 adsorbed on the Pt site can be easily activated when another CO coordinates with one O atom at the other end (Intermediate IV). The cycle is completed with the release of CO2. It is worth noting that the LH mechanism is usually dominated for supported Pt nanoparticle catalysts (except for Pt/CeO2 case where MvK is dominant21), which involves adsorption of CO and dissociative adsorption of O2 on metal active sites, followed by the reaction of adsorbed CO and O species to form CO233. A modified LH scheme was proposed for CO oxidation on Pt single atoms supported on FeOx (Pt1/FeOx), in which CO adsorbing on the Pt atom reacts with O from FeOx support15. Meanwhile, the LH-based mechanisms are energetically unfavorable on Pt1/TiO2 NA due to a high activation energy of ~1.8 eV (174 kJ mol−1) for the dissociation of the adsorbed O2 molecule with the help of the Pt site and one surrounding Ti site (Supplementary Fig. 14).
The redox property of Pt1/TiO2 NW was studied using H2 temperature-programmed reduction (H2-TPR) (Fig. 2f). In the H2-TPR profile of Pt1/TiO2 NW, the peaks at 103 and 508 °C are assigned to the reductions of isolated Pt atoms and bulk TiO2, respectively. We observed an additional H2-consumption peak at 186 °C for Pt1/TiO2 NW, which is absent in the profile of Pt NP/TiO2 NA. This peak can be assigned to the reduction of TiO2 induced by isolated Pt atoms. It has been reported that the presence of single-atom Pt can lower the reduction temperature of metal oxide support due to the strong metal-support interaction15. The enhanced reducibility of Pt1/TiO2 NA compared to other TiO2 based-DOCs might allow excursions into fuel-rich and fuel-lean conditions although more thorough evaluation is necessary.
Low-temperature diesel oxidation over single-atom Pt on TiO2 nanowire arrays
To evaluate their catalytic oxidation activity, the Pt1/TiO2 NA monoliths were tested under simulated engine exhausts based on clean diesel combustion (CDC) and low-temperature diesel combustion (LTC-D) protocols developed by US DRIVE24. The samples were tested at a high gas hourly space velocity (GHSV) of 60,000 h−1 to assess their close-to-reality performance under these protocoled conditions. We note that in both conditions, the composition of total hydrocarbons (THC) on a C1 basis in the exhausts is ~56% C2H4, 33% C3H6, and 11% C3H8. Samples were hydrothermally degreened at 550 oC for 4 h and at 700 oC for 4 h for evaluation in the CDC and LTC-D conditions, respectively, representing a ~5000 mile-on-road condition.
Fig. 3a shows the light-off curves for Pt1/TiO2 NA with Pt loading of 0.71 g L−1 in the CDC simulated exhaust. The T90 temperatures of CO, C2H4, C3H6, and THC are 164, 172, 173, and 178 °C, respectively, all well below 200 oC, at which most catalyst systems in today’s vehicles are ineffective34. For benchmark, we employed a commercial DOC sample with PGM loading of 4.66 g L−1, obtained from a brand new heavy-duty pick-up truck (year model 2012). The temperature T90 for THC of Pt1/TiO2 NA is ~172 °C lower than that of the commercial DOC (350 °C), despite ~5 times less PGM loading (Supplementary Fig. 15). On Pt1/TiO2 NA, both ethylene and propylene oxidation occur almost simultaneously. On the other hand, the oxidation of ethylene, the main hydrocarbon component in the simulated exhausts (56%), on the commercial DOC sample is sluggish, leading to the slow conversion of THC and consequently a much higher T90 than that of Pt1/TiO2 NA. Additionally, the conversions of both C3H6 and C2H4 on the Pt1/TiO2 NA integrated monoliths quickly reach more than 99 % with no plateau in the high conversion region that was observed in the commercial DOC, suggesting that the catalytic reaction kinetics over Pt1/TiO2 nano-array are not mass-transfer limited even at a high space velocity of 60,000 h−135.
a Light-off curves for Pt1/TiO2 NA (0.71 gPt L−1) in the CDC simulated exhaust. b Comparison of the DOC activity in the CDC simulated exhaust between P1/TiO2 NA (0.71 gPt L−1) and Pt NP/TiO2 NA (1.73 gPt L−1). c Durability of the Pt1/TiO2 NA in the simulated exhaust. d, e Sulfur-poisoning effects on the DOC activity of Pt1/TiO2 NA (1.73 gPt L−1) in the LTC-D simulated exhaust. f Transient (upper panel) and cumulative (lower panel) THC emission in the transient gas conditions mimicking a HDD FTP as running on a HDD certified 2010 Cummins ISB (6.7 L) 320 hp engine for fresh (blue) and aged (red) Pt1/TiO2 NA (0.53 gPt L−1). A commercial DOC monolith with double PGM loading (1.06 g L−1) was employed as the reference.
