Abstract
The rice orthologue of maize domestication gene Teosinte branched 1 (Tb1) affects tillering. But, unlike maize Tb1 gene, it was not selected during domestication. Here, we report that an OsTb1 duplicate gene (OsTb2) has been artificially selected during upland rice adaptation and that natural variation in OsTb2 is associated with tiller number. Interestingly, transgenic rice overexpressing this gene shows increased rather than decreased tillering, suggesting that OsTb2 gains a regulatory effect opposite to that of OsTb1 following duplication. Functional analyses suggest that the OsTb2 protein positively regulates tillering by interacting with the homologous OsTb1 protein and counteracts the inhibitory effect of OsTb1 on tillering. We further characterize two functional variations within OsTb2 that regulate protein function and gene expression, respectively. These results not only present an example of neo-functionalization that generates an opposite function following duplication but also suggest that the Tb1 homologue has been selected in upland rice.
Similar content being viewed by others
Introduction
Modern civilization is built on a foundation of domesticated crops and animals that have been the main source of calories for humans for more than 10,000 years. Multiple domesticated crops often share similar domestication traits compared to their wild relatives, such as loss of seed shattering and dormancy, increased fruit size, or alterations of plant architecture; this phenomenon is referred to as domestication syndrome. An increase in apical dominance is an important example of domestication syndrome that occurs in many gramineous crops. Domesticated maize, sorghum, rice, wheat, and foxtail millet all show an increase in apical dominance and a corresponding reduction in shoot branching compared to their wild counterparts1. The well-established domestication gene Teosinte branched 1 (Tb1) was originally found to result in increased apical dominance in maize2. QTLs containing Tb1 orthologous loci in sorghum, foxtail millet, wheat and pearl millet were later discovered to account for tiller variation under domestication3,4,5. However, these studies based on QTL analysis do not unequivocally demonstrate whether the underlying causal gene is a Tb1 orthologue or not. In rice, the Tb1 orthologous gene OsTb1, located on chromosome 3, was shown by mutant analysis to impact tiller branching but was suggested to not be related to rice domestication6,7 because this locus was not selected during domestication. A previous whole-genome scan for domestication genes in rice conducted by large-scale genome resequencing also detected no signals for artificial selection around this region8,9. Although it has been realized that the increase in apical dominance constitutes parallel morphological evolution in cereal crops, it remains elusive whether this parallel domestication has a similar genetic basis.
There are two rice subspecies Oryza sativa japonica and indica that exhibit different tillering abilities, with the japonica subspecies tending to have fewer tillers than indica. Rice also has two ecotypes, upland and irrigated ecotypes, which are adapted to rainfed upland conditions and well-watered conditions, respectively. Our previous analysis of upland rice genomes and irrigated rice genomes revealed that another gene, which is a paralogue of OsTb1 located on rice chromosome 9 (hereafter referred to as OsTb2), is highly differentiated between the two ecotypes10. Upland rice varieties are generally japonica and tend to exhibit taller plant architecture, better-developed roots and fewer tillers compared to their irrigated counterpart (Supplementary Fig. 1). It has been known that in upland rice fewer tillers is an adaptive architecture because upland varieties with a small number of tillers tend to have longer deep roots and larger panicles than those with profuse tillers11,12. Given that the two ecotypes have apparently different tillering abilities10, it is tempting to speculate that OsTb2 might regulate rice tillering.
Gene duplication is a major way whereby new genes originate. OsTb2 and OsTb1 are highly homologous and appear to be the two most closely related gene copies in the rice genome that likely diverged from a gene duplication event. After duplication, the new gene copy will be functionally redundant with the old copy in the short term, while in the long term, it can become a pseudogene or may be lost13. Alternatively, in some scenarios, the new copy obtains a new function during evolution, a process referred to as neo-functionalization14,15. It is important to test whether OsTb2 has retained the same function as OsTb1 in repressing tillering. DWARF14 (D14) is a gene involved in strigolactone signalling and negatively regulates rice tillering16. Previous studies showed that OsTb1 represses tillering by interacting with OsMADS57 to promote D14 expression17. Whether OsTb2 plays a similar role in regulating rice tillering remains to be elucidated.
In this study, we present evidence that OsTb2 has evolved a function opposite to that of its paralogue OsTb1. Unlike OsTb1, which is a tillering inhibitor, OsTb2 is a positive regulator of tillering. We show that OsTb2 likely functions by interfering with the inhibitory effect of OsTb1 on tillering. Moreover, we find that a 3 base pair (bp) indel in the coding region of OsTb2 is divergent between the japonica and indica subspecies and that in japonica, the 3 bp insertion enhances the function of OsTb2 in promoting tillering. More interestingly, another functional variation is a T to C mutation that has been selected and fixed in upland rice. By reducing the expression of OsTb2, this derived C allele has likely contributed to the dryland adaptation of upland rice by reducing tillers and increasing grain yield per panicle, generating an upland-adaptive plant architecture that was favoured and selected by humans. Our findings not only provide another vivid example of gene neo-functionalization but also demonstrate that paralogous genes with opposite functions might be selected during domestication and breeding.
Results
OsTb2 is differentiated between upland and irrigated rice
Upland and irrigated rice ecotypes display significant differentiation in tillering ability. In our previous comparative genomic study10, we found that Os09g0410500 on chromosome 9, a homologue of the maize Tb1 gene, was highly differentiated between the two ecotypes. The rice orthologue of the maize Tb1 gene, OsTb1, is located on chromosome 3, showing the highest homology to maize Tb1 among the rice genes (See Methods). We therefore referred to the Tb1 homologue on chromosome 9 as OsTb2. FST and XP-CLR were used to assess the artificial selection signature around OsTb2 (40 kb upstream to 40 kb downstream) (see Methods). Both FST and XP-CLR displayed a peak signal around OsTb2, and the empirical P-values of both tests are below 5‰ (Fig. 1), indicating OsTb2 was probably selected during the differentiation of upland japonica and irrigated japonica rice.
Peak signals (red arrows) were found by FST (a) and XP-CLR (b) assessment, respectively. The artificial selection signals were detected based on the FST value (a) and the cross-population composite likelihood ratio test (XP-CLR, b). The FST value and XP-CLR score were calculated window by window (see Methods), and we then choose the windows with genome-wide top 5‰ values as candidate regions with selection signals. The dotted horizontal lines indicate the threshold of genome-wide top 5‰ value.
