Abstract
Axially chiral molecules are among the most valuable substrates in organic synthesis. They are typically used as chiral ligands or catalysts in asymmetric reactions. Recent progress for the construction of these chiral molecules is mainly focused on the transition-metal-catalyzed transformations. Here, we report the enantioselective NHC-catalyzed (NHC: N-heterocyclic carbenes) atroposelective annulation of cyclic 1,3-diones with ynals. In the presence of NHC precatalyst, base, Lewis acid and oxidant, a catalytic C–C bond formation occurs, providing axially chiral α-pyrone−aryls in moderate to good yields and with high enantioselectivities. Control experiments indicated that alkynyl acyl azoliums, acting as active intermediates, are employed to atroposelectively assemble chiral biaryls and such a methodology may be creatively applied to other useful NHC-catalyzed asymmetric transformations.
Similar content being viewed by others
Introduction
Axial chirality, a key stereogenic element, is widely observed in natural products1,2,3 and often determines the pharmacological properties in biologically active molecules (e.g., Maxi-K channel openers, (R)-Streptonigrin; Fig. 1)4. Among them, axially chiral biaryls are recognized as one of fundamental entities of chiral ligands, catalysts, and other useful reagents5. Over the past few decades, numerous efforts have been devoted to constructing these axially chiral biaryls, but successful examples are relatively scarce in contrast to their great potential in various applications6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25. In 1984, Meyers and coworkers reported the first example of central-to-axial chirality conversion in biarylic systems26. Later on, the direct asymmetric cross-coupling of two aryls has proven to be a feasible method27,28,29,30,31,32,33. However, the poor enantiocontrol and low coupling efficiency greatly limit their applications. More recently, an elegant route to synthesize axially chiral biaryls was demonstrated via an aromatic ring formation34,35. Despite these advances, this field is still in its infancy and efficient synthetic routes still need to be identified.
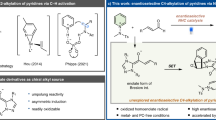
Representative molecules and synthetic protocols. a Two representative axially chiral molecules. b NHC-catalyzed transformations via the use of unsaturated acyl azolium intermediate. c Our synthetic proposal via [3+3] atroposelective annulation. NHCs react with ynals to generate chiral alkynyl acyl azolium intermediates to further react with cyclic 1,3-diones
Chiral N-heterocyclic carbenes (NHCs) as versatile catalysts have been well studied in last few decades36,37,38,39,40,41,42,43, but most of the reports are only focused on the assembly of central chirality. Herein, we report a highly enantioselective NHC-catalyzed [3+3] atroposelective annulation of ynals with cyclic 1,3-diones44, thus paving a route toward axially chiral biaryls. It is noteworthy that the NHC-bounded alkynyl acyl azoliums as active intermediates are generated from ynals in contrast to unsaturated acyl azoliums (Fig. 1) made from ynals via an internal redox reaction, which have been intensively investigated in organic reactions, such as esterification, Claisen rearrangement, cycloaddition, etc45,46,47,48,49,50,51,52,53,54,55. Our mechanistic studies have completely ruled out the route, involving the formation of unsaturated acyl azolium followed by a central-to-axial chiral conversion.
Results
Reaction optimization
We began our study with the model reaction of 5,5-dimethylcyclohexane-1,3-dione (1a) and 3-(2-methoxynaphthalen-1-yl)propiolaldehyde (2a). Key results are briefly summarized in Table 1. Using nBu4NOAc as the base, Mg(OTf)2 as the additive56,57, E as the oxidant, and toluene as the solvent, a number of chiral NHC catalysts A−D58,59,60,61,62 were initially screened. No desired product was detected in the presence of widely used NHC catalysts B and C. Pleasingly, chiral triazolium NHC precatalyst with N-2,4,6-(Br)3C6H2 substituent (Table 1, D) provided axially chiral 3aa with a moderate er, but albeit in a low yield (Table 1, entry 5). Along with the formation of 3aa, byproducts of 4aa, 5aa, and 6aa, which resulted from different unexpected intermediates and reaction pathways, were produced simultaneously. Given the significance of reaction conditions to the success of a focused catalytic transformation, we carried out a comprehensive optimization of reaction parameters. As outlined in Table 1, addition of 1a and 2a to a mixture of catalyst A (15 mol%), oxidant E (1.5 equiv.), and nBu4NOAc (2.0 equiv.) with Mg(OTf)2 (20 mol%), provided 3aa in 70% yield and 91:9 er (Table 1, entry 1).
