Abstract
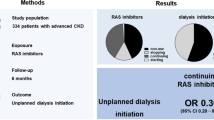
It is controversial whether renin-angiotensin system inhibitors (RASIs) should be stopped in patients with advanced chronic kidney disease (CKD). Recently, it was reported that stopping RASIs in advanced CKD was associated with increased mortality and cardiovascular (CV) events; however, it remains unclear whether stopping RASIs before dialysis initiation affects clinical outcomes after dialysis, which this study aimed to evaluate. In this multicenter prospective cohort study in Japan, we included 717 patients (mean age, 67 years; 68% male) who had a nephrology care duration ≥90 days, initiated hemodialysis, and used RASIs 3 months before hemodialysis initiation. The multivariable adjusted Cox models were used to compare mortality and CV event risk between 650 (91%) patients who continued RASIs until hemodialysis initiation and 67 (9.3%) patients who stopped RASIs. During a median follow-up period of 3.5 years, 170 (24%) patients died and 228 (32%) experienced CV events. Compared with continuing RASIs, stopping RASIs was unassociated with mortality (adjusted hazard ratio [aHR]: 0.82; 95% confidence interval [CI]: 0.50–1.34) but was associated with higher CV events (aHR: 1.59; 95% CI: 1.06–2.38). Subgroup analyses showed that the risk of stopping RASIs for CV events was particularly high in patients aged <75 years, with a significant interaction between stopping RASIs and age. This study revealed that patients who stopped RASIs immediately before dialysis initiation were associated with subsequent higher CV events. Active screening for CV disease may be especially beneficial for these patients.

Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) patients are at higher risk for cardiovascular (CV) events and mortality than the general population and require optimal management to reduce these risks [1, 2]. Renin-angiotensin system inhibitors (RASIs) have been reported to reduce the risk of kidney failure, CV events, and all-cause mortality in patients with CKD [3]. Thus, guidelines recommend using RASIs for advanced CKD (i.e., CKD stage 4 or 5) unless they are intolerable due to hyperkalemia or worsening of kidney function. However, the benefits of using RASIs for advanced CKD are less certain because most clinical trials excluded participants with advanced CKD [2, 4,5,6].
Several studies investigated whether RASIs should be stopped or continued in patients with advanced CKD [7,8,9,10,11,12,13]. Stopping RASIs in patients with advanced CKD was associated with all-cause mortality [8, 9] and CV events [8, 9, 11]. However, the observational period of these studies was until the initiation of dialysis; thus, whether stopping RASIs before dialysis initiation affects prognosis after the initiation of dialysis is uncertain.
This study aimed to evaluate whether stopping RASIs immediately before dialysis initiation affects subsequent outcomes, such as mortality and CV events, in patients registered with the Aichi cohort study of the prognosis in patients newly initiated into dialysis (AICOPP), which is a multicenter, prospective cohort study.
Materials and methods
Study population
We used data from the AICOPP, including 1520 incident dialysis patients. Details of the AICOPP have been previously described [14]. The cohort included patients who initiated dialysis between October 2011 and September 2013 at 17 facilities in Aichi, Japan. We screened patients aged ≥20 years and enrolled those who were discharged alive after hospitalization for dialysis initiation. Written informed consent was obtained from all patients. In our study of patients registered with AICOPP, we excluded patients referred to nephrologists <90 days prior to dialysis initiation or whose duration of nephrologist care was unknown, and patients who opted for peritoneal dialysis.
We recruited patients who had used angiotensin converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) 3 months prior to hemodialysis (HD) initiation and those who had data of ACEIs or ARBs at the time of HD initiation for survival analysis.