Since high population differentiation of a gene region can be caused by multiple other factors such as allele surfing, hierarchical population structure etc.18, we further checked the allele frequency spectra of SNPs from the vicinity of the putative causal mutations (see the next section for details about the putative causal mutations). We observed a U-shape pattern of the derived allele frequency spectra (AFS) in upland japonica, and the pattern decayed with the increasing distance from the causal mutations (Supplementary Fig. 2), which is a signal of the hitch-hiking effect19,20. We further performed a nonparametric Kolmogorov-Smirnov test to show that with the increasing distance from the focal mutation, the U-shape AFS pattern of SNPs in the sliding windows also decays and become similar to the background AFS pattern (Supplementary Fig. 3), supporting the hitch-hiking event. Moreover, we used the Hudson-Kreitman-Aguadé (HKA) test to screen for genome-wide recently selected genes (see Methods), and OsTb2 was found to be among the 301 selected genes (HKA test P-value = 0.019). Performing genome scan using the SweeD program also uncovered a significant likelihood value (ranking top 1.6%) in upland japonica, but an insignificant likelihood value (ranking top 17.3%) in irrigated japonica (Supplementary Fig. 4). These multiple lines of evidences strongly support that OsTb2 was under selection during the cultivation of upland japonica rice. As shown by our previous phylogenetic analysis10, upland japonica evolved from irrigated japonica. The artificial selection signature therefore suggested that OsTb2 might have been selected during evolution from irrigated rice to upland rice. Considering that the OsTb1 homologues, found in maize and other crops, have been reported to account for the change in apical dominance and that upland rice accessions actually have a significantly lower tillering ability than irrigated accessions10, we were interested in determining whether OsTb2 also impacts tiller number in rice and whether it was selected during the improvement of upland rice.
OsTb2 is associated with rice tillering ability
To identify the polymorphic sites of OsTb2, we sequenced this gene in 84 upland and 82 irrigated accessions (Supplementary Data 1) using Sanger sequencing. In total, seven SNPs and two indels were identified (Fig. 2a). To investigate the association between OsTb2 and tillering ability, we grew 132 of the above sequenced accessions and collected phenotypic data on their tiller numbers at 40 and 50 days after germination (DAG). We then tested the association between the SNPs/indels and tiller number phenotypes. As shown in Table 1, among the nine polymorphic sites, only Indel I and SNP3 were significantly associated with tiller number at both 40 DAG and 50 DAG. Moreover, these associations presented the smallest P-values among all the variants, indicating that Indel I and/or SNP3 is likely to be the functional variant(s) (Table 1). The two variants produce three haplotypes (Fig. 2b). Considering that hitch-hiking variants tightly linked with causal variants also have the potential to be associated with phenotypes, it remains to be determined whether only one or both variants are functional.
a Structure and polymorphic sites of OsTb2. Two indels and seven SNPs were found in the OsTb2 gene. Indel I and SNP3 (bold with asterisk) are both significantly associated with tiller numbers. b Indel I and/or SNP3 in the OsTb2 gene may be functional variants for which three haplotypes were observed in the germplasm. The 3bp-/T haplotype is present in indica and wild rice, and thus likely to be the ancestral haplotype. The 3bp+/T haplotype is mainly found in irrigated japonica, and the 3bp+/C haplotype is specific to upland japonica, consistent with the evolutionary viewpoint that upland japonica evolved from irrigated japonica.
Association analysis can sometimes yield a false-positive result due to population structure21. Thus, we further tested the association between OsTb2 alleles and tiller number in segregating populations. We used an F8 recombinant inbred line (RIL) population obtained by crossing the upland variety IRAT104 and the irrigated variety IR64, which segregate for both the Indel I and SNP3 markers. We genotyped the 134 lines in the F8 RIL populations using derived cleaved amplified polymorphic sequence (dCAPS) markers22 (see Methods) and grew those lines in irrigated and upland conditions to observe their phenotypes. Severely distorted segregation phenomena were observed for both Indel I and SNP3 loci. For the Indel I locus, 32 lines had a 3bp+ (3 bp insertion) genotype, while 96 lines had a 3bp− (3 bp deletion) genotype (the other six lines were heterozygous). When tiller numbers were compared between the Indel I-3bp+ lines and Indel I-3bp− lines, we observed a significant increase in tillers for the Indel I-3bp+ lines compared to lines with the Indel I-3bp− genotype (Student’s t-test, P = 0.039; Table 2). For the SNP3 site, the RIL-F8 population was so skewed towards SNP3-T that we found only four lines with SNP3-C, making it difficult to statistically test its association with tiller number. However, we found one individual, RIL116, that was heterozygous for both Indel I and SNP3. Therefore, we selfed this individual to produce a near-isogenic F2 population segregating for both Indel I and SNP3.
The derived near-isogenic F2 population included 451 individuals, all of which were grown to be phenotyped and genotyped. In this near-isogenic F2 population, distorted segregation was also observed for SNP3. Among the 451 individuals, we identified 135 individuals with the SNP3-T genotype, but only 60 individuals with the SNP3-C genotype, and the rest were heterozygous. For Indel I, 60 homologous 3bp− individuals and 75 homologous 3bp+ individuals were genotyped. All of these individuals were phenotyped twice (40 and 50 DAG). A conditional association study was then conducted to examine the association between SNP3/Indel I and tiller number. The results showed that the SNP3-C genotype had significantly fewer tillers than did the SNP3-T genotype under the Indel I-3bp+ condition (Student’s t-test, P = 6.15E-05 at 50 DAG; Table 3), while Indel I-3bp+ plants had significantly more tillers than Indel I-3bp− plants (Student’s t-test, P = 0.0193 at 50 DAG; Table 3). This result further confirmed that the derived Indel I-3bp + allele in japonica corresponded to an increase in tiller number, while the derived SNP3-C allele in upland japonica was associated with a reduced number of tillers. The findings that at 40 DAG, the SNP3 locus, but not the Indel I locus, was marginally significantly associated with tiller number (Student’s t-test, P = 0.1191; Table 3) and that the Indel I locus became significant only at 50 DAG suggested that, consistent with what we observed in the natural population association study, SNP3 might exhibit a function around the early tillering stage (40 DAG) and that Indel I probably affects tillering around the late tillering stage (50 DAG).
OsTb2 3bp+ can increase rice tiller number
We examined OsTb2 expression patterns in different tissues at 40 DAG and 50 DAG stages using qRT-PCR. The results showed that OsTb2 was predominantly expressed in the basal tiller node with a relatively lower expression in leaf blade and sheath. Higher expression of OsTb2 in the basal tiller node at 40 DAG than 50 DAG indicated that OsTb2 starts to function from the early stage of tillering (Supplementary Fig. 5). To validate the function of OsTb2, we cloned the gene sequences of IRAT104 (OsTb23bp+) and IR64 (OsTb23bp−) into the overexpression vector pCUBI-1390, driven by the Ubiquitin promoter, which was then transformed into Nipponbare. Multiple positive transgenic lines were obtained by hygromycin B screening. Gene expression was greatly increased in the OsTb23bp+-OE1~6 and OsTb23bp−-OE1~6 lines compared to the control lines, as shown in Supplementary Fig. 6a. Two transgenic lines for each genotype as well as control lines (both negative lines and WT) were then planted in dryland and irrigated environments in two growth seasons for phenotypic examination. Accordingly, it was found that the transgenic lines overexpressing OsTb23bp+ had significantly more tillers than the control lines (Fig. 3 and Supplementary Fig. 6b, c), supporting the hypothesis that unlike its homologue Tb1, which is a tiller suppressor, OsTb2 is a tiller enhancer. The transgenic lines overexpressing OsTb23bp− had slightly fewer (but not significantly) tillers, implying that the OsTb23bp− genotype had a limited effect on tiller number.
a Phenotypes of transgenic plants overexpressing two genotypes (3 bp+ and 3bp-) of OsTb2 at 30 DAG under irrigated conditions. WT, wild type. b Phenotypes of transgenic plants overexpressing two genotypes of OsTb2 at 65 DAG under irrigated conditions. WT, wild type. c Tiller number of transgenic plants overexpressing both OsTb23bp+ and OsTb23bp− at different DAG under irrigated conditions. d Tiller numbers of transgenic plants overexpressing both OsTb23bp+ and OsTb23bp− at different DAT under dryland conditions. Each value in c, d represents the mean ± s.d. (n = 50 plants). Student’s t-test analysis indicated a significant difference (compared with the WT control, *P < 0.05, **P < 0.01).
