Abstract
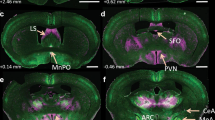
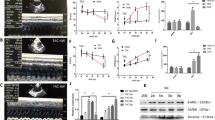
The renin-angiotensin system in the brain plays a pivotal role in modulating sympathetic nerve activity and contributes to the pathogenesis of hypertension. Angiotensin II (Ang II) type 1 receptor (AT1R)-associated protein (ATRAP) promotes internalization of AT1R while suppressing pathological overactivation of AT1R signaling. However, the pathophysiological function of ATRAP in the brain remains unknown. Therefore, this study aims to investigate whether ATRAP in the paraventricular nucleus (PVN) is involved in neurogenic hypertension pathogenesis in Ang II-infused rats. The ATRAP/AT1R ratio, which serves as an indicator of tissue AT1R hyperactivity, tended to decrease within the PVN in the Ang II group than in the vehicle group. This suggests an Ang II-induced hyperactivation of the AT1R signaling pathway in the PVN. Lentiviral vectors were generated to stimulate ATRAP expression. At 6 weeks of age, rats were microinjected with LV-Venus (Venus-expressing lentivirus) or LV-ATRAP (Venus-ATRAP-expressing lentivirus). The rats were then randomly divided into four groups: (1) Vehicle/LV-Venus, (2) Vehicle/LV-ATRAP, (3) Ang II/LV-Venus, and (4) Ang II/LV-ATRAP. Two weeks after microinjection, vehicle or Ang II was administered systemically for 2 weeks. In the Ang II/LV-ATRAP group, systolic blood pressure at 1 and 2 weeks following administration was significantly lower than that in the Ang II/LV-Venus group. Furthermore, urinary adrenaline levels tended to decrease in the Ang II/LV-ATRAP group than in the Ang II/LV-Venus group. These findings suggest that enhanced ATRAP expression in the PVN suppresses Ang II-induced hypertension, potentially by suppressing hyperactivation of the tissue AT1R signaling pathway and, subsequently, sympathetic nerve activity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data were included in this study. These datasets are available from the corresponding authors upon request.
References
Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–66.
Tanaka M. Improving obesity and blood pressure. Hypertens Res. 2020;43:79–89.
Hasegawa S, Inoue T, Nakamura Y, Fukaya D, Uni R, Wu CH, et al. Activation of sympathetic signaling in macrophages blocks systemic inflammation and protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2021;32:1599–615.
Xiao L, do Carmo LS, Foss JD, Chen W, Harrison DG. Sympathetic enhancement of memory T-cell homing and hypertension sensitization. Circ Res. 2020;126:708–21.
Dupont AG, Légat L. GABA is a mediator of brain AT1 and AT2 receptor-mediated blood pressure responses. Hypertens Res. 2020;43:995–1005.
Johnson AK, Xue B. Central nervous system neuroplasticity and the sensitization of hypertension. Nat Rev Nephrol. 2018;14:750–66.
Guyenet PG, Stornetta RL, Souza G, Abbott SBG, Brooks VL. Neuronal networks in hypertension: recent advances. Hypertension. 2020;76:300–11.
Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res. 2012;35:132–41.
Dampney RA, Michelini LC, Li DP, Pan HL. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol. 2018;315:H1200–H1214.
Sharma NM, Haibara AS, Katsurada K, Nandi SS, Liu X, Zheng H, et al. Central Ang II (angiotensin II)-mediated sympathoexcitation: role for HIF-1α (hypoxia-inducible factor-1α) facilitated glutamatergic tone in the paraventricular nucleus of the hypothalamus. Hypertension. 2021;77:147–57.
Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol. 2009;296:H1425–1433.
Chen A, Huang BS, Wang HW, Ahmad M, Leenen FH. Knockdown of mineralocorticoid or angiotensin II type 1 receptor gene expression in the paraventricular nucleus prevents angiotensin II hypertension in rats. J Physiol. 2014;592:3523–36.
Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–8.
Chen XY, Lin C, Liu GY, Pei C, Xu GQ, Gao L, et al. ACE2 gene combined with exercise training attenuates central AngII/AT1 axis function and oxidative stress in a prehypertensive rat model. J Appl Physiol. 2022;132:1460–7.
Su C, Xue J, Ye C, Chen A. Role of the central renin‑angiotensin system in hypertension (Review). Int J Mol Med. 2021;47:95.
Tamura K, Azushima K, Kinguchi S, Wakui H, Yamaji T. ATRAP, a receptor-interacting modulator of kidney physiology, as a novel player in blood pressure and beyond. Hypertens Res. 2022;45:32–39.
Daviet L, Lehtonen JY, Tamura K, Griese DP, Horiuchi M, Dzau VJ. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J Biol Chem. 1999;274:17058–62.
Lopez-Ilasaca M, Liu X, Tamura K, Dzau VJ. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol Biol Cell. 2003;14:5038–50.
Azuma K, Tamura K, Shigenaga A, Wakui H, Masuda S, Tsurumi-Ikeya Y, et al. Novel regulatory effect of angiotensin II type 1 receptor-interacting molecule on vascular smooth muscle cells. Hypertension. 2007;50:926–32.
Wakui H, Tamura K, Tanaka Y, Matsuda M, Bai Y, Dejima T, et al. Cardiac-specific activation of angiotensin II type 1 receptor-associated protein completely suppresses cardiac hypertrophy in chronic angiotensin II-infused mice. Hypertension. 2010;55:1157–64.