Substrate scope
With the most efficient catalytic conditions in hand, we next examined the substrate scope (Fig. 2). The R substituent of cyclic 1,3-dione 1 was investigated firstly. Substrates equipped with cyclic substituents (e.g., four- and six-membered rings) on cyclic 1,3-dione scaffold gave the corresponding products 3ba and 3ca in good yields but only with moderate er. In addition, reactions for cyclic 1,3-dione substrates bearing alkyl chains in different length proceeded smoothly under standard reaction conditions (3da−fa). While substrate cyclic 1,3-dione (2g) bearing a long alkyl chain was used, a good yield and high er value were achieved (Fig. 2, 3ga, 70% yield and 95:5 er).
To address the stability of the products, we conducted several experiments and the related results verified that the rotation barrier of the chiral axis was high enough to prevent the racemization of product 3gh during the reaction or its purification: with ΔG≠rot = 119.7 KJ mol−1 at 85 °C, the half-life of rotation is 7.41 h at 85 °C (Fig. 3; for details, see Supplementary Discussion).
Determination of the enantiomerization barrier. Reaction conditions: 3 mg of enantio-enriched 3gh were refluxed in 15 mL of toluene at 85 °C. Samples of 7 µL of this solution were injected on Chiralpak IC (heptane/iPrOH = 80/20, 1 mL min−1, UV detection at 254 nm) to monitor the percentage decrease of the second eluted enantiomer over time
Further investigation on the scope of ynals was conducted (Fig. 4). The steric and electronic effects on the aromatic ring of ynals were evaluated by the variation of substituent patterns. When examined substrates bear electron-withdrawing or electron-donating groups at 3-, 4-, 6-, 7-, or 8-substituted positions on naphthalene rings, moderate to good yields and high er values were regularly obtained (3gd−gr). When a substituted phenyl ring replaced the naphthalene ring in ynals, high er could still be achieved (3gs and 3gt). The absolute configuration of 3au was determined to be (S) by X-ray crystallography, and other products were assigned by analogy.
To demonstrate the utility of above synthesized products, we successfully converted 8 into commonly used axially biaryls 10. As shown in Fig. 5, Diels–Alder reaction of 8 and 9 afforded the corresponding axially chiral naphthyl–phenyl products 10 in acceptable yields and no racemization was observed.
Mechanistic studies
The origins of chemo- and stereo-selectivity of this reaction are rationalized by the postulated mechanism illustrated in Fig. 6 (Path A). The addition of NHC catalyst to ynal 2 yields an NHC-bounded Breslow intermediate I63,64. Breslow intermediate I then undergoes oxidation to generate the firstly proposed intermediate, alkynyl acyl azolium II, which subsequently reacts with cyclic 1,3-dione 1 to form intermediate III. III undergoes Michael addition to the alkynyl azolium moeity to form the allenolate intermediate and after subsequent proton transfer from the 1,3-dione to the allene, intermediate IV is reached. Next O–C bond is formed to create V and the NHC can be released and finally generated product 3. As the generation of NHC-bounded unsaturated acyl azolium intermediates from ynals has been reported by Zeitler45, Lupton46,47, Bode48,49,50,51, Scheidt52, and others53,54,55, an alternative pathway may involve the direct annulation of NHC-bounded unsaturated acyl azolium intermediate VI with cyclic 1,3-dione 1 leading to byproduct 4. However, as highlighted in Fig. 7 (Eq. (1)), the oxidative dehydrogenation of 4aa to 3aa does not proceed in the presence of oxidant alone or under standard reaction conditions. As such, 3aa cannot be generated from the α,β-unsaturated acyl azolium intermediate.