Irrigated japonica often has fewer tillers than irrigated indica rice likely due to their different genetic composition. Our transgenic experiments showed that the japonica-specific OsTb23bp+ could partially increase tiller number (Fig. 3b, d) and thus could alleviate tiller suppression by other genes in the japonica background. This allele might have been fixed in japonica because it could alleviate tiller suppression or due to random genetic drift.
Indel I alters OsTb2’s regulatory effects on D14 expression
We then wondered how the Indel I mutation might alter the function of OsTb2 in affecting tiller number. Considering that Indel I-3bp+ causes a single amino acid insertion in the TCP binding domain of this OsTb2 transcription factor and that in silico prediction hinted that this insertion might have changed the peptide secondary structure (Supplementary Fig. 7), we hypothesized that I-3bp+ may have altered the function of OsTb2 by altering its structure.
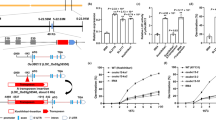
As our data showed that OsTb23bp+ had a function (i.e., promoting tillering) antagonistic to the Tb1 orthologue OsTb1, we next asked whether OsTb2 influenced gene expression in an opposite manner. To determine whether the 3 bp insertion affected the function of the OsTb2 transcription factor, we carried out a transient expression assay using a luciferase reporter system. D14 expression could promote apical dominance and reduce tillers. We found that the extent of D14::LUC expression was reduced by cotransformation with OsTb2 (Fig. 4a). The transient expression assays showed that both OsTb23bp+ and OsTb23bp− indeed impacted the expression of D14, and OsTb23bp+ exerted a significantly greater inhibitory effect than OsTb23bp− (Fig. 4a). It was previously shown that OsTb1 represses tillering by increasing the expression of D14. Therefore, it is likely that OsTb2 represses D14 by counteracting the positive regulation of D14 transcription by OsTb1. The yeast one-hybrid (Y1H) assay verified that OsTb2 could not bind to D14 promoter directly (Fig. 4c), which implied that OsTb2 reduced expression in other ways. To determine how OsTb2 repressed D14 expression, transient expression assays were further used to test whether OsTb2 plays a role in repressing D14 via the OsTb1-OsMADS57 pathway. When OsTb2 was coexpressed with OsTb1 and OsMADS57, the expression of the cotransformed reporter gene D14pro::LUC indicated that OsTb2 may neutralize the inhibition of OsTb1 on OsMADS57, which directly binds the D14 promoter to inhibit its transcription (Fig. 4b, d). The results also showed that the two Indel I genotypes resulted in significant differences in D14 expression (Student’s t-test, POsTb2 = 0.0218, Fig. 4a): the 3bp+ genotype corresponded to a lower level of D14, which was consistent with the 3bp+ genotype yielding more tillers. Therefore, we concluded that OsTb2 reduces the expression of D14, which then consequently increase tiller number. The 3 bp insertion that occurred in japonica promoted the repression of D14 by OsTb2, thus representing a genotype yielding an increased tiller number.
a Effects of OsTb23bp+ and OsTb23bp− on the transcriptional regulation of D14 in rice protoplasts. b Effects of OsTb23bp+ on the transcriptional regulation of D14 via the OsTb1-OsMADS57-D14 pathway in Nicotiana benthamiana. OsGRF4 is used as a negative control for detecting the effects of OsTb2 on D14pro::LUC activity. Each relative luciferase activity of D14pro::LUC value in a and b represents the mean ± s.d. (n = 10 biologically independent samples). Student’s t-test analysis indicated a significant difference (*P < 0.05, **P < 0.01). c In the yeast one-hybrid assay, OsTb2 fusion proteins fail to activate D14pro::LacZ reporter gene expression in yeast. The right diagrammatic drawing indicates the yeast strains transformed with related plasmids. d A schematic model depicts that OsTb2 interacts with OsTb1 to regulate the expression of D14. OsMADS57 directly represses the expression of D14; OsTb2 interacts with OsTb1 to alleviate OsTb1’s inhibition on OsMADS57, which consequently reduces the expression of D14 and increases tillers.
OsTb2 binds to OsTb1 and offset OsTb1’s tiller suppression
TCP genes encode plant-specific transcription factors with a bHLH motif that allows DNA binding and protein–protein interactions, forming homodimers or heterodimers23,24. Therefore, we asked whether OsTb1 interacts with OsTb2 in planta. BiFC assays indicated that the interaction between OsTb2 and OsTb1 occurred in the Nicotiana benthamiana nucleus (Fig. 5), which was consistent with the nuclear subcellular localization of OsTb2 (Supplementary Fig. 8). Therefore, OsTb2 was able to form a heterodimer with OsTb1. CoIP tests revealed that the protein complexes pulled down using anti-α-GFP agarose were recognized by an anti-α-MYC antibody in lines cotransformed with GFP-OsTb2 and MYC-OsTb1 (Fig. 5); i.e., OsTb2 could bind to OsTb1 in planta. It was previously found that OsTb1 represses tillering by increasing the expression of D1417. Therefore, OsTb2 probably represses D14 expression by counteracting the positive regulatory effect of OsTb1 on D14, ultimately increasing tiller number.
a YFPN-OsTb1 and CFPC-OsTb2 were expressed in a pairwise manner in rice protoplasts and exhibited a direct interaction in the nucleus, in which AHL22-mRFP was used as the nuclear marker protein; CFPC-GUS and YFPN-GUS fusion proteins were used as negative controls and were coexpressed with YFPN-OsTb1 and CFPC-OsTb2, respectively, in rice protoplasts. DIC indicates differential interference contrast transmission; the merged image is also shown; scale bar, 20 µm. b Coimmunoprecipitation assays of OsTb1 and OsTb2. Protein extracts from rice protoplasts harbouring MYC-OsTb1 and GFP-OsTb2 were coimmunoprecipitated by anti-GFP beads and detected by anti-GFP and anti-MYC antibodies. Protoplasts transformed with single MYC-OsTb1 or GFP-OsTb2 were used as a negative control. The source data underlying Fig. 5b are provided as a Source Data file.
