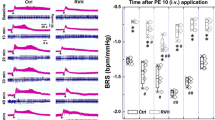
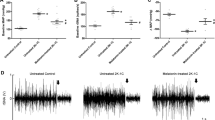
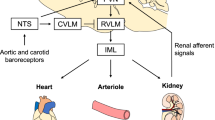
Abstract
Sympathoexcitation, under the regulatory control of the brain, plays a pivotal role in the etiology of hypertension. Within the brainstem, significant structures involved in the modulation of sympathetic nerve activity include the rostral ventrolateral medulla (RVLM), caudal ventrolateral medulla (CVLM), nucleus tractus solitarius (NTS), and paraventricular nucleus (paraventricular). The RVLM, in particular, is recognized as the vasomotor center. Over the past five decades, fundamental investigations on central circulatory regulation have underscored the involvement of nitric oxide (NO), oxidative stress, the renin-angiotensin system, and brain inflammation in regulating the sympathetic nervous system. Notably, numerous significant findings have come to light through chronic experiments conducted in conscious subjects employing radio-telemetry systems, gene transfer techniques, and knockout methodologies. Our research has centered on elucidating the role of NO and angiotensin II type 1 (AT1) receptor-induced oxidative stress within the RVLM and NTS in regulating the sympathetic nervous system. Additionally, we have observed that various orally administered AT1 receptor blockers effectively induce sympathoinhibition by reducing oxidative stress via blockade of the AT1 receptor in the RVLM of hypertensive rats. Recent advances have witnessed the development of several clinical interventions targeting brain mechanisms. Nonetheless, Future and further basic and clinical research are needed.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–7.
Grassi G. Sympathetic neural activity in human hypertension and related diseases. Am J Hypertens. 2010;23:1052–60.
Grassi G, Seravalle G, Quarti-Trevano F. The ‘neurogenic hypothesis’ in hypertension: current evidence. Exp Physiol. 2010;95:581–6.
Esler M. The 2009 Carl Ludwig lecture: pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol. 2010;108:227–37.
Mauo K, Lambert GW, Esler MD, Rakugi H, Ogihara T, Schlaich MP. The role of sympathetic nervous system activity in renal injury and end-stage renal disease. Hypertens Res. 2010;33:521–8.
Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–64.
Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46.
Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–88.
Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep. 2003;5:262–8.
Dampney RAL, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol. 2003;23:597–616.
Campos RR, Bergamschi CT. Neurotransmission alterations in central cardiovascular control in experimental hypertension. Curr Hypertens Rev. 2006;2:193–8.
Carlson SH, Wyss JM. Neurohormonal regulation of the sympathetic nervous system: new insights into central mechanisms of action. Curr Hypertens Rep. 2008;10:233–40.
Dampney RAL, Horiuchi J, Killinger S, Sheriff MJ, Tan PSP, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharm Physiol. 2005;32:419–25.
Coote JH. Landmarks in understanding the central nervous control of the cardiovascular system. Exp Physiol. 2007;92:3–18.
Agarwal SK, Gelsema AJ, Calaresu FR. Inhibition of rostral VLM by baroreceptor activation is relayed through caudal VLM. Am J Physiol. 1990;258:R1271–8.
Krukoff TL. The central action of nitric oxide in the regulation of autonomic functions. Brain Res Rev. 1999;30:52–65.
Patel K, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med. 2001;226:814–24.
Zanzinger J. Role of nitric oxide in the neural control of cardiovascular function. Cardiovasc Res. 1999;43:639–49.
Tai MH, Wang LL, Wu KLH, Chan JYH. Increased superoxide anion in the rostral ventrolateral medulla contributes to hypertension in spontaneously hypertensive rats via interactions with nitric oxide. Free Rad Biol Med. 2005;38:450–62.
Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–41.
Hirooka Y. Role of reactive oxygen species in the brainstem in neural mechanisms of hypertension. Auton Neurosci. 2008;142:20–4.
Campos RR. Oxidative stress in the brain and arterial hypertension. Hypertens Res. 2009;32:1047–8.
Hirooka Y, Sagara Y, Kishi T, Sunagawa K. Oxidative stress and central cardiovascular regulation: pathogenesis of hypertension and therapeutic aspects. Circ J. 2010;274:827–35.
Hirooka Y. Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hypertens Res. 2011;34:407–12.
Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol. 2011;300:R818–26.
Haspula D, Clark MA. Neuroinflammation and sympathetic overactivity: mechanisms and implications in hypertension. Auton Neurosci. 2018;210:10–17.
Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, Demkow U. Brain inflammation and hypertension: the chicken or the egg? J Neuroinflammation. 2015;12:85.
Waki H, Gouraud SS, Maeda M, Raizada MK, Paton JFR. Contributions of vascular inflammation in the brainstem for neurogenic hypertension. Respir Physiol Neurobiol. 2011;178:422–8.
Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev. 2017;97:1127–64.
Iyonaga T, Shinohara K, Matsuura T, Hirooka Y, Tsutsui H. Brain perivascular macrophages contribute to the development of hypertension in stroke-prone spontaneously hypertensive rats via sympathetic activation. Hypertens Res. 2019;43:99–110.
Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303.
Takesue K, Kishi T, Hirooka Y, Sunagawa K. Activation of microglia within the paraventricular nucleus of the hypothalamus is NOT involved in the maintenance of established hypertension. J Cardiol. 2017;69:84–8.
Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–802.
Talman WT, Dragon DN. Transmission of arterial baroreflex signals depends on neuronal nitric oxide synthase. Hypertension. 2004;43:820–4.
Talman WT. NO and central cardiovascular control: a simple molecule with a complex story. Hypertension. 2006;48:552–4.
Hirooka Y, Polson JW, Dampney RAL. Pressor and sympathoexcitatory effects of nitric oxide in the rostral ventrolateral medulla. J Hypertens. 1996;14:1317–24.
Zanzinger J, Seller H. Species differences in the distribution of nitric oxide synthase in brain stem regions that regulate sympathetic activity. Brain Res. 1997;764:265–8.
Chen SY, Mao SP, Chai CY. Role of nitric oxide on pressor mechanisms within the dorsomedial and rostral ventrolateral medulla in anesthetized cats. Clin Exp Pharm Physiol. 2001;28:155–63.
Morimoto S, Sasaki S, Miki S, Kawa T, Nakamura K, Itoh H, et al. Nitric oxide is an excitatory modulator in the rostral ventrolateral medulla in rats. Am J Hypertens. 2000;13:1125–34.
Tseng CJ, Liu HY, Lin HC, Ger LP, Tung CS, Yen MH. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension. 1996;27:36–42.
Huang CC, Chan SH, Hsu KS. cGMP/protein kinase G-dependent potentiation of glutamatergic transmission induced by nitric oxide in immature rat rostral ventrolateral medulla neurons in vitro. Mol Pharm. 2003;64:521–32.
Sakai K, Hirooka Y, Matsuo I, Eshima K, Shigematsu H, Shimokawa H, et al. Overexpression of eNOS in NTS causes hypotension and bradycardia in vivo. Hypertension. 2000;36:1023–8.
Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901.
Kishi T, Hirooka Y, Ito K, Sakai K, Shimokawa H, Takeshita A. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension. 2002;39:264–8.
Kishi T, HIrooka Y, Kimura Y, Sakai K, Ito K, Shimokawa H, et al. Overexpression of eNOS in RVLM improves impaired baroreflex control of heart rate in SHRSP. Hypertension. 2003;41:255–60.
Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–84.
Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, et al. Nitric oxide actions in the paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol Regul Integr Comp Physiol. 1994;266:R306–13.
Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol. 1997;273:R864–72.
Zheng H, katsurada K, Nandi S, Li Y, Patel KP. A critical role for the paraventricular nucleus of the hypothalamus in the regulation of the volume reflex in normal and various cardiovascular disease states. Curr Hypertens Rep. 2022;24:235–46.
Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contributes to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004;109:2357–62.
Kishi T, Hirooka Y, Shimokawa H, Takeshita A, Sunagawa K. Atorvastatin reduced oxidative stress in the rostral ventrolateral medulla of stroke-prone spontaneously hypertensive rats. Clin Exp Hypertens. 2008;30:3–11.
Kishi T, Hirooka Y, Konno S, Sunagawa K. Sympathoinhibition induced by centrally administered atorvastatin is associated with alteration of NAD(P)H oxidase and Mn superoxide dismutase activity in rostral ventrolateral medulla of stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharm. 2010;55:184–90.
Kishi T, Sunagawa K. Experimental ‘jet lag’ causes sympathoexcitation via oxidative stress through AT1 receptor in the brainstem. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1969–72.
Kishi T, Hirooka Y, Ogawa K, Konno S, Sunagawa K. Calorie restriction inhibits sympathetic nerve activity via anti-oxidant effect in the rostral ventrolateral medulla of obesity-induced hypertensive rats. Clin Exp Hypertens. 2011;33:240–5.
