Abstract
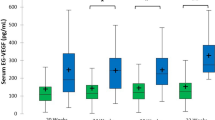
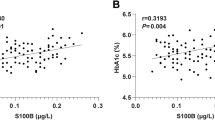
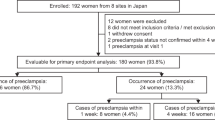
Our aims were to obtain the gestational-age-specific median of common logarithmic placental growth factor (PlGF) values in the first trimester in women with a singleton pregnancy in order to generate the gestational-age-specific multiple of the median (MoM) of log10PlGF at 9–13 weeks of gestation, to evaluate screening parameters of MoM of log10PlGF at 9–13 weeks of gestation to predict preterm preeclampsia (PE), and to construct an appropriate prediction model for preterm PE using minimum risk factors in multivariable logistic regression analyses in a retrospective sub-cohort study. Preterm PE occurred in 2.9% (20/700), and PE in 5.1% (36/700). Serum PlGF levels were measured using Elecsys PlGF®. MoMs of log10PlGF at 9–13 weeks of gestation in Japanese women with a singleton pregnancy followed a normal distribution. We determined the appropriate cut-off value of MoM of log10PlGF to predict preterm PE at around a10% false-positive rate (0.854). The MoM of log10PlGF < 0.854 yielded sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio (95% confidence interval [CI]), and negative likelihood ratio (95% CI) of 55.0%, 91.9%, 17.5%, 98.5%, 6.79 (4.22–10.91), and 0.49 (0.30–0.80), respectively. The combination of MoM of log10PlGF and presence of either chronic hypertension or history of PE/gestational hypertension (GH) yielded sensitivity and specificity of 80.0 and 85.7%, respectively, to predict preterm PE. In conclusion, the automated electrochemiluminescence immunoassay for serum PlGF levels in women with singleton pregnancy at 9–13 weeks of gestation may be useful to predict preterm PE.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
O’Gorman N, Wright D, Rolnik DL, Nicolaides KH, Poon LC. Study protocol for the randomised controlled trial: combined multimarker screening and randomised patient treatment with ASpirin for evidence-based PREeclampsia prevention (ASPRE). BMJ Open. 2016;6:e011801.
Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–22.
Ong CY, Lao TT, Spencer K, Nicolaides KH. Maternal serum level of placental growth factor in diabetic pregnancies. J Reprod Med. 2004;49:477–80.
Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:732–9.
Cowans NJ, Stamatopoulou A, Spencer K. First trimester maternal serum placental growth factor in trisomy 21 pregnancies. Prenat Diagn. 2010;30:449–53.
Cowans NJ, Stamatopoulou A, Tørring N, Spencer K. Early first-trimester maternal serum placental growth factor in trisomy 21 pregnancies. Ultrasound Obstet Gynecol. 2011;37:515–9.
Wortelboer EJ, Koster MP, Kuc S, Eijkemans MJ, Bilardo CM, Schielen PC, et al. Longitudinal trends in fetoplacental biochemical markers, uterine artery pulsatility index and maternal blood pressure during the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2011;38:383–8.
Vandenberghe G, Mensink I, Twisk JW, Blankenstein MA, Heijboer AC, van Vugt JM. First trimester screening for intra-uterine growth restriction and early-onset pre-eclampsia. Prenat Diagn. 2011;31:955–61.
Donalson K, Turner S, Morrison L, Liitti P, Nilsson C, Cuckle H. Maternal serum placental growth factor and α-fetoprotein testing in first trimester screening for Down syndrome. Prenat Diagn. 2013;33:457–61.
Tsiakkas A, Duvdevani N, Wright A, Wright D, Nicolaides KH. Serum placental growth factor in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound Obstet Gynecol. 2015;45:591–8.
Huang T, Dennis A, Meschino WS, Rashid S, Mak-Tam E, Cuckle H. First trimester screening for Down syndrome using nuchal translucency, maternal serum pregnancy-associated plasma protein A, free-β human chorionic gonadotrophin, placental growth factor, and α-fetoprotein. Prenat Diagn. 2015;35:709–16.
Han J, Liu H, Xu ZP, Cuckle H, Sahota D, Li DZ, et al. Maternal serum PlGF (placental growth factor) in Chinese women in the first trimester undergoing screening for Down syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;201:166–70.
Jayasena CN, Abbara A, Comninos AN, Narayanaswamy S, Gonzalez Maffe J, Izzi-Engbeaya C, et al. Novel circulating placental markers prokineticin-1, soluble fms-like tyrosine kinase-1, soluble endoglin and placental growth factor and association with late miscarriage. Hum Reprod. 2016;31:2681–8.
Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15.
Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53:812–8.
Tsiakkas A, Cazacu R, Wright A, Wright D, Nicolaides KH. Maternal serum placental growth factor at 12, 22, 32 and 36 weeks’ gestation in screening for pre-eclampsia. Ultrasound Obstet Gynecol. 2016;47:472–7.