SNP3 C-allele confers reduced OsTb2 expression and tillers
Since SNP3 causes a mutation in the 3’UTR of the OsTb2 transcript, it is likely that this SNP might alter the expression level of this gene. To determine whether the SNP3 in 3’UTR contribute transcription regulation of OsTb2, we grew 39 rice lines with different SNP3 genotypes and checked the expression of OsTb2 in the tillering node at 40 DAG and 50 DAG (Supplementary Data 2). C-type cultivars consistently showed significantly lower OsTb2 expression at 50 DAG under both dryland (soil water content, 16.8%) and irrigated conditions (Fig. 6a), suggesting that C-allele of SNP3 reduce the expression of OsTb2 (Fig. 6a). Consistently, C-type lines produce significantly fewer tillers at both 40 DAG and 50 DAG than T-type lines under both irrigated and dryland conditions (Fig. 6b). Pearson correlation analysis showed that the expression level of OsTb2 is significantly positively correlated with tiller number at 50 DAG under dryland (Fig. 6c, Student’s t-test, P = 0.003; R = 0.48) and irrigated (Fig. 6d, Student’s t-test, P = 0.044; R = 0.35) conditions. Fewer tillers in upland rice represent an adaptive trait because it increases the root/shoot ratio25,26 and results in longer deep roots as well as larger panicles11,12. To examine whether the C-allele of OsTb2 selected in upland rice brings about higher grain yield per panicle. We compare the grain yield per panicle data collected in the past three growth seasons between C-type and T-type lines. The results consistently showed that C-type allele is significantly associated with larger yield per panicle in all three growth season under dryland condition (Fig. 6e). We also conducted Pearson Correlation Analysis for the expression of OsTb2 and yield data (see Methods). We found that OsTb2 expression is negatively correlated with yield per panicle (Fig. 6f; Student’s t-test, P = 0.08, R = 0.29), consistent with our findings that the lower OsTb2 expression confers by the C-allele results in higher yield per panicle under dryland condition. We also tried to examine the correlation between SNP3 and gene expression using our RILs by growing 12 lines in irrigated and dryland conditions in the 2nd season of 2017. However, unfortunately, the dryland came across an extreme drought (soil water content 8.1% at 50 DAG) in that season. As shown in Supplementary Figure 9, we observed the C-allele corresponds to lower gene expression in irrigated conditions, consistent with what we observe in Fig. 6. But unexpectedly, we saw a rapid induction of the C-allele in the extreme drought (Supplementary Fig. 9). We speculated that the extreme drought might have triggered another feedback pathway to compensate the over-suppression of tillering (Supplementary Fig. 9b). The detailed mechanism for this OsTb2 induction under extreme drought remains to be elucidated by future studies.
a For the 39 cultivars, C-type cultivars have lower OsTb2 expression than the T-type at 40 DAG and 50 DAG under both irrigated and dryland conditions. b C-type is associated with fewer tillers under irrigated and dryland conditions. The expression of OsTb2 is significantly positively correlated with tiller numbers in the 39 cultivars at 50 DAG under dryland (c) and irrigated (d) conditions. e C-type cultivars have significant higher yield per panicle than T-type cultivars in three growth seasons under dryland conditions. f The expression of OsTb2 is negatively correlated with grain yield per panicle in the 39 cultivars in the 3rd season under dryland condition. ‘R’ refers to Pearson product-moment correlation coefficient; ‘P’ is P-value. Student’s t-test analysis indicated a significant difference (*P < 0.05, **P < 0.01). Each dot in a, b and e represents the mean value of about 8 plants and n > 15 cultivars for statistics.
Discussion
Plants modify their development to adapt to the environment, protecting themselves from detrimental conditions by triggering a variety of signalling pathways27. Axillary buds are indeterminate structures that can be developmentally controlled in response to endogenous or environmental cues28. OsTb2 from our study is identical to the RETARDED PALEA1 gene reported in a previous study, which showed that this gene plays a role in palea development and floral zygomorphy in rice29. In the present study, we comprehensively analysed the function of OsTb2 and showed that OsTb2 modulates the development of axillary buds and was artificially selected during the adaptation of upland rice. Our transgenic experiments and association analysis supported the hypothesis that contrary to OsTb1, OsTb2 suppresses apical dominance and counteracts tillering inhibition by OsTb1, leading to an increased tiller number. We found two functional mutations in OsTb2, i.e. the 3bp indel-I that distinguishes japonica and indica subspecies and the SNP3 that differentiates upland japonica and irrigated rice (including irrigated japonica and indica).
In general, japonica rice exhibits fewer tillers than indica rice, and our data showed that the japonica-specific OsTb23bp+ genotype could increase the number of tillers, while the indica-species OsTb23bp− genotype could not. This mutation was probably fixed in japonica rice because it could alleviate tiller suppression by other genes in the japonica background or due to random genetic drift. We’ve shown using transient expression assays that OsTb23bp+ and OsTb23bp− alleles encode proteins with different activities on D14 expression likely by affecting OsTb2 protein activity (Fig. 4a). Also, using in silico prediction, we showed that this indel variant would alter the protein secondary structures of OsTb2, which likely affects its function (Supplementary Fig. 7).
The upland rice ecotype evolved from irrigated japonica rice10 and adapted to rainfed upland conditions. It has long been well established that in upland rice fewer tillers is an adaptive trait. For example, Fukai et al. screened 1081 rice lines and found that well-adapted upland rice tends to have a small number of well-developed tillers. These lines developed a few large tillers with longer roots11, while the lines with profuse tillers tend to have shorter roots and their tillers were not well developed under upland conditions. Also, Kato et al. examined the rice lines adapted to aerobic dryland soils and found that plant architecture with a few large tillers is a more suitable architecture than that with profuse tillers12. Consistently, we found that the upland-specific SNP3-C allele has been fixed in upland rice and is associated with tiller reduction in both natural and segregating populations (Tables 1–3). Compared with SNP3-T allele, the SNP3-C allele is associated with lower expression of OsTb23bp+ under both rainfed upland and irrigated conditions (Fig. 6). Our transgenic data and field experiments based on inbred rice lines both showed that the expression of OsTb2 positively regulates tiller number, as opposed to the function of OsTb1. So, we concluded that the C-type SNP in the 3’-UTR of OsTb2 is a causal mutation that confers the adaptive fewer tillers in upland rice and was fixed by artificial selection during dryland adaptation.
It should be mentioned that in a growth season in 2017 where our upland field experienced an extreme drought, we observed an unexpected induction of OsTb2 expression associated with the C-type allele (Supplementary Fig. 9), contrary to what was observed in the irrigated field in that same growth season as well as what we observe in both irrigated and upland fields in this growth season of 2019, we reasoned that it was because the drought stress was so extreme that another feedback pathway might be triggered to compensate the over-suppression of tillering. The detailed mechanism of how C-allele was dramatically induced under extreme drought remains to be elucidated by future studies; this could represent another unknown regulatory pathway. Interestingly, in our association analyses in segregating populations (Tables 2, 3), we repeatedly observed the significant association between SNP3 and tiller number from 40 DAG, but the association between indel-I and tiller numbers only got significant from 50 DAG. The detailed mechanism for this fact is not clear, but given that OsTb2 has a much higher expression in 40 DAG than in 50 DAG (supplementary Fig. 5), it is very likely that in 40 DAG the effect of gene expression variation caused by SNP3 masks the effect of protein activity difference caused by Indel-I.