Baseline variables
Baseline demographic and clinical data, including blood and urine test results, were collected immediately before or during hospitalization for HD initiation. Body mass index (BMI) was calculated using the following formula: BMI = weight(kg)/height(m)2. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL, casual blood glucose ≥200 mg/dL, HbA1c (NGSP) ≥ 6.5%, use of insulin, or use of oral hypoglycemic agents. A history of CV disease (CVD) was defined as a history of heart failure requiring hospitalization, coronary artery intervention, heart bypass surgery, stroke, aortic disease requiring surgery, or peripheral artery disease requiring hospitalization. Urgent dialysis was defined as emergency dialysis or dialysis initiation using an indwelling vascular catheter when faced with a risk to life. Emergency dialysis initiation was referred to as unscheduled initiation. The estimated glomerular filtration rate (eGFR) was calculated using the Japanese Society of Nephrology’s equation: eGFR = 194 × serum creatinine–1.094 × age–0.287 (×0.739 for women). The use of diuretics before dialysis initiation included regular loop diuretics, thiazide-type diuretics, or spironolactone [14, 15]. The use of RASIs was defined as the use of ACEIs or ARBs. We divided patients into four groups according to the patterns of RASIs use 3 months before and at the time of HD initiation: continued, stopped, did not use, and started during the 3 months prior to HD initiation [16]. Patients with missing this information were assigned to the unknown group (Supplementary Table 1).
Outcomes
The study outcomes were all-cause mortality and CV events after HD initiation. CV events were defined as heart failure, acute coronary syndrome, stroke, or peripheral artery disease requiring hospitalization [15]. Outcome data of all patients in this cohort were collected by reviewing the medical records of the AICOPP group or by sending letters to each dialysis clinic where patients were transferred for maintenance HD. Patients were followed up from the day of dialysis initiation, until either death, failure to follow-up, kidney transplantation, recovery from dialysis therapy, or the end of follow-up on September 30, 2016 [17].
Statistical analysis
The baseline characteristics of the cohort for survival analysis were summarized into two groups: continuing RASIs and stopping RASIs, with normally and non-normally distributed variables and categorical variables as mean (standard deviation [SD]), median (interquartile range [IQR]), and number (percentage), respectively. Group differences were assessed using Student’s t test, Wilcoxon rank sum test, and chi-square test for continuous variables with approximately normal distributions, non-normally distributed variables, and categorical data, respectively.
We estimated survival across the two groups using the Kaplan–Meier method. Differences in survival estimates between the two groups were assessed using the log-rank test. We performed analyses using multiple imputations by chained equations in the multivariable models to handle missing data. We performed chained equations with 100 imputations and combined the estimates of the analysis per dataset using Rubin’s rule. After multiple imputations, we constructed the multivariable Cox proportional hazard models to assess the risk of mortality and CV events associated with stopping RASIs and clinical characteristics. Three models were explored: Model 1 was unadjusted; Model 2 was adjusted for age, sex, BMI, history of diabetes mellitus, and history of CVD; and Model 3 additionally accounted for serum creatinine, serum potassium, eGFR decline (the change in eGFR for 3 months before dialysis initiation), and use of ion exchange resin, antiplatelet drugs, β-blockers, diuretics, and urgent dialysis. As sensitivity analyses for CV events in the stopping RASIs group, we performed Fine and Gray competing-risk analyses considering non-CV death as a competing risk. In case of significant association between mortality or CV events, and stopping RASIs, subgroup analyses according to age (<75 vs. ≥75 years), sex, diabetes mellitus, and history of CVD were performed.
In all analyses, a two-sided p-value of <0.05 was considered statistically significant. All the statistical analyses were performed using Stata version 17 (StataCorp, College Station, Texas, USA).
Results
Patient characteristics
Of the 1118 patients, 717 were included for the survival analysis (Fig. 1). Table 1 shows the baseline characteristics of the analytical cohorts in the two groups. The characteristics of the eligible patients were similar to those of patients excluded due to non-using, newly starting RASIs or unknown data (Supplementary Table 1). The mean ± SD age of 717 study participants at HD initiation was 67 ± 13 years, and 68% were male. The numbers of patients with continuing and stopping RASIs were 650 (91%) and 67 (9.3%), respectively. Patients who stopped RASIs had a shorter duration of nephrology care, more frequent urgent dialysis, faster eGFR decline, and higher serum C-reactive protein levels than those who continued RASIs. Missing data were found in ≤5% of all variables (Supplementary Table 2).