Chaemsaithong P, Pooh RK, Zheng M, Ma R, Chaiyasit N, Tokunaka M, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol. 2019;221:650.e1–650.e16.
Mazer Zumaeta A, Wright A, Syngelaki A, Maritsa VA, Da Silva AB, Nicolaides KH. Screening for pre-eclampsia at 11-13 weeks’ gestation: use of pregnancy-associated plasma protein-A, placental growth factor or both. Ultrasound Obstet Gynecol. 2020;56:400–7.
Boutin A, Gasse C, Guerby P, Giguère Y, Tétu A, Bujold E. First-trimester preterm preeclampsia screening in nulliparous women: the great obstetrical syndrome (GOS) Study. J Obstet Gynaecol Can. 2021;43:43–9.
Law KS, Wei TY. Effect of low-dose aspirin in preventing early-onset preeclampsia in the Taiwanese population-a retrospective cohort study. Int J Womens Health. 2021;13:1095–101.
Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161.e1–161.e11.
Ohkuchi A, Hirashima C, Suzuki H, Takahashi K, Yoshida M, Matsubara S, et al. Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res. 2010;33:422–7.
https://www.info.pmda.go.jp/downfiles/ivd/PDF/700025_23100EZX00013000_A_04_02.pdf Elecsys sFlt-1® [package insert]. Accessed 2 March 2023.
https://www.info.pmda.go.jp/downfiles/ivd/PDF/700025_23100EZX00014000_B_02_02.pdf Elecsys PlGF® [package insert]. Accessed 2 March 2023.
Chaemsaithong P, Sahota D, Pooh RK, Zheng M, Ma R, Chaiyasit N, et al. First-trimester pre-eclampsia biomarker profiles in Asian population: multicenter cohort study. Ultrasound Obstet Gynecol. 2020;56:206–14.
Goto M, Koide K, Tokunaka M, Takita H, Hamada S, Nakamura M, et al. Accuracy of the FMF Bayes theorem-based model for predicting preeclampsia at 11-13 weeks of gestation in a Japanese population. Hypertens Res. 2021;44:685–91.
Watanabe K, Matsubara K, Nakamoto O, Ushijima J, Ohkuchi A, Koide K, et al. Outline of the new definition and classification of “Hypertensive Disorders of Pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens Res Pregnancy. 2018;6:33–7.
Ohkuchi A, Suzuki H, Matsubara K, Watanabe K, Saitou T, Oda H, et al. Exponential increase of the gestational-age-specific incidence of preeclampsia onset (COPE study): a multicenter retrospective cohort study in women with maternal check-ups at <20 weeks of gestation in Japan. Hypertens Res. 2022;45:1679–89.
Confidence Interval Calculator. https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.pedro.org.au%2Fwp-content%2Fuploads%2FCIcalculator.xls&wdOrigin=BROWSELINK. Accessed 2 March 2023.
Risk assessment: Risk of preeclampsia. https://fetalmedicine.org/research/assess/preeclampsia/first-trimester. Accessed 2 March 2023.
Wright D, Tan MY, O’Gorman N, Poon LC, Syngelaki A, Wright A, et al. Predictive performance of the competing risk model in screening for preeclampsia. Am J Obstet Gynecol. 2019;220:199.e1–199.e13.
Vatten LJ, Åsvold BO, Eskild A. Angiogenic factors in maternal circulation and preeclampsia with or without fetal growth restriction. Acta Obstet Gynecol Scand. 2012;91:1388–94.
O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. 2016;214:103.e1–103.e12.
O’Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, Wright A, et al. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;49:751–5.
Tan MY, Syngelaki A, Poon LC, Rolnik DL, O’Gorman N, Delgado JL, et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2018;52:186–95.
Ohkuchi A, Hirashima C, Arai R, Takahashi K, Suzuki H, Ogoyama M, et al. Temporary hypertension and white coat hypertension in the first trimester as risk factors for preeclampsia. Hypertens Res. 2019;42:2002–12.
Acknowledgements
A.O. acknowledges the kind gift of Elecsys PlGF, and cooperation of Roche Diagnostics for PlGF measurement. A.O. also acknowledges OMRON Healthcare Co. Ltd., because clinic BP of all pregnant women who sought maternal checkups at our hospital was measured using Omron HEM-906®, and home blood pressure (HBP) was measured using HBP monitoring devices (Omron HEM-5001® and Omron HEM-7080IC®) in some women with suspected H.T., based on a research contract with OMRON Healthcare Co., Ltd. (RinA-Dai07-30 and RinA-Dai15-215).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ohkuchi, A., Takahashi, K., Hirashima, C. et al. Automated electrochemiluminescence immunoassay for serum PlGF levels in women with singleton pregnancy at 9–13 weeks of gestation predicts preterm preeclampsia: a retrospective cohort study. Hypertens Res 47, 1196–1207 (2024). https://doi.org/10.1038/s41440-023-01534-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01534-1