Control experiments. (1) 4aa failed to undergo oxidation to form 3aa in the presence of DQ. (2) The absolute configuration of 3au was determined to be (S) by X-ray crystallography. (3) Under standard conditions, the reaction of 1g with 2s yielded 3gs and 7. However, we found that 7 was not directly generated from 3gs under currrent conditions
During the process of optimization, byproduct 5 was found clearly and confirmed by NMR spectra, presumably generated through the Knoevenagel condensation of 3 with 1.0 equivalent of 1. To examine this hypothesis, a controlled experiment was carried out (Fig. 7, Eq. (3)). Surprisingly, the er value of 7 is not consistent with the er value of 3gs (59:41 er vs. 96:4 er) and this observation indicates that an alternative pathway may be operating (Fig. 6, Path C). Building upon intermediate IV, we suggest that the Knoevenagel condensation process generates intermediate VI which subsequently leads to 5 via annulation. Moreover, there is an interesting observation found during the optimization of reaction conditions with Lewis acids (Table 1, entry 6). When Mg(OTf)2 is omitted from the reaction condition, the yield of byproduct 6 increases to 18%, which can be explained by the fact that 1 can now do a direct ‘O’ attack to the alkynyl on intermediate II, because the Mg2+ ion is not there to coordinate 1 and II. Therefore, Mg2+ plays a critical role as it reduces the ketoenolate’s ‘O’ attack (transition state VIII, Path D) and promotes the ‘C’ attack (intermediate III, Path A, Fig. 6).
Preliminary computational studies were conducted on steps III to V in Path A assuming an acetate ligand on the magnesium ion to provide insights into the observed enantioselectivity. It was found that the energies of all transition states from III to the allenalate are higher than those of the rest of processes and we thus hypothesize that the enentioselectivity is determined in this intramolecular C–C bond forming reaction. Interestingly, in contrast to other studies on the α,β-unsaturated acyl azolium analogs, this step creats two components of axial chirality, namely the allenolate and the 2-methoxynaphthalen-1-yl moiety, in addition to one chiral center of the 1,3-dione. The twisted alkynyl acyl azolium plane allows the ketoenolate group to stay away from the indane ring (Fig. 8), whose role is to discriminate the strain energy during the formation of the allenolate center rather than the intuitive effect to block the approach of the nucleophile.
Discussion
In summary, we have successfully developed an NHC-catalyzed atroposelective annulation of cyclic 1,3-diones with ynals, providing chiral α-pyrone-aryls in moderate to good yields with high enantioselectivities. This protocol features good functional group tolerance, and allows the rapid assembly of axially chiral molecules from simple and readily available starting materials under mild conditions. Our computational investigation suggests that the enantioselectivity is determined during the Michael addition of the ketoenolate to the alkynyl azolium moiety. Further investigations on axially chiral compounds as hits in medicinal chemistry or as chiral ligands or catalysts in asymmetric synthesis, as well as a detailed mechanistic study, are currently underway in our laboratories.
Methods
Synthesis of 3
In a glovebox, a flame-dried Schlenk reaction tube equipped with a magnetic stir bar, NHC precatalyst A (9.2 mg, 0.015 mmol), nBu4NOAc (60.2 mg, 0.20 mmol), oxidant DQ (62.0 mg), cyclic 1,3-dione 1 (0.11 mmol), ynal 2 (0.10 mmol), and freshly distilled toluene (2.0 mL) were added. The reaction mixture was stirred at room temperature for 24 h. The mixture was then filtered through a pad of Celite washed with DCM. After the solvent was evaporated, the residue was purified by flash column chromatography to afford the desired product 3.
Computational details
All structures and energies were computed using the Gaussian 09 program package version D.0165. The B3LYP functional together with the 6-31g(d,p) basis set was used. All structures were optimized to a minimum confirmed by frequency calculations and all transition state structures were confirmed by identifying one imaginary frequency and intrinsic reaction coordinate (IRC) analysis.