Flow diagram illustrating patient enrollment for the present study. A total of 1118 patients were included in the baseline data (Supplementary Table 1), and 717 in the survival analysis. ACEIs angiotensin-converting enzyme inhibitors, ARBs angiotensin II receptor blockers
Associations of stopping RASIs with mortality and CV events
The median [IQR] observation period of mortality was 3.5 [2.9–4.1] years, and 170 (24%) patients died. During follow-up, there were 74 (10%) censored cases comprising 15 patients who received kidney transplants and 59 patients who were lost to follow-up. Crude mortality rates (per 100 person-years) were 7.1 and 8.9 in patients who continued and stopped RASIs, respectively (Table 2). The Kaplan–Meier curves showed no difference in survival rates between the two groups. (p = 0.35; Fig. 2A). The multivariable analysis also showed no significant association between continuing or stopping RASIs and mortality; the multivariable-adjusted hazard ratios (HRs) was 0.82 (95% confidence interval [CI], 0.50–1.34).
The median [IQR] observation period of CV events was 3.2 [1.5–3.9] years, and 228 (32%) patients experienced CV events. Crude CV event rates (per 100 person-years) were 10.9 and 17.7 in patients who continued and stopped RASIs, respectively (Table 2). The Kaplan–Meier curves showed a decreased CV event-free rate in the stopping group (p = 0.014; Fig. 2B). In the multivariable analysis, stopping RASIs was associated with increased CV event risk; the multivariable-adjusted HRs was 1.59 (95% CI, 1.06–2.38). The results of the competing risk analysis were similar to those of the Cox regression analysis (subdistribution HR, 1.66; 95% CI, 1.05–2.62).
Subgroup analyses
Although the association between stopping RASIs and CV events was mostly consistent across subgroups, only the age category (<75 vs. ≥75 years) had a significant interaction regarding CV events (p = 0.012); the risk of stopping RASIs was especially higher in patients with <75 years (Fig. 3).
Subgroup analyses of CV events. The plots with capped spikes indicate the multivariable-adjusted hazard ratios with 95% confidence intervals for CV events of stopping RASIs compared with continuing RASIs. There was a significant interaction with relatively higher hazard ratios of CV events between stopping RASIs and age (<75 or ≥75 years). CVD cardiovascular disease, CV cardiovascular, RASIs renin-angiotensin system inhibitors
Discussion
In this multicenter, prospective cohort study of patients initiating HD, we assessed the association of stopping RASIs during the 3 months prior to HD initiation with mortality and CV events. We found that stopping RASIs was associated with higher risk of CV events after HD initiation and was not associated with mortality. The subgroup analysis for CV events revealed that the association was consistent except for the subgroup of age ≥75 years; CV risk of stopping RASIs was especially higher in the subgroup with age <75 years, with a significant interaction.
Several studies showed that stopping RASIs in advanced CKD was associated with mortality [8, 9] and CV events [8, 9, 11]. However, dialysis initiation was a censoring event in these studies [8, 9, 11]; thus, whether stopping RASIs immediately prior to dialysis initiation adversely affects prognosis after dialysis initiation remains uncertain. A previous multicenter randomized control study showed that stopping RASIs in patients with advanced CKD was not associated with long-term rate of eGFR decrease [10]. CV events and death were secondary outcomes, and they were similar in both the stopping and continuing RASIs groups. To our knowledge, this is the first study to evaluate the association between stopping RASIs before dialysis initiation on mortality and CV events after HD initiation. Patients who stopped RASIs within 3 months prior to HD initiation had higher CV event rates than those who continued RASIs. This result supports the guideline from China for pre-dialysis to continue RASIs in advanced CKD even just before dialysis initiation [18]. In the Evidence-based Clinical Practice Guideline for CKD from Japan, CVD screening at least before the initiation of dialysis is recommended; however, there is not enough evidence to suggest that universal CVD screening is beneficial [2]. Our results suggest that active screening for CVD may be especially beneficial for patients stopping RASIs immediately before dialysis initiation.