So far there have been quite a few genes known to control the tiller formation in rice, such as MOC130,31,32, SPL genes33,34, miR15635, LB4D36, DWARF10 37, D1416, D5338, RCN8/939, and OsTb140. Some of these genes have strong effects on rice tillering. However, to our knowledge, OsTb2 is the only gene regulating tiller number in rice determined to have been subjected to artificial selection. The reason that OsTb2, rather than other genes, was selected during upland rice adaptation remains elusive. It might have been a chance occurrence. Alternatively, artificial selection may have preferred to act on Tb1 homologues, given that they have contributed to morphological evolution across different cereal crops. This situation may have arisen because this family of genes can be easily modulated for phenotypic evolution without a considerable detriment to other agronomic traits. This question remains to be further explored. It should be mentioned that, as a regulatory gene, the effects of OsTb2 on tillering seems to be moderate or even minor in some conditions, because the effect sizes of the two variants (Indel 1 and SNP3) range from 0.47 to 2.77 in the segregating populations (Table 2, Table 3, Supplementary Table 1) and the phenotypes of the transgenic lines are significant but not dramatic (Fig. 3). But selection genes do not have to be large-effect genes. For example, a recent work reported that a minor-effect gene controlling seed dormancy was parallel selected in the domestication of soybean, rice and tomato41. The large-effect genes, such as MOC1, might cause too severe phenotypes that are not suitable for agricultural production, and consequently are not favored by human selection.
Strigolactone (SLs) signalling and biosynthesis are involved in the regulation of branching in plants42,43. The D14 gene functions in the MAX/RMS/D pathway of SL biosynthesis16,42. It was previously reported that OsTb1 regulates tiller development in rice by modulating D14 expression indirectly17; we now report that OsTb2 can interact with OsTb1 and may regulate D14 expression indirectly by counteracting OsTb1. Consequently, OsTb2 may be involved in balancing the D14-mediated SL signalling pathway. Recent studies indicate that TCPs in Oryza sativa (rice), Sorghum bicolor, and Arabidopsis thaliana act downstream of the auxin and MORE AUXILIARY GROWTH (MAX) pathways44,45,46,40. Additional studies are needed to understand whether the regulation of tiller number by OsTb2 is also associated with auxin pathways.
Evolutionary novelties often originate from gene duplication. In this study, we found that OsTb2, as a duplicate gene of OsTb1, does not function as a tiller inhibitor but evolved a function opposite that of OsTb1, adding an example to the classical concept of neo-functionalization15,47,48,49,50. There was actually a similar report that detailed the interaction between two isoforms of an important BRANCHED1 (BRC1) transcription factor in potato51. In that case, the regular long form inhibits lateral branching, similar to BRC1 in other species, but a modified protein that originates from alternative BRC1 splicing inhibits the long form and promotes lateral branching51. In our study, two Tb1 homologues, OsTb1 and OsTb2, were shown to have antagonistic effects on rice tiller number, similar to the model of the regulation of lateral branching in potato by BRC1 isoforms and the regulation of flowering time in beets that is controlled by the interplay of two paralogs of the Arabidopsis FLOWERING LOCUS T (FT) gene with antagonistic functions52.
While the Mayans had a lucky break discovering plants with the Tb1 transposon51, we are now on the cusp of understanding TCP genes and plant branching. In this context, there is the prospect that regulating OsTb2 or other Tb1 homologues will lead to superior outcomes in the adaptation and breeding of rice and other cereal crops.
Methods
Plant materials and phenotyping
The 84 upland and 82 irrigated accessions included in this study were collected from different regions worldwide (Supplementary Data 1). One 134 F8 recombinant inbred lines (RILs) were generated from F2 plants that were obtained by crossing the upland variety IRAT104 and the irrigated variety IR64. We identified RIL116, which was heterozygous for both Indel I and SNP3, and selfed this individual to produce a near-isogenic F2 population segregating for both Indel I and SNP3. The derived near-isogenic F2 population included 451 individuals, all of which were grown to be phenotyped and genotyped.
Phenotyping was performed in both irrigated and dryland conditions (i.e., preventing soil submergence in water to simulate a rainfed upland environment) for three growth seasons at Xishuangbanna, Yunnan province (1st season refers to the second season of 2015; 2nd season refers to the second season of 2017; 3rd season refers to the first season of 2019). For the irrigated condition, seeds were germinated in a seedbed, and seedlings were then transplanted to a paddy field, where water was ponded on the soil surface throughout the growth and developmental period. For the rainfed upland condition, we conducted direct seeding by dibbling seeds in dry soil. To fully simulate rainfed conditions, no irrigation was applied in the upland condition. When rain came, we drained any excess water to prevent soil submergence. For each accession, we planted three replicates and each replicate have 12 individuals in two rows (6 individuals in each row), with a row spacing of 30 centimetres and a plant spacing of 20 centimetres. For each line, approximately eight individuals were randomly selected and phenotyped. The tiller numbers of the accessions and RILs were surveyed at 40 and 50 DAG, and yield per panicle of the accessions were investigated. The soil water content of dryland was measured by soil moisture meters (TZS-W, Zhejiang Top Instrument Co.Ltd) at 40 and 50 DAG.
Identification of OsTb2 using a population genetic approach
OsTb2 was reported from our previous work10. We performed a whole-genome scan for genes with the top FST and XP-CLR signals. We first determined the allele frequencies of the SNP alleles in the upland and irrigated japonica populations (Supplementary Data 1) using the resequencing data reported in our previous work10. Then based on the allele frequencies, we calculated the FST value between upland and irrigated populations using the method described by Nei53. In the genome scan, we used 20-kb sliding windows with 2-kb sliding step. The FST value for each window was obtained by averaging the FST values over SNP sites in that window. To calculate the XP-CLR score, we used the software XP-CLR54 and allele frequencies from upland and irrigated populations. A window size of 0.1 cM, a 2-kb grid size and a maximum SNP number of 150 for each window was used. OsTb2 was found to be located in regions with the top 5‰ FST and XP-CLR signals between upland japonica and irrigated japonica accessions, which have significantly different tillering abilities10. When running the BLAST program against the rice genome using the maize Tb1 gene sequence, the orthologue OsTb1 has the highest identity and the paralogue OsTb2 has the second highest identity. To further substantiate OsTb2 is a paralogue, we downloaded maize and rice genes in this family from the Panther gene family database and use the MUSCLE software to infer the phylogenetic relationship among these genes (Supplementary Fig. 10)55. We also used the MCscan software to do synteny analysis (Supplementary Fig. 11)56. Our results supported that OsTb2 is a paralogue rather than an orthologue of the maize Tb1 gene.
Evolutionary analyses detecting OsTb2 as under selection
For allele frequency spectrum (AFS) analysis, we resequenced the upland and irrigated japonica accessions at higher depth of about 15× for more accurate allele frequency estimation. Using the SNP information around the OsTb2 gene region (from 80 kb downstream to 80 kb upstream) and indica rice as outgroup, we generated the derived AFSs for SNPs from windows, which are 10 kb, 20 kb, …, and 80 kb away from the putative causal mutations of the OsTb2 gene for both upland and irrigated rice populations and then checked if the AFSs display a U-shape pattern, a signal of the hitch-hiking effect. The raw reads that map this gene region can be provided upon request. We further used a nonparametric test (Kolmogorov-Smirnov test) to examine if the U-shape pattern decays with the increasing distance from the focal mutation.