Data availability
For 1H, 13C NMR, and high-performance liquid chromatography spectra of the compounds in this manuscript, see Supplementary Figs. 1–167. For the details of the synthetic procedures, see Supplementary Methods. The supplementary crystallographic data for this paper could be obtained free of charge from The Cambridge Crystallographic Data Centre (3au: CCDC 1501039) via https://www.ccdc.cam.ac.uk/
References
Bringmann, G., Gìnther, C., Ochse, M., Schupp, O. & Tasler, S. in Progress in the Chemistry of Organic Natural Products, Vol. 82 (eds. Herz, W., Falk, H., Kirby, G. W., Moore, R. E.) 1–129 (Springer, Berlin, 1998).
Kozlowski, M. C., Morgan, B. J. & Linton, E. C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 38, 3193–3207 (2009).
Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 111, 563–639 (2011).
LaPlante, S. R., Edwards, P. J., Fader, L. D., Kakalian, A. & Hucke, O. Revealing atropisomer axial chirality in drug discovery. ChemMedChem. 6, 505–513 (2011).
Noyori, R. & Takaya, H. BINAP: an efficient chiral element for asymmetric catalysis. Acc. Chem. Res. 23, 345–350 (1990).
Bringmann, G. et al. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 44, 5384–5427 (2005).
Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 44, 3418–3430 (2015).
Bringmann, G., Walter, R. & Weirich, R. in Methods of Organic Chemistry (Houben Weyl) 4th edn, Vol. E21a (eds Helmchen, G., Hoffmann, R. W., Mulzer, J. & Schaumann, E.) 567 (Thieme, Stuttgart, Germany, 1995).
Bringmann, G., Walter, R. & Weirich, R. The directed synthesis of biaryl compounds: modern concepts and strategies. Angew. Chem. Int. Ed. 29, 977–991 (1990).
Lipshutz, B. H., Kayser, F. & Liu, Z.-P. Asymmetric synthesis of biaryls by intramolecular oxidative couplings of cyanocuprate intermediates. Angew. Chem. Int. Ed. 33, 1842–1844 (1994).
Nishii, Y., Wakasugi, K., Koga, K. & Tanabe, Y. Chirality exchange from sp3 central chirality to axial chirality: benzannulation of optically active diaryl-2,2-dichlorocyclopropylmethanols to axially chiral α-arylnaphthalenes. J. Am. Chem. Soc. 126, 5358–5359 (2004).
Vorogushin, A. V., Wulff, W. D. & Hansen, H.-J. Stereoselectivity of the benzannulation reaction: efficient central-to-axial chirality transfer. J. Am. Chem. Soc. 124, 6512–6513 (2002).
Guo, F., Konkol, L. C. & Thomson, R. J. Enantioselective synthesis of biphenols from 1,4-diketones by traceless central-to-axial chirality exchange. J. Am. Chem. Soc. 133, 18–20 (2011).
Li, G.-Q. et al. Organocatalytic aryl–aryl bond formation: an atroposelective [3,3]-rearrangement approach to BINAM derivatives. J. Am. Chem. Soc. 135, 7414–7417 (2013).
Link, A. & Sparr, C. Organocatalytic atroposelective aldol condensation: synthesis of axially chiral biaryls by arene formation. Angew. Chem. Int. Ed. 53, 5458–5461 (2014).
Chen, Y.-H. et al. Atroposelective synthesis of axially chiral biaryldiols via organocatalytic arylation of 2-naphthols. J. Am. Chem. Soc. 137, 15062–15065 (2015).
Brandes, S., Bella, M., Kjarsgaard, A. & Jøgensen, K. A. Chirally aminated 2-naphthols—organocatalytic synthesis of non-biaryl atropisomers by asymmetric Friedel–Crafts amination. Angew. Chem. Int. Ed. 45, 1147–1151 (2006).