A secondary function of a DOC catalyst is to oxidize NO to NO2 to help downstream functionalities such as NOx selective catalytic reduction (SCR) catalyst and regeneration of diesel particulate filters1,2. In this regard, the Pt1/TiO2 NA also outperforms the commercial sample, with a maximum NO-to-NO2 conversion of 47% for the Pt/TiO2 nano-array at a low temperature 291 oC as compared to 27% at 427 oC for the commercial DOC (Supplementary Fig. 15).
To evaluate and validate the practical diesel oxidation reactivity of isolated Pt atom active sites, we employed Pt-nanoparticle (NP) supported on mesporous SiO2 monolith and TiO2 NA monolith (Supplementary Fig. 16) as two references. These reference catalysts were prepared by etching of cordierite honeycombs followed by Pt dip-coating, and atomic layer deposition of Pt over TiO2 NA monolith28, respectively. It is noted the low-temperature DOC activity of Pt NP/TiO2 nano-array is good, but inferior to Pt1/TiO2 NA, despite a 2.5-time higher PGM loading (Fig. 3b). The temperatures T90 of Pt NP/TiO2 NA for CO, C3H6, C2H4, and THC are 193, 196, 196, and 223 °C, respectively, ~23–45 °C higher than that of Pt1/TiO2 NA. When using a Pt NP loading of 0.71 gPt L−1 on the etched cordierite substrate that spontaneously created a layer of mesoporous SiO2 support, similarly inferior catalytic oxidation performances were observed over CO and C3H6, with ~35–45 °C higher T90 temperatures than those of the Pt1/TiO2 NA sample of the same Pt loading (Supplementary Fig. 16c). This evidence clearly illustrates the improved reactivity for CO and HC oxidation of isolated Pt atoms, compared to Pt NP active sites although direct turnover frequency (TOF) measurements are difficult or impossible with these steep light-off curves and under the complex flow conditions.
To evaluate the durability of Pt1/TiO2 NA during reactive conditions, the catalyst was tested under simulated exhaust at 330 oC with cyclic exposures to 820 °C for 10 min (Fig. 3c). No deactivation was observed of the catalyst with the conversions of CO and THC remaining at 100% and >90%, respectively, during a ~36 h evaluating period. The excellent durability of Pt1/TiO2 NA is attributed to the stable atomically distributed Pt sites, which even retained after hydrothermal aging at 700 °C for 100 h and the CDC simulated exhaust treatment, as revealed in the single-atom structural analyses earlier (Fig. 1f, g).
Sulfur-containing compounds, present in most vehicle fuels and lubricants, can be oxidized to SO2 during combustion, interacting with and deactivating PGM supported catalysts36,37,38. Therefore, it is necessary to test sulfur-poisoning effects on Pt1/TiO2 NA for the practical DOC applications. The sample Pt1/TiO2 NA with Pt loading of 1.73 g L−1 was chosen for the USDRIVE’s testing protocol for sulfur tolerance based on the LTC-D simulated exhaust24. Under the LTC-D condition, this sample performed better than the commercial DOC sample (5 oC lower in T90 for THC oxidation and much higher max NO-to-NO2 conversion (71% vs. 25%)) despite 2.7 times lower in PGM loading (1.73 vs 4.66 g L−1) (Supplementary Fig. 17). After an exposure to 1 gsulfur L−1 of SO2, the sample showed only a slight deactivation for CO and unsaturated HC with a T90 increase of ~19 °C (Fig. 3d, e and Supplementary Fig. 18). Interestingly, low-temperature catalytic propane oxidation was improved, likely due to the formation of interfacial sulfate species that facilitate propane chemisorption on Pt36, thus decreasing T90 of THC by 9 °C (Fig. 3e and Supplementary Fig. 18). Under simulated exhaust, propane contributes to 11% of THC and propane oxidation often starts after unsaturated HCs. Therefore, T90 of THC of a DOC is largely dependent on low-temperature propane oxidation activity, as reflected by increased T88 but decreased T90 of THC after S-poisoning (Supplementary Fig. 18). After de-sulfation in H2 stream, the oxidation catalytic activity for CO was even better and that for unsaturated HCs was almost fully recovered.