We applied the Hudson-Kreitman-Aguadé (HKA)57 and population branch statistic (PBS)58 to identify candidate genes having recently reached fixation. Three populations (irrigated japonica, upland japonica and indica) were used to calculate pairwise FST values of SNPs. For all the 44,643 genes, mean FST were generated using SNPs only located in coding regions. Then a classical transformation by Cavalli-Sforza Tpop1−2 = −log (1 – FST) was obtained to estimate the divergence time T between Population1 (Pop1) and Population2 (Pop2) in units scaled by population size. The length of population branch can be obtained by Eq. 1:
Then we recorded the SNPs number (A) of each population and the number (B) of fixed SNPs (the sites with FST > 0.9 for the population compared with both two other populations), performed the HKA test by comparing the ratio of A/B to the genome-wide average and testing the null hypothesis A/B(gene) = A/B(genome-wide) using a Pearson’s Chi-square test on the 2 × 2 contingency table. Finally, genes with PBS value ranking genome-wide top 5% and a significant nominal P-value (<0.05) for the HKA test were considered as sweeps candidates. ORF evidence and notes were extracted from rice annotation database.
SweeD59 was used for detecting selective sweeps in the upland and irrigated japonica populations with the following settings (−folded −grid 40000). And the regions with top 5% composite likelihood ratio statistic60 were identified as having significant selection signatures.
Identification of variations around OsTb2 and genotyping
DNA fragments around the OsTb2 gene were amplified from the 130 accessions by tb2-up-f/tb2-up-r and tb2-f/tb2-r primers (Supplementary Table 2), and subjected to Sanger sequencing. Seven SNPs and two indels were identified by alignment with MEGA software.
Based on the sequence around SNP3 and Indel I, we designed dCAPS for the genotypes of these loci. For SNP3, a 124 bp fragment was amplified via PCR by tb2-SNP3-f/tb2-SNP3-r primers (Supplementary Table 2) and then cut using the restriction enzyme Bsl I. Two bands (99 bp and 25 bp) were observed in the gel for to the C-genotype, while the T-genotype could not be digested. For Indel I, 200 bp PCR products were obtained with (tb2-indel-gate-f/tb2-indel-r) primers (Supplementary Table 2) and then digested with the restriction enzyme Bsl I. Two bands (130 bp and 70 bp) were observed in the gel for to 3bp+ genotype, while 3bp- type could not be cut.
Association analysis and conditional association analysis
Association analysis was used to test the association between SNP3, Indel I and tiller number. The genotypes of the alleles of 130 accessions were determined using Sanger sequencing, and the genotypes of the RILs of NILs were determined using dCAPS markers (Supplementary Table 2). The accessions were then classified into three different genotypes (two homozygotes and one heterozygote). Student’s t-test was subsequently performed to compare the tillers between the two homozygous groups. Conditional analysis: A total of 52 accessions with the T-genotype for the SNP3 site, but different Indel I genotypes were used to test the association between the Indel I genotypes and phenotypes; 50 accessions with the 3bp+ genotype for Indel I, but different genotypes for the SNP3 locus were used to assess the association between SNP3 genotypes and tiller numbers.
Quantification of gene expression using real-time PCR
We conducted quantitative PCR to survey the expression level of OsTb2 in different genotypes including 39 cultivars (Supplementary Data 2) and 12 lines from 134 RILs according 3bp+/C and 3bp+/T genotype. Total RNA was extracted from the tiller node tissues of the plant materials at 40 DAG or 50 DAG. After digesting the RNA samples with DNase I (Fermentas), we performed reverse transcription with the Fermentas K1632 Revert Aid H minus First-Strand cDNA kit. We used SYBR-Green Supermix (Bio-Rad) to conduct real-time PCR and analysed the samples in the ABI 7000 Sequence Detection System. β-actin (actin-f/actin-r) was used as an internal control. The OsTb2-specific qPCR primers for the transcript included tb2-qPCR-f and tb2-qPCR-r (Supplementary Table 2).
Vector construction and genetic transformation
The coding region of OsTb2 was amplified from rice (IRAT104 and IR64 cultivars, which were the parents of the RIL population used to identify this gene) cDNA by PCR using Kpn I and Bam H I linker primers (Supplementary Table 2). The resulting OsTb2 fragment was inserted into the Kpn I and Bam H I sites of pCUbi139061, generating Ubipro::OsTb2. All the vectors were introduced into Agrobacterium tumefaciens strain EHA105 and then transferred into Nipponbare plants via Agrobacterium-mediated callus transformation62. Phenotyping of the T2 transgenic lines was performed using the above methods at 25 DAG to 73 DAG.
Subcellular localization of GFP-OsTb2 fusion proteins
The open reading frames (ORFs) of OsTb2 were inserted into pMDC43 as C-terminal fusions with the green fluorescent protein (GFP) reporter gene driven by the CaMV 35 s promoter63. These constructs were transformed into the leaves of 3-week-old tobacco (Nicotiana benthamiana) by A. tumefaciens infiltration64. DAPI staining was used to identify the nucleus. The resulting green fluorescence of protoplasts expressing GFP-OsTb2 was observed using a confocal laser-scanning microscope (LSM700, Zeiss, Jena, Germany).
Bimolecular fluorescence complementation assay
Complementary DNAs of OsTb2 and OsTb1 were cloned into the bimolecular fluorescence complementation (BiFC) vectors pnYFP-X and pcCFP-X, respectively, with GUS also cloned as a negative control. The constructs were cotransformed into Nicotiana benthamiana protoplasts for transient expression. Protoplast isolation from tobacco leaf tissues and PEG-mediated transformation were performed according to Bart et al.65. Cells were incubated at 28 °C in the dark overnight. A confocal laser-scanning microscope (LSM700, Zeiss, Jena, Germany) was used to observe the green fluorescence of protoplasts. The 35S::GFP construct and AHL22 were used as a control and a nuclear marker protein, respectively66.
Protein coimmunoprecipitation assay
The recombinant constructs GFP-OsTb2 and MYC-OsTb1 were introduced into rice protoplasts, and protein extracts were prepared as described by He67. The protein extracts were precipitated with anti-GFP agarose beads (CMC Scientific, http://www.cmcscientific.com) overnight. Then, proteins bound to the beads were resolved by SDS-PAGE and detected by Western blotting using anti-GFP (dilution at 1:1000; ab1218, abcam), anti-MYC (dilution at 1:1000; ab264433, abcam) primary antibodies (MBL, http://www.mblintl.com/) and HRP-labelled goat anti-mouse secondary antibody (dilution at 1:5000; ab97023, abcam).
Transient expression assays in rice protoplasts
For the D14 promoter repression assay, two forms of OsTb2 were used in the system. The full-length OsTb2 cDNAs were fused into the pRTVcMyc vector, driven by the 35 s promoter, to generate pRTVcMyc-OsTb23bp+ and pRTVcMyc-OsTb23bp−. To generate the D14pro::LUC reporter gene, the D14 promoter (D14pro) was amplified. The plasmid carrying the GUS gene under the control of the 35 s promoter was used as a normalization control. The presented values represent the means ± s.d. of six technical replicates. Cotransformation of the D14pro::LUC reporter and pRTVcMyc-OsTb23bp+ or pRTVcMyc-OsTb23bp− was performed according to He et al.67 to identify the effect of OsTb2 in the transient assay. The Renilla luciferase reporter gene (REN) under the control of CaMV35S promoter was used as an internal control to normalize the data for eliminating variations in the experiment.