Shirakawa, S., Wu, X. & Maruoka, K. Catalytic asymmetric synthesis of axially chiral O-Iodoanilides by phase-transfer catalyzed alkylations. J. Am. Chem. Soc. 134, 916–919 (2012).
Shirakawa, S., Liu, S. & Maruoka, K. Kinetic resolution of axially chiral 2-amino-1,1′-biaryls by phase-transfer-catalyzed N-allylation. Angew. Chem. Int. Ed. 52, 14200–14203 (2013).
Gao, H. et al. Practical organocatalytic synthesis of functionalized non-C2-symmetrical atropisomeric biaryls. Angew. Chem. Int. Ed. 55, 566–571 (2016).
De, C. K., Pesciaioli, F. & List, B. Catalytic asymmetric benzidine rearrangement. Angew. Chem. Int. Ed. 52, 9293–9295 (2013).
Ma, G., Deng, J. & Sibi, M. P. Fluxionally chiral DMAP catalysts: kinetic resolution of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 53, 11818–11821 (2014).
Yu, C., Huang, H., Zhang, Y. & Wang, W. Dynamic kinetic resolution of biaryl lactones via a chiral bifunctional amine thiourea-catalyzed highly atropo-enantioselective transesterification. J. Am. Chem. Soc. 138, 6956–6958 (2016).
Zhang, J.-W. et al. Discovery and enantiocontrol of axially chiral urazoles via organocatalytic tyrosine click reaction. Nat. Commun. 7, 10677 (2016).
Zheng, S.-C. et al. Organocatalytic atroposelective synthesis of axially chiral styrenes. Nat. Commun. 8, 15238 (2017).
Meyers, A. & Wettlaufer, D. The complete intramolecular transfer of a central chiral element to an axial chiral element. Oxid. (S)-4-naphthyldihydroquinolines to (S)-4-naphthylquinolines. J. Am. Chem. Soc. 106, 1135–1136 (1984).
Yin, J. & Buchwald, S. L. A catalytic asymmetric suzuki coupling for the synthesis of axially chiral biaryl compounds. J. Am. Chem. Soc. 122, 12051–12052 (2000).
Hayashi, T., Hayashizaki, K., Kiyoi, T. & Ito, Y. Asymmetric synthesis catalyzed by chiral ferrocenylphosphine-transition-metal complexes. 6. Practical asymmetric synthesis of 1,1’-binaphthyls via asymmetric cross-coupling with a chiral [(alkoxyalkyl)ferrocenyl]monophosphine/nickel catalyst. J. Am. Chem. Soc. 110, 8153–8156 (1988).
Saito, S., Kano, T., Muto, H., Nakadai, H. & Yamamoto, H. Asymmetric coupling of phenols with arylleads. J. Am. Chem. Soc. 121, 8943–8944 (1999).
Uozumi, Y., Matsuura, Y., Arakawa, T. & Yamada, Y. M. A. Asymmetric Suzuki–Miyaura coupling in water with a chiral palladium catalyst supported on an amphiphilic resin. Angew. Chem. Int. Ed. 48, 2708–2710 (2009).
Shen, X., Jones, G. O., Watson, D. A., Bhayana, B. & Buchwald, S. L. Enantioselective synthesis of axially chiral biaryls by the Pd-catalyzed Suzuki−Miyaura reaction: substrate scope and quantum mechanical investigations. J. Am. Chem. Soc. 132, 11278–11287 (2010).
Yamamoto, T., Akai, Y., Nagata, Y. & Suginome, M. Highly enantioselective synthesis of axially chiral biarylphosphonates: asymmetric Suzuki–Miyaura coupling using high-molecular-weight, helically chiral polyquinoxaline-based phosphines. Angew. Chem. Int. Ed. 50, 8844–8847 (2011).
Xu, G., Fu, W., Liu, G., Senanayake, C. H. & Tang, W. Efficient syntheses of korupensamines A, B and michellamine B by asymmetric Suzuki-Miyaura coupling reactions. J. Am. Chem. Soc. 136, 570–573 (2014).