To demonstrate the scalability for practical conditions, a full-size Pt1/TiO2 NA monolith with a Pt loading of 0.53 g L−1 and dimensions of Ф 5 cm × 7.5 cm was evaluated under highly transient feed gas conditions, mimicking a heavy-duty diesel (HDD) federal test procedure (FTP) as running on a HDD certified 2010 Cummins ISB (6.7 L) 320 hp engine. The transient feed gas composition is listed in Supplementary Fig. 19. The Pt1/TiO2 NA sample was aged at 650 °C for 100 h in 10% steam/air flow to a representative end of life condition for a North American HDD application. Fig. 3f and Supplementary Fig. 20 show the transient response and cumulative emission of THC and CO, respectively, for the fresh and aged Pt1/TiO2 samples. The Pt1/TiO2 samples do not show reactivity during the first 200 s because of low temperature, high exhaust flow rate, and high concentration of HCs and CO. When the temperature increases, both fresh and aged samples showed good activity despite the drastic changes in both concentration and flow rate of emissions. It is noted that the HC oxidation activity of the aged sample is almost as good as the fresh sample, with only a small difference observed in the transient and cumulative HC emissions. The performance of the aged sample is mainly due to the stable single-atom Pt sites. As revealed by HAADF STEM (Fig. 1f, g and Supplementary Fig. 9), we observed a high density of isolated Pt atoms on the surface of TiO2 nanowires even after hydrothermal aging at 700 °C for 100 h followed by the simulated exhaust treatment. However, some small pores (<7 nm) are closed during hydrothermal aging, as indicated by HAADF STEM and N2 isotherms, thus access is inhibited on a portion of Pt sites (Supplementary Fig. 2). Such loss of Pt active sites leads to slow response to CO at high flow rate and concentration (at 500, 930, and 1100 s), and consequently an increase in the CO emission.
Discussions
The remarkable low-temperature performance of the isolated Pt single atoms supported on TiO2 nano-arrays can be attributed to the combination of atomically dispersed Pt and the unique structure of long-range ordered mesoporous TiO2 arrays. Single Pt atoms clearly show better diesel oxidation activity than the Pt NP counterpart, as demonstrated in the reactivity comparison under the simulated CDC exhaust conditions (Fig. 3b and Supplementary Fig. 15). First, the enhanced low-temperature reactivity of Pt single-atom catalyst is explained by the distinct electronic structures that modify interaction with adsorbate molecules or by the involvement of supports that may alter reaction pathways15,18. The LH and MvK mechanisms are often reported for supported Pt catalysts. However, low-temperature CO oxidation on Pt1/TiO2 NA favors the ER mechanism with very low activation energy. Second, the introduction of macro-pores in the mesoporous NW structures improves mass transport properties39,40. In the Pt1/TiO2 NA, the space between the mesoporous nanobundles acts as a large channel for fast transport of the reactants along the bundles before the reactants diffuse into the mesoporous network to the Pt active sites (Fig. 1a). Well-spaced support also helps to inhibit sintering of Pt, contributing to the hydrothermal robustness. The Pt1/TiO2 NA structures, which combine durable and reactive single Pt atoms and hierarchically porous NA support, are expected to have broader applications in the field of catalysis and materials science.
In summary, we demonstrate a single-atom Pt catalyst anchored on a highly stable mesoporous rutile titania nanowire array forest grown in field-size honeycomb monolith. With over 80% PGM usage reduction compared to that of the commercial benchmark, such a single-atom Pt catalyst has displayed remarkable oxidation activities for CO and hydrocarbons with 90% conversion at temperatures as low as ~160 °C under simulated exhaust conditions. Such excellent low-temperature activities are sustained over hydrothermal aging and sulfation as the active single-atom Pt species retain on the stable titania nanowire arrays, as a result of a strong electrostatic interaction between Pt single-atom species and TiO2 nanowire surface. A simple and cost-effective solvothermal process in conjunction of dip-coating and impregnation methods provides a viable strategy for scalable manufacturing of durable Pt single-atom catalysts on the nano-array-integrated monoliths.
Methods
Catalyst preparation
TiO2 nanowire arrays are grown on ceramic monoliths via a nonpolar solvent/hydrophilic solid substrate interfacial reaction under hydrothermal conditions41,42. Typically, the washed and TiO2 seeded cordierite honeycomb substrates with size up to 7.5 cm × 7.5 cm × 5 cm are placed within a sealed Teflon reactor (1 L), containing 500 mL of a non-polar solvent, 50 mL of a Ti (IV) alkoxide precursor, 5 mL of titanium tetrachloride (1 M in toluene), and 50 mL of hydrochloric acid (37 wt.%). At room temperature, titanium (IV) precursors and water are separated since the Ti precursors are dissolved in the nonpolar solvent. Under hydrothermal conditions, water diffuses away from the high-energy water/nonpolar solvent interface to the hydrophilic TiO2 nuclei on ceramic wall, where water reacts with Ti precursors, resulting in growth-crystallization of TiO2. The presence of Cl− anions is crucial for the anisotropic growth, as they tend to absorb on the rutile (110) plane, thus inhibiting further crystal growth of this plane. After being taken out for the reactor, all the samples are sonicated in acetone, ethanol, and water for 3 h to remove organic compounds and Cl− residues from the synthesis before Pt loading.