Dual-luciferase assays in tobacco leaves
The effector plasmids pMDC43-OsTb2, pMDC43-OsTb1, and pMDC43-OsMADS57 were cloned as described above. The reporter plasmid pGreen-D14pro-LUC encodes two luciferases, firefly luciferase controlled by the D14 promoter and the Renilla luciferase controlled by the constitutive 35 s promoter. The D14pro, fused to the minimum 35 s promoter, was PCR amplified from the 35 s template and cloned into the Hind III/Bam HI sites of the vector pGreen-0800-LUC. pGreen-D14pro-LUC was transformed into Agrobacterium (strain EHA105) carrying the helper plasmid pSoup-P19, which also encodes a repressor of co-suppression68. The Agrobacterium strain containing both the reporter pGreen-D14pro-LUC and the helper pSoup-P19 was used either alone or mixed with the Agrobacterium strain containing the effector plasmids pMDC43-OsTb2, pMDC43-OsTb1, and pMDC43-OsMADS57, as shown in Fig. 4b. pMDC43-OsGRF4 was used as a negative control effector. Overnight cultures of Agrobacterium were collected by centrifugation resuspended, and infiltrated as described above62. After 3 days, using commercial Dual-LUC reaction (DLR) reagents according to the manufacturer’s instructions (Promega) leaf samples were collected for the Dual-LUC assay. Specifically, we excised leaf discs from the site (ca. 1-2 cm in diameter) of Agrobacterium infection, ground using liquid nitrogen, and homogenized using 100 μl of Passive Lysis buffer (Promega). Then, we mixed 20 μl of the crude extract with 100 μl of Luciferase Assay buffer (Promega), and examined the firefly luciferase activity (LUC) using a luminometer (BG-1, GEM Biomedical Inc). After the measurement of firefly luciferase activity, 100 μl of Stop and Glow buffer (Promega) was added to quench the firefly luciferase and initiate the Renilla luciferase reaction.
Yeast one-hybrid assay using the pLacZi2u/pB42AD system
We followed the previous reported procedure69. Briefly, the coding sequence of OsTb2 was inserted into the MCS of pB42AD to generate an AD-fusion construct (pB42AD-OsTb2), and D14pro was inserted into the MCS of the pLacZi2μ reporter plasmid (pLacZi2μ-D14pro). The pB42AD-OsTb2 plasmid was cotransformed with pLacZi2μ-D14pro, including the LacZ reporter gene driven by a D14pro fragment, for testing in EGY48 yeast strain. Transformants were grown on SD (galactose+raffinose)/-Ura/-Trp/X-gal plates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A reporting summary for this Article is available as a Supplementary Information file. The datasets generated and analyzed during the current study are available from the corresponding author upon request. DNA-seq data were deposited in in the National Center for Biotechnology Information (NCBI) under the SRA accession number PRJNA595072. The source data underlying Fig. 5b as well as Tables 1, 2, and 3 are provided as a Source Data file.
References
Dixon, L. E. et al. TEOSINTE BRANCHED1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum). Plant Cell 30, 563–581 (2018).
Doebley, J., Stec, A. & Hubbard, L. The evolution of apical dominance in maize. Nature 386, 485–488 (1997).
Doust, A. N. & Kellogg, E. A. Effect of genotype and environment on branching in weedy green millet (Setaria viridis) and domesticated foxtail millet (Setaria italica) (Poaceae). Mol. Ecol. 15, 1335–1349 (2006).
Hart, G. E., Schertz, K. F., Peng, Y. & Syed, N. H. Genetic mapping of Sorghum bicolor (L.) Moench QTLs that control variation in tillering and other morphological characters. Theor. Appl. Genet. 103, 1232–1242 (2001).
Poncet, V. et al. Genetic control of domestication traits in pearl millet (Pennisetum glaucum L., Poaceae). Theor. Appl. Genet. 100, 147–159 (2000).
Doust, A. Architectural evolution and its implications for domestication in grasses. Ann. Bot. 100, 941–950 (2007).
Goto, Y. et al. Tillering behavior of the rice fine culm 1 mutant. Plant Prod. Sci. 8, 68–70 (2005).
Chepyshko, H., Lai, C. P., Huang, L. M., Liu, J. H. & Shaw, J. F. Multifunctionality and diversity of GDSL esterase/lipase gene family in rice (Oryza sativa L. japonica) genome: new insights from bioinformatics analysis. BMC genomics 13, 309 (2012).
Xu, X. et al. Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat. Biotech. 30, 105–111 (2012).
Lyu, J. et al. A genomic perspective on the important genetic mechanisms of upland adaptation of rice. BMC Plant Biol. 14, 160 (2014).
Fukai, S. & Cooper, M. Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Res 40, 67–86 (1995).
Kato, Y. & Katsura, K. Rice adaptation to aerobic soils: physiological considerations and implications for agronomy. Plant Prod. Sci. 17, 1–12 (2014).
Reams, A. B. & Roth, J. R. Mechanisms of gene duplication and amplification. Csh. Perspect. Biol. 7, a016592 (2015).
Hughes, T. E., Langdale, J. A. & Kelly, S. The impact of widespread regulatory neofunctionalization on homeolog gene evolution following whole-genome duplication in maize. Genome Res. 24, 1348–1355 (2014).
Moore, R. C. & Purugganan, M. D. The evolutionary dynamics of plant duplicate genes. Curr. Opin. Plant Biol. 8, 122–128 (2005).
Arite, T. et al. D14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 50, 1416–1424 (2009).
Guo, S. et al. The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat. Commun. 4, 1566 (2013).
de Villemereuil, P. D. & Gaggiotti, O. E. A new FST-based method to uncover local adaptation using environmental variables. Methods Ecol. Evol. 6, 1248–1258 (2015).
Kimura, M. & Ohta, T. Distribution of allelic frequencies in a finite population under stepwise production of neutral alleles. Proc. Natl Acad. Sci. USA 72, 2761–2764 (1975).
Fay, J. C. & Wu, C. I. Hitchhiking under positive Darwinian selection. Genetics 155, 1405–1413 (2000).
Clayton, D. et al. Population structure, differential bias and genomic control in a large-scale, case-control association study. Nat. Genet. 37, 1243–1246 (2005).
Michaels, S. D. & Amasino, R. M. A robust method for detecting single-nucleotide changes as polymorphic markers by PCR. Plant J. 14, 381–385 (1998).
Manassero, N. G., Viola, I. L., Welchen, E. & Gonzalez, D. H. TCP transcription factors: architectures of plant form. Biomol. Concept 4, 111–127 (2013).
Martin-Trillo, M. & Cubas, P. TCP genes: a family snapshot ten years later. Trends Plant Sci. 15, 31–39 (2010).
Asch, F., Dingkuhn, M., Sow, A. & Audebert, A. Drought-induced changes in rooting patterns and assimilate partitioning between root and shoot in upland rice. Field Crop Res. 93, 223–236 (2005).
Mašková, T. & Herben, T. Root: shoot ratio in developing seedlings: how seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol. Evol. 8, 7143–7150 (2018).
Chen, L. P. et al. OsMADS57 together with OsTB1 coordinates transcription of its target OsWRKY94 and D14 to switch its organogenesis to defense for cold adaptation in rice. N. Phytol. 218, 219–231 (2018).
Janssen, B. J., Drummond, R. S. & Snowden, K. C. Regulation of axillary shoot development. Curr. Opin. Plant Biol. 17, 28–35 (2014).