Gutnov, A. et al. Cobalt(I)-catalyzed asymmetric [2+2+2] cycloaddition of alkynes and nitriles: synthesis of enantiomerically enriched atropoisomers of 2-arylpyridines. Angew. Chem. Int. Ed. 43, 3795–3797 (2004).
Shibata, T., Fujimoto, T., Yokota, K. & Takagi, K. Iridium complex-catalyzed highly enantio- and diastereoselective [2+2+2] cycloaddition for the synthesis of axially chiral teraryl compounds. J. Am. Chem. Soc. 126, 8382–8383 (2004).
Enders, D. & Balensiefer, T. Nucleophilic carbenes in asymmetric organocatalysis. Acc. Chem. Res. 37, 534–541 (2004).
Phillips, E. M., Chan, A. & Scheidt, K. A. Discovering new reactions with N-heterocyclic carbene catalysis. Aldrichimica Acta 42, 55–66 (2009).
Moore, J. L. & Rovis, T. Carbene catalysts. Top. Curr. Chem. 291, 77–144 (2009).
Bugaut, X. & Glorius, F. N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 41, 3511–3522 (2012).
Ryan, S. J., Candish, L. & Lupton, D. W. Acyl anion free n-heterocyclic carbene organocatalysis. Chem. Soc. Rev. 42, 4906–4917 (2013).
Mahatthananchai, J. & Bode, J. W. On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums. Acc. Chem. Res. 47, 696–707 (2014).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Flanigan, D. M., Romanov-Michailidis, F., White, N. A. & Rovis, T. Organocatalytic reactions enabled by N‑heterocyclic carbenes. Chem. Rev. 15, 9307–9387 (2015).
Candish, L., Levens, A. & Lupton, D. W. N-heterocyclic carbene catalysed redox isomerisation of esters to functionalized benzaldehydes. Chem. Sci. 6, 2366–2370 (2015).
Zeitler, K. Stereoselective synthesis of (E)-α,ß-unsaturated esters via carbene-catalyzed redox esterification. Org. Lett. 8, 637–640 (2006).
Ryan, S. J., Candish, L. & Lupton, D. W. N-heterocyclic carbene-catalyzed generation of α,ß-unsaturated acyl imidazoliums: synthesis of dihydropyranones by their reaction with enolates. J. Am. Chem. Soc. 131, 14176–14177 (2009).
Ryan, S. J., Candish, L. & Lupton, D. W. N-heterocyclic carbene-catalyzed (4+2) cycloaddition/decarboxylation of silyl dienol ethers with α,ß-unsaturated acid fluorides. J. Am. Chem. Soc. 133, 4694–4697 (2011).
Wanner, B., Mahatthananchai, J. & Bode, J. W. Enantioselective synthesis of dihydropyridinones via NHC-catalyzed Aza-Claisen reaction. Org. Lett. 13, 5378–5381 (2011).
Kravina, A. G., Mahatthananchai, J. & Bode, J. W. Enantioselective, NHC-catalyzed annulations of trisubstituted enals and cyclic N-sulfonylimines via α,ß-unsaturated acyl azoliums. Angew. Chem. Int. Ed. 51, 9433–9436 (2012).
Kaeobamrung, J., Mahatthananchai, J., Zheng, P. & Bode, J. W. An enantioselective claisen rearrangement catalyzed by N-heterocyclic carbenes. J. Am. Chem. Soc. 132, 8810–8812 (2010).
Mahatthananchai, J., Kaeobamrung, J. & Bode, J. W. Chiral N-heterocyclic carbene-catalyzed annulations of enals and ynals with stable enols: a highly enantioselective Coates−Claisen rearrangement. ACS Catal. 2, 494–503 (2012).
Maki, B. E., Chan, A., Phillips, E. M. & Scheidt, K. A. Tandem oxidation of allylic and benzylic alcohols to esters catalyzed by N-heterocyclic carbenes. Org. Lett. 9, 371–374 (2007).
De Sarkar, S., Grimme, S. & Studer, A. NHC catalyzed oxidations of aldehydes to esters: chemoselective acylation of alcohols in presence of amines. J. Am. Chem. Soc. 132, 1190–1191 (2010).