Kishi T, Hirooka Y, Sunagawa K. Sympathoinhibition caused by orally administered telmisartan through inhibition of AT(1) receptor in the rostral ventrolateral medulla. Hypertens Res. 2012;35:940–6.
Kishi T, Hirooka Y, Katsuki M, Ogawa K, Shinohara K, Isegawa K, et al. Exercise training causes sympathoinhibition through antioxidant effect in the rostral ventrolateral medulla of hypertensive rats. Clin Exp Hypertens. 2012;34:278–83.
Kishi T, Sunagawa K. Combination therapy of atorvastatin and amlodipine inhibits sympathetic nervous system activation and improves cognitive function in hypertensive rats. Circ J. 2012;76:1934–41.
Konno S, Hirooka Y, Araki S, Koga Y, Kishi T, Sunagawa K. Azelnidipine decreases sympathetic nerve activity via antioxidant effect in the rostral ventrolateral medulla of stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharm. 2008;52:555–60.
Koga Y, Hirooka Y, Araki S, Nozoe M, Kishi T, Sunagawa K. High salt intake enhances blood pressure increase during development of hypertension via oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertens Res. 2008;31:2075–83.
Nishihara M, Hirooka Y, Matsukawa R, Kishi T, Sunagawa K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens. 2012;30:97–106.
Konno S, Hirooka Y, Kishi T, Sunagawa K. Sympathoinhibitory effect of telmisartan through the reduction of oxidative stress in rostral ventrolateral medulla of obesity-induced hypertensive rat. J Hypertens. 2012;30:1992–9.
Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension. 2007;50:360–7.
Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation. 2009;119:978–86.
Oliveira-Sales EB, Dugaich AP, Carillo BA, Abreu NP, Boim MA, Martins PJ, et al. Oxidative stress contributes to renovascular hypertension. Am J Hypertens. 2008;21:98–104.
Oliveira-Sales EB, Nishi EE, Carillo BA, Dolnikoff MS, Bergamaschi CT, Campos RR. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am J Hypertens. 2009;22:484–92.
Oliveira-Sales EB, Colombari DSA, Davisson RL, Kasparov S, Hirata AE, Campos RR, et al. Kidney-induced hypertension depends on superoxide signaling in the rostral ventrolateral medulla. Hypertension. 2010;56:290–6.
Nishi EE, Almedia VR, Amaral FG, Simon KA, Futuro-Neto HA, Pontes RB, et al. Melatonin attenuates renal sympathetic overactivity and reactive oxygen species in the brain in neurogenic hypertension. Hypertens Res. 2019;42:1683–91.
Chan SH, Chan JY. Brain stem NOS and ROS in neural mechanisms of hypertension. Antioxid Redox Signal. 2014;20:146–63.
Xia WJ, Liu KL, Wang XM, Yang Y, Meng T, Qiao JA, et al. Hypothalamic paraventricular nucleus hydrogen sulfide exerts antihypertensive effects in spontaneously hypertensive rats via the Nrf2 pathway. Am J Hypertens. 2023 https://doi.org/10.1093/ajh/hpad012.
Niu LG, Sun N, Liu KL, Su Q, Qi J, Fu LY, et al. Genistein alleviates oxidative stress and inflammation in the hypothalamic paraventricular nucleus by activating the Sirt1/Nrf2 pathway in high salt-induced hypertension. Cardiovasc Toxicol. 2022;22:898–909.
Nozoe M, Hirooka Y, Koga Y, Araki S, Konno S, Kishi T, et al. Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J Hypertens. 2008;26:2176–84.
Kishi T, Hirooka Y, Konno S, Ogawa K, Sunagawa K. Angiotensin II type 1 receptor activated caspase-3 through ras/mitogen-activated protein kinase/extracellular signal-regulated kinase in the rostral ventrolateral medulla is involved in sympathoexcitation in stroke-prone spontaneously hypertensive rats. Hypertension. 2010;55:291–7.
Chan SH, Hsu KS, Huang CC, Wang LL, Qu CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–80.
Hu L, Zhu DN, Yu Z, Wang JQ, Sun ZJ, Yao T. Expression of angiotensin type 1 (AT1) receptor in the rostral ventrolateral medulla in rats. J Appl Physiol. 2002;92:2153–61.
Reja V, Goodchild AK, Phillips JK, Pilowsky PM. Upregulation of angiotensin AT1 receptor and intracellular kinase gene expression in hypertensive rats. Clin Exp Pharm Physiol. 2006;33:690–5.