Yuan, Z. et al. RETARDED PALEA1 controls palea development and floral zygomorphy in rice. Plant Physiol. 149, 235–244 (2009).
Li, X. Y. et al. Control of tillering in rice. Nature 422, 618–621 (2003).
Lin, Q. B. et al. Rice APC/CTE controls tillering by mediating the degradation of MONOCULM 1. Nat. Commun. 3, 752 (2012).
Liao, Z. G. et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 10, 2738 (2019).
Jiao, Y. Q. et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42, 541–544 (2010).
Miura, K. et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 42, 545–549 (2010).
Liu, Q. et al. The alteration in the architecture of a T-DNA insertion rice mutant osmtd1 is caused by up-regulation of MicroRNA156f. J. Integr. Plant Biol. 57, 819–829 (2015).
Liang, F. et al. Genetic analysis and fine mapping of a novel semidominant dwarfing gene LB4D in rice. J. Integr. Plant Biol. 53, 312–323 (2011).
Zhang, S. Y. et al. The interactions among DWARF10, auxin and cytokinin underlie lateral bud outgrowth in rice. J. Integr. Plant Biol. 52, 626–638 (2010).
Wei, L. R., Xu, J. C., Li, X. B., Qian, Q. & Zhu, L. H. Genetic analysis and mapping of the dominant dwarfing gene D-53 in rice. J. Integr. Plant Biol. 48, 447–452 (2006).
Jiang, H., Guo, L. B., Xue, D. W., Zeng, D. L. & Qian, Q. Genetic analysis and gene-mapping of two reduced-culm-number mutants in rice. J. Integr. Plant Biol. 48, 341–347 (2006).
Takeda, T. et al. The OsTB1 gene negatively regulates lateral branching in rice. Plant J. 33, 513–520 (2003).
Wang, M. et al. Parallel selection on a dormancy gene during domestication of crops from multiple families. Nat. Genet. 50, 1435–1441 (2018).
Zhou, F. et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504, 406–410 (2013).
Ongaro, V. & Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 59, 67–74 (2008).
Aguilarmartínez, J. A., Pozacarrión, C. & Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell. 19, 458–472 (2007).
Finlayson, S. A. Arabidopsis Teosinte Branched1-like 1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant Cell Physiol. 48, 667–677 (2007).
Kebrom, T. H. & Finlayson, S. A. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol. 140, 1109 (2006).
Ohno, S. Evolution by Gene Duplication. (Springer Berlin, Heidelberg, 1970).
Freeling, M. & Volff, J. N. The evolutionary position of subfunctionalization, downgraded. Genome Dyn. 4, 25–40 (2008).
Lynch, M. & Conery, J. S. The evolutionary fate and consequences of duplicate genes. Science 290, 1151–1155 (2000).
Mcgrath, C. L. & Lynch, M. Evolutionary Significance of Whole-Genome Duplication. (Springer Berlin, Heidelberg, 2012).
Brewer, P. B. Plant architecture: the long and the short of branching in potato. Curr. Biol. 25, 724–725 (2015).
Pin, P. A. et al. An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 330, 1397–1399 (2010).
Marks, J. in Molecular evolutionary genetics (ed. M. Nei) (Columbia University Press. 1987).
Chen, H., Patterson, N. & Reich, D. Population differentiation as a test for selective sweeps. Genome Res. 20, 393–402 (2010).
Madeira, F. et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47, 636–641 (2019).
Wang, Y. P. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, 49 (2012).
Hudson, R. R., Kreitman, M. & Aguadé, M. A test of neutral molecular evolution based on nucleotide data. Genetics 116, 153–159 (1987).
Yi, X. et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329, 75–78 (2010).
Pavlos, P., Daniel, I., Alexandros, S. & Nikolaos, A. SweeD: likelihood-based detection of selective sweeps in thousands of genomes. Mol. Biol. Evol. 30, 2224–2234 (2013).
Nielsen, R. et al. Genomic scans for selective sweeps using SNP data. Genome Res. 15, 1566–1575 (2005).
Peng, H. et al. A putative leucine-rich repeat receptor kinase, OsBRR1, is involved in rice blast resistance. Planta 230, 377–385 (2009).
Duan, Y. et al. An efficient and high-throughput protocol for Agrobacterium-mediated transformation based on phosphomannose isomerase positive selection in Japonica rice (Oryza sativa L.). Plant Cell Rep. 31, 1611–1624 (2012).
Curtis, M. D. & Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003).
Sparkes, I. A. & Al, E. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1, 2019–2025 (2006).
Bart, R., Chern, M., Park, C. J., Bartley, L. & Ronald, P. C. A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods 2, 13 (2006).
Xiao, C., Chen, F., Yu, X., Lin, C. & Fu, Y. F. Over-expression of an AT-hook gene, AHL22, delays flowering and inhibits the elongation of the hypocotyl in Arabidopsis thaliana. Plant Mol. Biol. 71, 39–50 (2009).
He, F., Chen, S., Ning, Y. & Wang, G. L. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Curr. Protoc. Plant Biol. 1, 373–383 (2016).
Hellens, R. P., Edwards, E. A., Leyland, N. R., Bean, S. & Mullineaux, P. M. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42, 819–832 (2000).
Lin, R. C. et al. Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318, 1302–1305 (2007).
Acknowledgements
We thank Prof. Kaixiong Ye of the University of Georgia and Prof. Kun Wang of the Northwestern Polytechnical University for their advice on data analyses. This work was supported by grants from the National Natural Science Foundation of China (U1602266 and 31601274), the ‘973’ project of China (No. 2013CB835200), and grants from the Yunnan Provincial Science and Technology Department (2018IC096, 2019ZG013, and 2019HC028).
Author information
Authors and Affiliations
Contributions
F.Y.H., J.L. and W.W. conceived the project. J.L., L.Y.H. and Y.S.Z. designed and performed the experiments. Shila.Z., G.F.H., J.Z., YCB, M.N. and Q.F. performed the genotyping and phenotyping analysis. W.M.H., Shila.Z., P.Z., Y.Z., Shile.Z. and H.C. performed the data analysis. J.L. and L.Y.H. wrote the manuscript, with L.J.W. revising the draft for submission and guiding the responses to referees and correction of the final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Shin-Han Shiu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lyu, J., Huang, L., Zhang, S. et al. Neo-functionalization of a Teosinte branched 1 homologue mediates adaptations of upland rice. Nat Commun 11, 725 (2020). https://doi.org/10.1038/s41467-019-14264-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-14264-1
This article is cited by
-
Transcriptome analysis and functional validation reveal the novel role of LhCYCL in axillary bud development in hybrid Liriodendron
Plant Molecular Biology (2024)
-
Genetic and functional mechanisms of yield-related genes in rice
Acta Physiologiae Plantarum (2024)
-
The genetic architecture of prolificacy in maize revealed by association mapping and bulk segregant analysis
Theoretical and Applied Genetics (2023)
-
The TCP transcription factor HvTB2 heterodimerizes with VRS5 and controls spike architecture in barley
Plant Reproduction (2022)
-
Broadening the horizon of crop research: a decade of advancements in plant molecular genetics to divulge phenotype governing genes
Planta (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.