Microwave-assisted dip-coating and Na-promoted wet incipient impregnation methods are employed to load Pt on the TiO2 nano-arrays. For the dip-coating method, the substrate is first submerged into the diluted Pt precursor solution (0.5 mg Pt precursor per mL). It is taken out and blow-dried using compression air stream. The substrate is then dried in a microwave oven for 1–3 min. These steps are repeated until appropriate amount of the metal precursor is absorbed. Finally, the sample is calcined in air at 500 °C for 2–4 h with a ramp rate of 2 oC min−1. In addition, a following 1-h stay at a different calcination temperature such as 700, 800, and 900 °C has been adopted to look into the induced porosity and surface area change and its effect on the single-atom loading and the catalyst stability. For impregnation, the substrate is submerged into diluted Pt precursor (50 μg mL−1) and NaOH (atomic ratio Na/Pt is 10/1), and aged for 12 h at 80 °C. The solution is then evaporated in open air at 50 oC until dry. During evaporation, the substrate is rotated every 15–20 min to enhance uniformity. Finally, the sample is dried at 150 °C for 12 h and calcined in air at 500 °C for 4 h with a ramp rate of 2 °C min−1.
To evaluate their hydrothermal stability in the probe reactions (CO and C3H6 oxidation) and the simulated exhaust (clean diesel combustion, CDC; and low-temperature combustion-diesel, LTC-D) tests, the monolithic catalysts are aged in a flow of 12% O2, 6% CO2, 6% H2O in N2 at 700 °C for 100 h. In the transient engine tests, the sample is aged in a flow on 10% H2O in air at 650 °C for 100 h. These aging conditions are chosen to represent a North America heavy-duty diesel application.
Catalytic activity evaluation
The catalytic activity measurements were performed in a continuous flow reactor equipped with a gas chromatography. The monolithic catalysts (5 × 5 channels × 2.5 cm long) were placed in a tubular quartz reactor with a surrounding electrical heating coil. The inlet temperature was measured using a 0.16-mm K-type thermocouple, which was fixed at <5 mm in front of the monolith. The utilization of the small thermocouple (0.16 mm) is to prevent disruption of gas flow. Typically, the catalytic activity of each sample was evaluated through total six reaction cycles, in which the inlet temperature was ramped up from 100 to 525 °C at 2 °C min−1 and naturally cool down to 100 °C before next testing cycles. The feed gas mixtures were controlled by mass flow controller before entering the blender and then the reactor at a gas hourly space velocity of 30,000 h−1. The feed gas always contains 12% O2 to mimic the O2 concentration in the CDC and LTC-D simulated exhaust, but varies CO and C3H6 concentrations (also were chosen to reflect the CO and total hydrocarbon concentration in the CDC and LTC-D simulated exhausts) in each cycle such that: cycle 1 and 2 contain 2000 ppm CO and 1000 ppm C3H6; cycle 3 contains 2000 ppm CO; cycles 4 contains 1000 ppm C3H6; cycle 5 contains 500 ppm C3H6; and cycle 6 contains 500 ppm CO and 500 ppm C3H6; all are balanced by N2. Sample Array-50-D was evaluated in an additional sequence (cycle 3→4→5→6→1→2), showing negligible difference in the catalytic activity, thus confirming the sequence of the tests does not affect the catalytic activity of TiO2 nano-array supported Pt catalysts.