Cheng, J., Huang, Z. & Chi, Y. R. NHC organocatalytic formal LUMO activation of α,ß-unsaturated esters for reaction with enamides. Angew. Chem. Int. Ed. 52, 8592–8596 (2013).
De Sarkar, S. & Studer, A. NHC-catalyzed Michael addition to α,ß-unsaturated aldehydes by redox activation. Angew. Chem. Int. Ed. 49, 9266–9269 (2010).
Cardinal-David, B., Raup, D. E. A. & Scheidt, K. A. Cooperative N-heterocyclic carbene/Lewis acid catalysis for highly stereoselective annulation reactions with homoenolates. J. Am. Chem. Soc. 132, 5345–5347 (2010).
Raup, D. E. A., Cardinal-David, B., Holte, D. & Scheidt, K. A. Cooperative catalysis by carbenes and Lewis acids in a highly stereoselective route to γ-lactams. Nat. Chem. 2, 766–771 (2010).
Kerr, M. S., Read de Alaniz, J. & Rovis, J. Highly enantioselective catalytic intramolecular Stetter reaction. J. Am. Chem. Soc. 124, 10298–10299 (2002).
Kerr, M. S., Read de Alaniz, J. & Rovis, T. Efficient synthesis of achiral and chiral 1,2,4-triazolium salts: bench stable precursors for N-heterocyclic carbenes. J. Org. Chem. 70, 5725–5728 (2005).
He, M., Struble, J. R. & Bode, J. W. Highly enantioselective azadiene Diels−Alder reactions catalyzed by chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 128, 8418–8420 (2006).
Rovis, T. Development of chiral bicyclic triazolium salt organic catalysts: the importance of the N-aryl substituent. Chem. Lett. 37, 2–7 (2008).
Mahatthananchai, J. & Bode, J. W. The effect of the N-mesityl group in NHC-catalyzed reactions. Chem. Sci. 3, 192–197 (2012).
Ukai, T., Tanaka, R. & Dokawa, T. A new catalyst for the acyloin condensation. J. Pharm. Soc. Jpn. 63, 296–304 (1943).
Breslow, R. On the mechanism of thiamine action. IV.1 Evidence from studies on model systems. J. Am. Chem. Soc. 80, 3719–3726 (1958).
Frisch, M. J. et al. Gaussian 09 Revision D.09 (Gaussian Inc., Wallingford, 2016).
Acknowledgements
Generous financial supports for this work were provided by: the National Natural Science Foundation of China (21672121), the “Thousand Plan” Youth program of China, the Tsinghua University, the Bayer Investigator fellow, the fellowship of Tsinghua-Peking centre for life sciences (CLS), and the China Postdoctoral Science Foundation (2015M570072) to J.W., and KAUST to K.-W.H.
Author information
Authors and Affiliations
Contributions
C.Z. conducted the main experiments; F.L. and D.G. prepared several starting materials, including substrates and NHC catalysts. K.M. and K.-W.H. conducted the computational studies. J.W. conceptualized and directed the project, and drafted the manuscript with the assistance from co-authors. All authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, C., Guo, D., Munkerup, K. et al. Enantioselective [3+3] atroposelective annulation catalyzed by N-heterocyclic carbenes. Nat Commun 9, 611 (2018). https://doi.org/10.1038/s41467-018-02952-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-02952-3
This article is cited by
-
N-Heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution
Nature Communications (2024)
-
Organocatalytic diastereo- and atroposelective construction of N–N axially chiral pyrroles and indoles
Nature Communications (2024)
-
Carbene-catalyzed enantioselective seleno-Michael addition as access to antimicrobial active Se-containing heterocycles
Science China Chemistry (2024)
-
Desymmetrization of N-Cbz glutarimides through N-heterocyclic carbene organocatalysis
Nature Communications (2022)
-
Carbene-catalyzed atroposelective synthesis of axially chiral styrenes
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.