Ohsawa M, Tamura K, Wakui H, Maeda A, Dejima T, Kanaoka T, et al. Deletion of the angiotensin II type 1 receptor-associated protein enhances renal sodium reabsorption and exacerbates angiotensin II-mediated hypertension. Kidney Int. 2014;86:570–81.
Kinguchi S, Wakui H, Azushima K, Haruhara K, Koguchi T, Ohki K, et al. Effects of ATRAP in renal proximal tubules on angiotensin-dependent hypertension. J Am Heart Assoc. 2019;8:e012395.
Uneda K, Wakui H, Maeda A, Azushima K, Kobayashi R, Haku S, et al. Angiotensin II type 1 receptor-associated protein regulates kidney aging and lifespan independent of angiotensin. J Am Heart Assoc. 2017;6:e006120.
Miyazaki T, Nakajima W, Hatano M, Shibata Y, Kuroki Y, Arisawa T, et al. Visualization of AMPA receptors in living human brain with positron emission tomography. Nat Med. 2020;26:281–8.
Kobayashi R, Wakui H, Azushima K, Uneda K, Haku S, Ohki K, et al. An angiotensin II type 1 receptor binding molecule has a critical role in hypertension in a chronic kidney disease model. Kidney Int. 2017;91:1115–25.
Nakajima W, Jitsuki S, Sano A, Takahashi T. Sustained enhancement of lateral inhibitory circuit maintains cross modal cortical reorganization. PLoS ONE. 2016;11:e0149068.
Yamaji T, Yamashita A, Wakui H, Azushima K, Uneda K, Fujikawa Y, et al. Angiotensin II type 1 receptor-associated protein deficiency attenuates sirtuin1 expression in an immortalised human renal proximal tubule cell line. Sci Rep. 2019;9:16550.
Miyazaki T, Takase K, Nakajima W, Tada H, Ohya D, Sano A, et al. Disrupted cortical function underlies behavior dysfunction due to social isolation. J Clin Investig. 2012;122:2690–701.
Abe E, Yamashita A, Hirota K, Yamaji T, Azushima K, Urate S, et al. Angiotensin II type-1 receptor-associated protein interacts with transferrin receptor-1 and promotes its internalization. Sci Rep. 2022;12:17376.
Dejima T, Tamura K, Wakui H, Maeda A, Ohsawa M, Kanaoka T, et al. Prepubertal angiotensin blockade exerts long-term therapeutic effect through sustained ATRAP activation in salt-sensitive hypertensive rats. J Hypertens. 2011;29:1919–29.
Shigenaga A, Tamura K, Wakui H, Masuda S, Azuma K, Tsurumi-Ikeya Y, et al. Effect of olmesartan on tissue expression balance between angiotensin II receptor and its inhibitory binding molecule. Hypertension. 2008;52:672–8.
Wakui H, Tamura K, Matsuda M, Bai Y, Dejima T, Shigenaga A, et al. Intrarenal suppression of angiotensin II type 1 receptor binding molecule in angiotensin II-infused mice. Am J Physiol Renal Physiol. 2010;299:F991–F1003.
Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018;19:876.
Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439.
McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, et al. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–18.
Dupont AG, Brouwers S. Brain angiotensin peptides regulate sympathetic tone and blood pressure. J Hypertens. 2010;28:1599–610.
Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63:572–9.
Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, et al. A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol. 2015;26:1619–33.
Gao HL, Yu XJ, Hu HB, Yang QW, Liu KL, Chen YM, et al. Apigenin improves hypertension and cardiac hypertrophy through modulating NADPH oxidase-dependent ROS generation and cytokines in hypothalamic paraventricular nucleus. Cardiovasc Toxicol. 2021;21:721–36.
Wang HW, Huang BS, White RA, Chen A, Ahmad M, Leenen FH. Mineralocorticoid and angiotensin II type 1 receptors in the subfornical organ mediate angiotensin II - induced hypothalamic reactive oxygen species and hypertension. Neuroscience. 2016;329:112–21.
Young CN, Davisson RL. Angiotensin-II, the brain, and hypertension: an update. Hypertension. 2015;66:920–6.
Acknowledgements
This work was supported with grants from the Yokohama Foundation for Advancement of Medical Science, Uehara Memorial Foundation, Japan Society for the Promotion of Science, Japan Kidney Association-Nippon Boehringer Ingelheim Joint Research Program, Japanese Association of Dialysis Physicians, Salt Science Research Foundation, Strategic Research Project of Yokohama City University, the Japan Agency for Medical Research and Development (AMED), Translational Research Program, Strategic Promotion for Practical Application of Innovative Medical Technology (TR-SPRINT) from AMED, Moriya Scholarship Foundation, Bayer Scholarship for Cardiovascular Research, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Yokohama City University research grant “KAMOME Project”.
Author information
Authors and Affiliations
Contributions
MS, SK, and HW designed and conducted the study. MS, HW, and KA wrote the manuscript. MS, SK, HW, KA, and WN performed the experiments. MS and SK analyzed the data. KF, TM, TT, and KT supervised the study. All authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sotozawa, M., Kinguchi, S., Wakui, H. et al. Enhancement of angiotensin II type 1 receptor-associated protein in the paraventricular nucleus suppresses angiotensin II-dependent hypertension. Hypertens Res 47, 67–77 (2024). https://doi.org/10.1038/s41440-023-01480-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01480-y
Keywords
This article is cited by
-
ATRAP in the paraventricular nucleus of the hypothalamus as another key player in the control of sympathetic outflow
Hypertension Research (2024)