The catalytic diesel oxidation activity of catalysts under simulated exhaust conditions was evaluated according to the protocol developed by the Advanced Combustion and Emission Control (ACEC) Technical Team of USDRIVE. The light-off measurements were conducted on a customized plug-flow reactor system. Micro-cores of catalyst samples were cut and loaded into cylindrical quartz tubing using a wrapping in order to ensure no gas could bypass the catalyst channels. The catalyst temperature was monitored using two thermocouples, one measuring the inlet temperature while located ~2 cm from the catalyst front, the other measuring the mid-catalyst temperature and located from the rear into the middle of the central channel of the micro-core. Space velocity was kept at 60,000 h−1 throughout all the tests. Gas concentrations were determined per ACEC Tech Team (USDRIVE) protocol. The composition of the “LTC-D” simulated exhaust is [O2] = 12%, [H2O] = 6%, [CO2] = 6%, [H2] = 400 ppm, [CO] = 2000 ppm, [NO] = 100 ppm, [C2H4] = 833.5 ppm, [C3H6] = 333 ppm, [C3H8] = 111 ppm, and Ar balance. The composition of the “CDC” simulated exhaust is [O2] = 12%, [H2O] = 6%, [CO2] = 6%, [H2] = 100 ppm, [CO] = 500 ppm, [NO] = 200 ppm, [C2H4] = 389 ppm, [C3H6] = 233.5 ppm, [C3H8] = 51.7 ppm, and Ar balance. Water was introduced via argon flow through a bubbler and heated lines held at 200 °C. Product gas stream was measured via MKS FTIR. Experiments were conducted first with a degreening step in only the O2, CO2, and H2O components of the gas stream at 700 °C for 4 h for LTC-D and 550 oC for 4 h CDC protocols, followed by an evaluation step from 100 to 500 °C at a rate of 2 °C min−1. Besides the regular simulated exhaust test, catalyst samples were also treated using the following simulated exhaust test treatment protocol to look into the single Pt atom species fate before and after experiencing the treatment on the Pt/TiO2 NA samples with both fresh state and hydrothermal aged condition. CDC simulated gas treatment: 12% O2 + 6% H2O + 6% CO2 + 100 ppm H2 + 500 ppm CO + 200 ppm NO + 1400 ppm HCs, with a ramp rate of 5 °C min−1 from room temperature to 500 °C, and then keeping at 500 °C for 1 h.
Sulfur-poisoning effects on the catalyst performance were evaluated following USDRIVE’s protocol. The catalyst was exposed to 5 ppm SO2 added to the full simulated exhaust at a space velocity of 30,000 h−1 and 300 °C catalyst inlet temperature for 5 h. A total sulfur exposure level of 1 g sulfur per liter of catalyst was estimated following this exposure condition. Poisoning was conducted following the pretreatment in which the catalyst was annealed at 600 oC for 20 min before cooled down to 300 °C in 12% O2, 6% H2O, 6% CO2, and N2 balance. After sulfur exposure, SO2 was removed from the feed and the sample cooled to 100 °C at which point the catalyst performance was evaluated. For de-sulfation, the catalyst was annealed at 700 °C in 3% H2 and 1% CO in N2 for 30 mins, followed by calcination in air at 500 °C for 2 h.
The Pt/TiO2 nano-array sample with a Pt loading of 0.53 g L−1 and dimensions of Ф 2″ × 3″ were tested under highly transient feed gas conditions mimicking a heavy-duty diesel (HDD) federal test procedure (FTP) as running on a HDD certified 2010 Cummins ISB (6.7 L) 320 hp engine. The characteristic of the transient feed gas is provided in Supplementary Fig. 19. The Pt/TiO2 nano-array and reference samples were aged at 650 °C for 100 h in 10% steam/air flow to a representative end of life condition for a North American HDD application.
Catalyst characterization
The X-ray absorption fine structure data was collected at the BL14W1 station at the Shanghai Synchrotron Radiation Facility (SSRF). The storage ring of SSRF was operated at 3.5 G eV with a maximum current of 260 mA. Pt foil and PtO2 were used as reference samples. Using Si (111) double-crystal monochromator, the data collection was carried out in fluorescence mode for Pt L3-edge with a 7-element detector Ge solid state detector. The energy was calibrated according to the absorption edge of Pt foil (edge energy: 11564 eV).
The EXAFS data was analyzed and fitted using the ATHENA module in the IFEFFIT software packages. Each k2-weighted EXAFS spectrum was processed by a two-step procedure: (i) subtraction of post-edge background from overall absorption; and (ii) normalization with respect to the edge-jump step. The k2-weighted χ(k) data of Pt L3-edge were then Fourier transformed to real (R) space through a hanning windows (dk = 1.0 Å−1) in order to separate the EXAFS contributions from different coordination shells. To determine the quantitative structural parameters around central atoms, the ARTEMIS module of IFEFFIT software packages was employed for least-squares curve parameter fitting.
Coordination number of model samples (Pt foil) was fixed as the nominal value. The obtained S02 of Pt foil was 0.80. It was fixed in the subsequent fitting of Pt L3-edge date. The fitted ranges for k and R spaces were selected to be k = 3–10.3 Å–1 (k2 weighted) with R = 1.0–2.2 Å (Pt1/TiO2 NW) or 1.4–3.2 Å (Pt NP/TiO2 NA).