Leenen FHH. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res. 2007;101:221–3.
Huang BS, Leenen FHH. The brain renin-angiotensin-aldosterone system: a major mechanism for sympathetic hyperactivity and left ventricular remodeling and dysfunction after myocardial infarction. Curr Heart Fail Rep. 2009;6:81–8.
Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am J Physiol. 2009;297:H1557–66.
Dupont AG, Brouwers S. Brain angiotensin peptides regulate sympathetic tone and blood pressure. J Hypertens. 2010;28:1599–610.
Nozoe M, Hirooka Y, Koga Y, Sagara Y, Kishi T, Engelhardt JF, et al. Inhibition of Rac1-derived reactive oxygen species in nucleus tractus solitarius decreases blood pressure and heart rate in stroke-prone spontaneously hypertensive rats. Hypertension. 2007;50:62–8.
Zimmerman MC, Zucker IH. Mitochondrial dysfunction and mitochondria-produced reactive oxygen species: new aspects for neurogenic hypertension? Hypertension. 2009;53:112–4.
Hirooka Y, Potts PD, Dampney RAL. Role of angiotensin II receptor subtypes in mediating the sympathoexcitatory effects of exogenous and endogenous angiotensin peptides in the rostral ventrolateral medulla. Brain Res. 1997;772:107–14.
Dampney RAL, Tan PSP, Sheriff MJ, Fontes MAP, Horiuchi J. Cardiovascular effects of angiotensin II in the rostral ventrolateral medulla: the push-pull hypothesis. Curr Hypertens Rep. 2007;9:222–7.
Shinohara K, Hirooka Y, Kishi T, Sunagawa K. Reduction of nitric oxide-mediated γ-amino butyric acid release in the rostral ventrolateral medulla is involved in superoxide-induced sympathoexcitation of hypertensive rats. Circ J. 2012;76:2814–21.
Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, et al. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analysis. J Biol Chem. 2012;287:2984–95.
Zanzinger J. Mechanisms of action of nitric oxide in the brain stem: role of oxidative stress. Auton Neurosci. 2002;98:24–7.
Sun J, Druhan LJ, Zweler JL. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch Biochem Biophys. 2010;494:130–7.
Kung LC, Chan SH, Wu KL, Ou CC, Tai MH, Chan JY. Mitochondrial respiratory enzyme complexes in rostral ventrolateral medulla as cellular targets of nitric oxide and superoxide interaction in the antagonism of antihypertensive action of eNOS transgene. Mol Pharm. 2008;74:1319–32.
Rhee SG. Cell signaling, H2O2, is a necessary evil for cell signaling. Science. 2006;312:1882–3.
Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–9.
Hirooka Y. Brain perivascular macrophages and central sympathetic activation after myocardial infarction: heart and brain interaction. Hypertension. 2010;55:610–1.
Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kB in the paraventricular nucleus. Hypertension. 2011;59:113–21.
Kishi T. Disruption of central antioxidant property of nuclear factor erythroid 2-related factor 2 worsens circulatory homeostasis with baroreflex dysfunction in heart failure. Int J Mol Sci. 2018;19:646.
Kishi T. Heart failure is a disruption of dynamic circulatory homeostasis mediated by the brain. Int Heart J. 2016;57:145–9.
Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res. 2012;35:132–41.
Matsuura T, Kumagai H, Kawai A, Onimaru H, Imai M, Oshima N, et al. Rostral ventrolateral medulla neurons of neonatal Wister-Kyoto and spontaneously hypertensive rats. Hypertension. 2002;40:560–5.
Oshima N, Kumagai H, Onimaru H, Kawai A, Pilowski PM, Iigaya K, et al. Monosynaptic excitatory connection from the rostral ventrolateral medulla to sympathetic preganglionic neurons revealed by simultaneous recording. Hypertens Res. 2008;31:1445–54.
Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in the rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation. 2012;9:212.
Gao L, Li Y, Schultz HD, Wang WZ, Finch M, Smith LM, et al. Downregulated Kv4.3 expression in the RVLM as a potential mechanism for sympathoexcitation in rats with chronic heart failure. Am J Physiol. 2010;298:H945–55.
Wang JM, Tan J, Leenen FHH. Central nervous system blockade by peripheral administration of AT1 receptor blockers. J Cardiovasc Pharm. 2003;41:593–9.
Tsuchihashi T, Kagiyama S, Matsumura K, Abe I, Fujishima M. Effects of chronic oral treatment with imidapril and TCV-116 on the responsiveness to angiotensin II in ventrolateral medulla of SHR. J Hypertens. 1999;17:917–22.