Regarding mortality, previous studies showed that stopping RASIs in advanced CKD was associated with mortality [8, 9], while some studies showed that it was not associated with mortality [10, 11]. In our study, there was no significant association between continuing or stopping RASIs and mortality. One possible explanation is that, since the stopping RASIs group is likely to experience a faster decline in eGFR and consequently an earlier initiation of dialysis, the analysis using the time of dialysis initiation as the baseline may introduce a “lead time bias” that may lead to a longer life expectancy.
There are limited studies on the influence of the stopping RASIs according to age in patients with advanced CKD. In an observational study of advanced CKD patients, compared with continuing RASIs, stopping RASIs had a higher CV events risk [9]. In their study, subgroup analysis was divided by age, and the CV events risk was compared between subgroup with age <70 years and ≥70 years. The subgroup with age <70 years who stopped RASIs tended to experience higher CV events rates; however, no significant interaction was observed between stopping RASIs and age. In our study, RASIs in the subgroup with age ≥75 years was not associated with CV events risk, whereas CV events risk of stopping RASIs was especially higher in the subgroup with age <75 years, with a significant interaction (p = 0.012; Fig. 3). Thus, the benefits of continuing RASIs may be limited for the older. However, further investigation is needed to draw conclusions.
This study had several limitations. First, because this was an observational study, we could not directly verify whether stopping RASIs within 3 months before HD initiation had a positive influence on increasing CV events after HD initiation. In our cohort, patients who stopped RASIs had a higher prevalence of urgent dialysis and rapid eGFR decline for 3 months before dialysis initiation (Table 1). However, several baseline characteristics, such as age, male sex, and history of CV events, were comparable between the two groups. Additionally, multivariable analysis was performed to eliminate the influence of potential confounders as much as possible. Nevertheless, the influence of unmeasured confounders may remain. Second, very little information was available 3 months before HD initiation. In this regard, we adjusted for eGFR decline 3 months before dialysis initiation, potassium level at HD initiation, potassium binder prescription at HD initiation, and urgent dialysis in the multivariate analysis; however, information regarding the reason for stopping RASIs was unavailable. For example, although hyperkalemia within 3 months of starting dialysis may have led to stopping RASIs, data on potassium levels at 3 months before HD initiation were not collected. Furthermore, CV events immediately before dialysis initiation could prompt stopping RASIs; however, information on the timing of the history of CV events was also not collected. Third, we could identify information on RASIs use only at 3 months before dialysis initiation and at the time of dialysis initiation. Fourth, the sample size was relatively small (stopping RASIs group, n = 67).
In conclusion, patients who stopped RASIs within 3 months before HD initiation had a higher risk of CV events after HD initiation. Active screening for CVD may be especially beneficial for these patients.
References
Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:572–86.
Japanese Society of Nephrology. Essential points from Evidence-based Clinical Practice Guidelines for Chronic Kidney Disease 2018. Clin Exp Nephrol. 2019;23:1–15.
Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: A Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–41.
Weir MR, Lakkis JI, Jaar B, Rocco MV, Choi MJ, Kramer HJ, et al. Use of renin-angiotensin system blockade in advanced CKD: An NKF-KDOQI controversies report. Am J Kidney Dis. 2018;72:873–84.
Mukoyama M, Kuwabara T. Role of renin-angiotensin system blockade in advanced CKD: to use or not to use? Hypertens Res. 2022;45:1072–5.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transpl. 2010;25:3977–82.
Qiao Y, Shin JI, Chen TK, Inker LA, Coresh J, Alexander GC, et al. Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med. 2020;180:718–26.
Fu EL, Evans M, Clase CM, Tomlinson LA, van Diepen M, Dekker FW, et al. Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J Am Soc Nephrol. 2021;32:424–35.