To conduct the H2 temperature programmed reduction (TPR), the samples were degassed in helium at ambient temperature for 20 min. TPR condition: 10% H2, balance Ar, 50 ml min−1, 10 °C min−1. To determine the surface area and pore size distribution of the catalysts, N2 adsorption–desorption isotherms and H2 chemisorption were carried out using a Micromeritics ASAP 2020 Physisorption analyzer at liquid nitrogen temperature. Brunauer–Emmett– Teller (BET) method and Barret–Joyner–Halenda (BJH) method were used to calculate the specific surface area and pore size, respectively. The samples to be measured were degassed at 350 °C for 4 h in vacuum before the measurement of N2 adsorption–desorption isotherm.
The morphology and structure of catalysts were characterized by electron microscopies. Scanning electron microscopy images were taken using FEI Teneo low vacuum SEM and a JEOL 6335F field emission SEM, operating at 10–20 kV. Transmission electron microscopy (TEM) including both bright field and high angular annual dark field (HAADF) images and energy-dispersive X-ray spectroscopy for composition distribution were taken using a FEI Talos STEM and a Tecnai F30 STEM. The aberration-corrected HAADF STEM images were obtained on two STEM/TEM systems: (1) a JEOL JEM 2200FS, attached with a CEOS (Heidelburg, Germany) probe corrector, with a nominal image resolution of 0.07 nm; and (2) a JEOL JEM-ARM200F, equipped with a CEOS probe corrector, with a guaranteed resolution of 0.08 nm. The chemical states of the TiO2 and Pt/TiO2 nanowire samples were characterized using a Thermo K-Alpha X-ray photoelectron Spectrometer with an Aluminum K-Alpha 1.486 KeV source. X-ray diffraction (XRD) patterns of the samples were collected by a Bruker D2 Phaser using Cu Kα radiation (λ = 0.15418 nm) operated at 40 kV and 40 mA. The XRD data were recorded for 2θ values from 10° to 80° with an interval of 0.02°.
H2 chemisorption was used to determine the platinum dispersion (DPt) at 35 °C on a Micromeritics ASAP 2020 analyzer. H2 reduction treatment of samples was conducted at 300 °C for 2 h, followed by the outgassing at 150 °C for 2 h prior to chemisorption experiment. A double isotherm method was used to determine the H2 uptake: first isotherm revealed the total hydrogen consumed (uptake), (HC)T, while the second one, acquired after evacuation for 2 h showed the “reversible” or weakly adsorbed hydrogen, (HC)r. The amount of “irreversible” or strongly adsorbed hydrogen, (HC)i, was determined by calculating the isotherm difference. The pressure range of isotherms was controlled at 0–12 kPa, where the extrapolation to zero pressure was used to measure gas uptake. The DPt was finally calculated by an atomic (HC)i/Pts = 1 ratio, where Pts implies a Pt atom on surface43.
To evaluate the mechanical stability of TiO2 nano-array integrated cordierite honeycomb samples, a sonication experiment was conducted on both TiO2 and Pt/TiO2 nano-array samples before and after hydrothermal aging at 700 oC for 100 h. The samples were sonicated in water at 40 kHz for 3 h and weighed before and after the sonication. Little morphology change (in SEM) was observed and the weight loss after the sonication process is less than 1 wt. % for both fresh and aged samples, indicating the sound honeycomb structure integrity and the superior adhesion of TiO2 and TiO2/Pt nano-arrays on the honeycomb monolith channel walls.
Density Functional Theory (DFT) calculations were performed as implemented in the Vienna Ab initio simulation package44,45, employing the Perdew, Burke and Ernzerhof exchange-correlation functional46. Models for rutile TiO2 (110) surfaces contain two layers along the y direction followed by a vacuum slab of more than 20 Angstrom. The (110) surface was constructed by repeating the cell of dimension 13.16 Å × 11.88 Å along the x and z directions, respectively. With this model, a k-point mesh of 2 × 1 × 2 and a plane-wave energy cutoff of 400 eV were used. As systems to be modeled involve isolated molecules like CO2 and O2, the long-range van der Waals interactions was included into the calculations using the scheme of Grimme47. Reaction pathways were searched using the nudge-elastic band method by Henkelman and Jónsson48.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Change history
09 March 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Binder, A. J. et al. Oxidation over a ternary oxide catalyst with high resistance to hydrocarbon inhibition. Angew. Chem. 127, 13461–13465 (2015).
Kim, C. H., Qi, G., Dahlberg, K. & Li, W. Strontium-doped Perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 327, 1624–1627 (2010).
Mellor, J. R. et al. The application of supported gold catalysts to automotive pollution abatement. Catal. Today 72, 145–156 (2002).