Nishimura Y, Ito T, Hoe KL, Saavedra JM. Chronic peripheral administration of the angiotensin II AT1 receptor antagonist candesartan blocks brain AT1 receptors. Brain Res. 2000;871:29–38.
Gohlke P, Weiss S, Jansen A, Wienen W, Stangier J, Rascher W, et al. AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharm Exp Ther. 2001;298:62–70.
McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, et al. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–18.
Leenen FHH, Yuan B. Prevention of hypertension by irbesartan in Dahl S rats relates to central angiotensin II type 1 receptor blockade. Hypertension. 2001;37:981–4.
Lin Y, Matsumura K, Kagiyama S, Fukuhara M, Fujii K, Iida M. Chronic administration of olmesartan attenuates the exaggerated pressor response to glutamate in the rostral ventrolateral medulla of SHR. Brain Res. 2005;1058:161–6.
Araki S, Hirooka Y, Kishi T, Yasukawa K, Utsumi H, Sunagawa K. Olmesartan reduces stress in the brain of stroke-prone spontaneously hypertensive rats assessed by an in vivo ESR method. Hypertens Res. 2009;32:1091–6.
Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: Results from the USCD Statin Study, a randomized trial. Arch Intern Med. 2008;168:721–7.
Sinski M, Lewandowsk J, Ciarka A, Bidiuk J, Abramczyk P, Dobosiewicz A, et al. Atorvastatin reduced sympathetic activity and increased baroreceptor reflex sensitivity in patients with hypercholesterolemia and systemic arterial hypertension. Kardiol Pol. 2009;67:613–20.
Kishi T, Hirooka Y. Sympathoinhibitory effects of atorvastatin in hypertension. Circ J. 2010;74:2552–3.
Siddiqi L, Joles JA, Oey PL, Blankestijn PJ. Atorvastatin reduced sympathetic activity in patients with chronic kidney disease. J Hypertens. 2011;29:2176–80.
Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–5.
Kishi T, Hirooka Y, Mukai Y, Shimokawa H, Takeshita A. Atorvastatin causes depressor and sympathoinhibitory effects with upregulation of nitric oxide synthase in stroke-prone spontaneously hypertensive rats. J Hypertens. 2003;21:379–86.
Hirooka Y, Kimura Y, Nozoe M, Sagara Y, Ito K, Sunagawa K. Amlodipine-induced reduction of oxidative stress in the brain is associated with sympatho-inhibitory effects in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2006;29:49–56.
Shinohara K, Hirooka Y, Ogawa K, Kishi T, Yasukawa K, Utsumi H, et al. Combination therapy of olmesartan and azelnidipine inhibits sympathetic activity associated with reducing oxidative stress in the brain of hypertensive rats. Clin Exp Hypertens. 2012;34:456–64.
Iwanami J, Mogi M, Iwai M, Horiuchi M. Inhibition of the renin-angiotensin system and target organ protection. Hypertens Res. 2009;32:229–37.
Mogi M, Horiuchi M. Effects of angiotensin II receptor blockers on dementia. Hypertens Res. 2009;32:738–40.
Krum H, Schlaich MP, Whitbourn R, Sobotka P, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81.
DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol. 2010;298:R245–53.
Simplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Simplicity HTN-2 Trail): a randomized controlled trial. Lancet. 2010;376:1903–9.
Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in cat. J Auton Nerv Syst. 1981;3:311–20.
Stella A, Golin R, Genovesi S, Zanchetti A. Renal reflexes in the regulation of blood pressure and sodium excretion. Can J Physiol Pharm. 1987;65:1536–9.
Ye S, Zhong H, Campese VM. Oxidative stress mediates the sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension. 2006;48:309–15.
Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2005;46:533–9.
Kishi T. Regulation of the sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 academic conference award from the Japanese Society of Hypertension. Hypertens Res. 2013;36:845–51.
Acknowledgements
A Grant-in-Aid for Scientific Research supported this study from the Japan Society for the Promotion of Science (15590757, 17590745, 19390231, and 22790709) and, in part, a Kimura Memorial Foundation Research Grant. I want to appreciate Prof. Yoshitaka Hirooka, Kenji Sunagawa, and Akira Takeshita significantly. Furthermore, I express special thanks to Satomi Konno.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kishi, T. Clarification of hypertension mechanisms provided by the research of central circulatory regulation. Hypertens Res 46, 1908–1916 (2023). https://doi.org/10.1038/s41440-023-01335-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01335-6