Bhandari S, Mehta S, Khwaja A, Cleland JGF, Ives N, Brettell E, et al. Renin–angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387:2021–32.
Yang A, Shi M, Lau ESH, Wu H, Zhang X, Fan B, et al. Clinical outcomes following discontinuation of renin-angiotensin-system inhibitors in patients with type 2 diabetes and advanced chronic kidney disease: a prospective cohort study. EClinicalMedicine. 2023;55:101751.
Walther CP, Winkelmayer WC, Richardson PA, Virani SS, Navaneethan SD. Renin-angiotensin system blocker discontinuation and adverse outcomes in chronic kidney disease. Nephrol Dial Transpl. 2021;36:1893–9.
Nakayama T, Mitsuno R, Azegami T, Sato Y, Hayashi K, Itoh H. A systematic review and meta-analysis of the clinical impact of stopping renin–angiotensin system inhibitor in patients with chronic kidney disease. Hypertens Res. 2023;46:1525–35.
Hishida M, Tamai H, Morinaga T, Maekawa M, Aoki T, Tomida H, et al. Aichi cohort study of the prognosis in patients newly initiated into dialysis (AICOPP): baseline characteristics and trends observed in diabetic nephropathy. Clin Exp Nephrol. 2016;20:795–807.
Inaguma D, Tanaka A, Shinjo H, Kato A, Murata M. Predialysis Vitamin D receptor activator treatment and cardiovascular events after dialysis initiation: a multicenter observational study. Nephron. 2016;133:35–43.
Nakayama T, Morimoto K, Uchiyama K, Kusahana E, Washida N, Azegami T, et al. Effects of renin-angiotensin system inhibitors on the incidence of unplanned dialysis. Hypertens Res. 2022;45:1018–27.
Hishida M, Shafi T, Appel LJ, Maruyama S, Inaguma D, Matsushita K. Lower levels of proteinuria are associated with elevated mortality in incident dialysis patients. PLoS One. 2019;14:1–13.
Chinese Experts Group of the Guideline for the Management of ‘CKD-PeriDialysis’, Chinese Non-government Medical Institutions Association. Chinese clinical practice guideline for the management of ‘CKD-PeriDialysis’-the periods prior to and in the early-stage of initial dialysis. Kidney Int Rep. 2022;7:S531–58.
Acknowledgements
We acknowledge the support provided by the investigators and members of the AICOPP.
Funding
Open Access funding provided by Nagoya University.
Author information
Authors and Affiliations
Contributions
YN, DI, TI, and SK participated in the design of the study and interpretation of data. YN, DI, TI, and SK participated in writing the manuscript. All authors were involved in drafting, reviewing, and approving the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
D.I. has received research funding from The Aichi Kidney Foundation. T.I. has received grants and honoraria from Kyowa Kirin; consulting fees from GlaxoSmithKline. S.M. has received grants and/or research funding from Chugai, Mitsubishi Tanabe, Ono, and ROHTO; honoraria from Alexion, Astellas, AstraZeneca, Bayer, Mitsubishi Tanabe, Kyowa Kirin, and Novartis.
Ethics statement
This study was approved by the Institutional Review Board of Nagoya Daini Red Cross Hospital and each participating facility in the AICOPP group (the approval number: 20110823-3) and was conducted in accordance with the Ethical guidelines for Clinical Research by the Japanese Ministry of Health, Labor, and Welfare (created July 30, 2003; full revision December 28, 2004; full revision July 31, 2008) and the Helsinki Declaration (revised 2013). The subjects received oral and written explanations of the purpose of the study and provided their written consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakamura, Y., Inaguma, D., Imaizumi, T. et al. Association between stopping renin-angiotensin system inhibitors immediately before hemodialysis initiation and subsequent cardiovascular events. Hypertens Res 47, 1372–1379 (2024). https://doi.org/10.1038/s41440-024-01616-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01616-8