Li, J., Chang, H., Ma, L., Hao, J. & Yang, R. T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—a review. Catal. Today 175, 147–156 (2011).
Liu, G. & Gao, P.-X. A review of NOx storage/reduction catalysts: mechanism, materials and degradation studies. Catal. Sci. Tech. 1, 552–568 (2011).
Dec, J. E. Advanced compression-ignition engines—understanding the in-cylinder processes. Proc. Comb. Inst. 32, 2727–2742 (2009).
Zhang, Y., Cattrall, R. W., McKelvie, I. D. & Kolev, S. D. Gold, an alternative to platinum group metals in automobile catalytic converters. Gold. Bull. 44, 145 (2011).
Herzing, A. A., Kiely, C. J., Carley, A. F., Landon, P. & Hutchings, G. J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 321, 1331–1335 (2008).
Carley, A. F. et al. CO bond cleavage on supported nano-gold during low temperature oxidation. Phys. Chem. Chem. Phys. 13, 2528–2538 (2011).
Zhu, Z. et al. Highly active and stable Co3O4/ZSM-5 catalyst for propane oxidation: effect of the preparation method. ACS Catal. 3, 1154–1164 (2013).
Xie, X., Li, Y., Liu, Z.-Q., Haruta, M. & Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458, 746–749 (2009).
Zhai, Y. et al. Alkali-stabilized Pt-OHx species catalyze low-temperature water-gas shift reactions. Science 329, 1633–1636 (2010).
Ding, K. et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Yang, M. et al. A common single-site Pt(II)–O(OH)x– species stabilized by sodium on “Active” and “Inert” supports catalyzes the water-gas shift reaction. J. Am. Chem. Soc. 137, 3470–3473 (2015).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Li, X. et al. Single-atom Pt as co-catalyst for enhanced photocatalytic H2 evolution. Adv. Mater. 28, 2427–2431 (2016).
Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335, 1209–1212 (2012).
Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Zhang, Z. et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 8, 16100 (2017).
Liu, J. Catalysis by supported single metal atoms. ACS Catal. 7, 34–59 (2017).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Wei, S. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 13, 856–861 (2018).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
USDRIVE. Aftertreatment Protocols For Catalyst Characterization and Performance Evaluation: Low Temperature Oxidation Catalyst Test Protocol. (2015).
Curran, S. J., Gao, Z. & Wagner, R. M. Reactivity-controlled compression ignition drive cycle emissions and fuel economy estimations using vehicle system simulations. Int. J. Engine Res. 16, 1014–1024 (2015).
Wang, Y.-G., Yoon, Y., Glezakou, V.-A., Li, J. & Rousseau, R. The role of reducible oxide–metal cluster charge transfer in catalytic processes: new insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics. J. Am. Chem. Soc. 135, 10673–10683 (2013).
Yang, Z. et al. Size-dependent CO and propylene oxidation activities of platinum nanoparticles on the monolithic Pt/TiO2-YOx diesel oxidation catalyst under simulative diesel exhaust conditions. Catal. Sci. Tech. 5, 2358–2365 (2015).
Hoang, S. et al. High performance diesel oxidation catalysts using ultra-low Pt loading on titania nanowire array integrated cordierite honeycombs. Catal. Today. 320, 2–10 (2019).
Wu, D. & Zhang, H. Mechanical stability of monolithic catalysts: scattering of washcoat adhesion and failure mechanism of active material. Ind. Eng. Chem. Res. 52, 14713–14721 (2013).
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Baxter, R. J. & Hu, P. Insight into why the Langmuir–Hinshelwood mechanism is generally preferred. J. Chem. Phys. 116, 4379–4381 (2002).
Newton, M. A., Ferri, D., Smolentsev, G., Marchionni, V. & Nachtegaal, M. Room-temperature carbon monoxide oxidation by oxygen over Pt/Al2O3 mediated by reactive platinum carbonates. Nat. Commun. 6, 8675 (2015).
Allian, A. D. et al. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 133, 4498–4517 (2011).
Zammit, M. et al., Future Automotive Aftertreatment Solutions: The 150 °C Challenge Workshop Report. (National Technical Information Service, U.S. Department of Commerce 2012).
Duprat, F. Light-off curve of catalytic reaction and kinetics. Chem. Eng. Sci. 57, 901–911 (2002).
Heck, R. M. & Farrauto, R. J. Automobile exhaust catalysts. Appl. Catal. A: Gen. 221, 443–457 (2001).
Johnson, J. Cleaner vehicles, gas to be required by EPA. Chem. Eng. N. 78, 9 (2000).
Kašpar, J., Fornasiero, P. & Hickey, N. Automotive catalytic converters: current status and some perspectives. Catal. Today 77, 419–449 (2003).
Johannessen, E., Wang, G. & Coppens, M.-O. Optimal Distributor networks in porous catalyst pellets. I. Molecular diffusion. Ind. Eng. Chem. Res. 46, 4245–4256 (2007).
Wang, G. & Coppens, M.-O. Calculation of the optimal macropore size in nanoporous catalysts and its application to DeNOx catalysis. Ind. Eng. Chem. Res. 47, 3847–3855 (2008).
Hoang, S., Guo, S., Hahn, N. T., Bard, A. J. & Mullins, C. B. Visible light driven photoelectrochemical water oxidation on nitrogen-modified TiO2 nanowires. Nano Lett. 12, 26–32 (2012).
Feng, X. et al. Vertically aligned single crystal TiO2 nanowire arrays grown directly on transparent conducting oxide coated glass: synthesis details and applications. Nano Lett. 8, 3781–3786 (2008).
Avila, M. S. et al. Effect of V2O5 loading on propane combustion over Pt/V2O5–Al2O3 Catalysts. Catal. Lett. 134, 118–123 (2010).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio Total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comp. Chem., 27, 1787–1799 (2006).
Henkelman, G., Uberuaga, B. P., Jónsson, H. & Climbing Image, A. Nudged Elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
We are grateful for the financial support from the Scalable Nanomanufacturing Program at the US National Science Foundation under CBET1344792 and the Vehicle Technologies Incubator Program at the US Department of Energy under DE-EE0006854. X.L. is partially supported by a ThermoFisher Scientific Graduate Fellowship. The SEM and partial TEM studies were performed using the facilities in the University of Connecticut ThermoFisher Scientific Center for Advanced Microscopy and Materials Analysis (CAMMA). Computational support from XSEDE is acknowledged through the Grant No. TG-DMR170031. J.L. acknowledges funding by the US National Science Foundation under CHE-1465057 and the use of the John M. Cowley Center for High Resolution Electron Microscopy at Arizona State University. Y.G. acknowledges the financial support from the National Natural Science Foundation of China (No. 21777051). The X-ray absorption fine structure data was collected at BL14W1 station in Shanghai Synchrotron Radiation Facility (SSRF). The authors acknowledge Shuo Zhang at SSRF and Chang-Yong Nam at the Brookhaven National Laboratory for technical support. The authors also thank the technical help and insightful discussion on the TPR experiments from Dr. Jilei Liu and the late Prof. Maria Flytzani-Stephanopoulos at the Tufts University.
Author information
Authors and Affiliations
Contributions
P-X.G. conceived the research. P-X.G. and S.H. designed the experiments and co-wrote the paper. S.H. synthesized and characterized the samples. Y.G. and S.H. performed most of the catalyst evaluation, data collection, and analyses. A.J.B., E.A.K., S.H., X.L., and T.J.T. conducted the bench reactor simulated exhaust testing and in situ DRIFTs, W.T. and X.L. performed temperature programmed testing and physico-chemical characterization, S.W. helped with the probe catalytic reaction testing, J.L. conducted the aberration-corrected STEM characterization. T.D.H. and R.R. performed the density functional theory calculation. Y.W. carried out the XANES and EXAFS measurements. X.L. and Y.D. performed the high resolution TEM and XPS characterization, J.Y. helped with the XANES and EXAFS sample preparation, T.J.P. was responsible for the transient cyclic dynamic testing. All the authors discussed the results and provided input on the paper.
Corresponding author
Ethics declarations
Competing interests
A U.S. non-provisional patent (Application No.: 16/465,981) on catalyst methods of making, with P.-X.G. and S.H. as co-inventors, has been filed by the University of Connecticut.
Additional information
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoang, S., Guo, Y., Binder, A.J. et al. Activating low-temperature diesel oxidation by single-atom Pt on TiO2 nanowire array. Nat Commun 11, 1062 (2020). https://doi.org/10.1038/s41467-020-14816-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-14816-w
This article is cited by
-
Atomic dispersion of bulk/nano metals to atomic-sites catalysts and their application in thermal catalysis
Nano Research (2023)
-
Comprehensive treatment strategy for diesel truck exhaust
Environmental Science and Pollution Research (2023)
-
Selective dissolution to synthesize densely populated Pt single atom catalyst
Nano Research (2023)
-
Sustainable production of hydrogen with high purity from methanol and water at low temperatures
Nature Communications (2022)
-
Advanced TEM Characterization for Single-atom Catalysts: from Ex-situ Towards In-situ
Chemical Research in Chinese Universities (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.