Abstract
Raised blood pressure affects around ten percent of pregnancies worldwide, causing maternal and perinatal morbidity and mortality. Self-monitoring of blood pressure during higher-risk or hypertensive pregnancy has been shown to be feasible, acceptable, safe, and no more expensive than usual care alone. Additionally, self-testing for proteinuria has been shown to be just as accurate as healthcare professional testing, creating the potential for monitoring of multiple indicators through pregnancy. The work suggests however, that an organisational shift is needed to properly use and see benefits from self-monitored readings. This paper describes the findings from a large programme of work examining the use of self-monitoring in pregnancy, summarising the findings in the context of the wider literature and current clinical context.

The BUMP Research Programme developed and tested self-monitoring and self-testing interventions for pregnancy. The work showed that self-monitoring during pregnancy was feasible, acceptable, safe, and no more expensive, but did not improve the detection or control of hypertension.
Similar content being viewed by others
Introduction
Raised blood pressure (BP) affects ~10% of pregnancies, and is associated with an increased risk of developing pre-eclampsia, and in the long-term an increased risk of chronic hypertension and cardiovascular disease [1, 2]. The early detection and subsequent management of pregnancy hypertension is important, in order to optimise pregnancy outcomes for the woman and infant. Unlike BP monitoring and management outside of pregnancy, BP can change rapidly during pregnancy and changes can be difficult to predict [1].
Self-monitoring of blood pressure (SMBP), where an individual measures their own BP outside of the clinical setting, is effective at detecting and lowering blood pressure in adults with hypertension, and now commonplace outside of pregnancy [3,4,5]. In pregnancy and the postnatal period, SMBP has the potential to improve the detection and management of raised BP whilst empowering women and potentially reducing clinic visits [6].
Interest in the opportunities for SMBP in pregnancy and the postnatal period is increasing. In the last five years, several studies have reported research around the use of SMBP during pregnancy, from settings around the world including Europe, Asia and North America. Early work from small observational studies suggested the potential for reduced morbidity and resource use, as well as acceptability and feasibility, yet evidence from large-scale randomised trials for the effectiveness of SMBP in pregnancy has been lacking until recently [7,8,9]. This article reviews the findings from the Blood Pressure Monitoring in Pregnancy (BUMP) programme of work, undertaken in the United Kingdom (UK), in this rapidly developing area [10,11,12,13].
Prevalence of self-monitoring of blood pressure
A large UK survey in 2019 of pregnant individuals found that overall, around one in five were currently self-monitoring their BP; of those who self-identified as hypertensive, half reported SMBP. However, many of those self-monitoring did not share their readings with their antenatal care team and were not necessarily using pregnancy-validated BP monitors [12]. These findings are in line with a Canadian-based study, which found more than 60% of pregnant women diagnosed with hypertension were already undertaking SMBP [14]. This evidence suggests SMBP has become relatively widespread, and so health care professionals (HCPs) should enquire about SMBP proactively and consider providing information on BP monitoring.
Self-monitoring thresholds in pregnancy are not clear
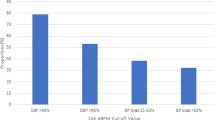
A systematic review and individual patient data analysis aimed to explore BP thresholds in pregnancy. The search found over 20 studies and analysed patient data from eight studies (n = 758), revealing a mean home-clinic difference of just ≤1.2 mmHg systolic BP throughout pregnancy. However, there was significant heterogeneity between studies (I2 > 80%); only one study was randomised, and only two of the studies used a monitor validated for use in pregnancy. Although the overall population difference was small (around 2 mmHg systolic), levels of ‘white coat hypertension’ were high, particularly towards the end of pregnancy, when around half of those with clinic hypertension had normal range home readings. The home-office difference was much greater in those with hypertension (predominantly over 10 mmHg systolic through pregnancy) [15]. Data from the OPTIMUM pilot trial and BUMP trials show similar findings [16, 17]. More recent systematic reviews (not including the BUMP trial data) report similar findings with significant clinical heterogeneity [9].
Trials of self-monitoring of blood pressure
Until recently, few data were available regarding SMBP as an intervention in pregnancy; a systematic review found just one previous randomised controlled trial of self-monitoring in an antenatal setting with normotensive women [18]. This included 80 low-risk pregnancy women who were randomised to undertake either weekly self-monitoring with reduced routine antenatal clinics or usual care (but did not evaluate the impact of self-monitoring on the timing of new diagnoses of pregnancy hypertension). We found one additional trial in which a US group randomised 300 low-risk pregnant women to remote monitoring with reduced clinic visits or usual care. The women randomised to remote care had reduced obstetric input but more nurse/midwife time was needed for the remote care. Other maternal and perinatal outcomes were similar between the groups. The BUMP pilot study and OPTIMUM-BP pilot trial established feasibility in the UK health service of SMBP in higher risk and hypertensive pregnancies and supported the development of the BUMP trials, which aimed to assess the place of self-monitoring in the diagnosis (BUMP1) and management (BUMP2) of hypertension in pregnancy [10, 11].
The BUMP trials were two fully powered randomised controlled trials of SMBP in pregnancy that recruited more than 3000 women at higher risk of pre-eclampsia, or with pregnancy hypertension. Participating women were randomly allocated to either usual care or usual care plus SMBP.
The BUMP1 trial recruited 2441 pregnant women at higher risk of pre-eclampsia around 20 weeks’ gestation with the aim of detecting raised BP earlier than standard clinic visits. The trial found that SMBP during higher-risk pregnancy appeared to be safe, but did not significantly improve earlier clinic-based detection of hypertension when used alongside usual care. Despite this, there was a signal of the potential of SMBP: self-monitoring did provide potential prior notice of hypertension in many women, with 61% (109/179) of those with hypertension reporting raised SMBP around one month prior to clinic diagnosis (median 29 days earlier). This suggests that SMBP in pregnancy could have been used to detect hypertension earlier, but the information was not necessarily acted upon by clinicians (or possibly by women). Further work is required to assess the place of self-monitoring of BP in this higher-risk population, for instance in remote consultations or alongside self-management, and to understand how to better engage HCPs in its use [11].
The BUMP2 trial recruited 850 women with gestational or chronic hypertension, with the aim of improving BP control (as had been found outside of pregnancy) [4]. The intervention of SMBP did not result in improved BP control as assessed by clinic systolic BP but was acceptable, with no difference in adverse maternal or infant outcomes, and was equivalent in cost to usual care alone [19]. As with using monitoring for earlier diagnosis, further co-interventions may be required in order to achieve improvements in BP control and other pregnancy outcomes [10].
For the BUMP2 trial, many women’s average blood pressure was above the NICE target of 135/85mmHgh based on their clinic readings, suggesting additional titration of antihypertensive medication might have been needed [10]. Additionally, a higher proportion than expected in the usual care arm (68%) reported prior SMBP, but these data were not available during the trial itself. These considerations, in combination with the survey results, suggest that SMBP is already part of women’s health behaviours but has not yet been fully or formally incorporated into care pathways [12].
Understanding the trial findings
A process evaluation of the trials showed that the majority of women and healthcare professionals involved in the trial found that SMBP enhanced their experiences of the clinical encounter and the HCP-woman relationship [20, 21]. However, the pursuit of normal readings, selective or delayed reporting of raised readings, and variable engagement by HCPs with home readings, could have influenced the impact of the BUMP intervention. Interviews and observations suggest that SMBP led to both earlier detection of rising BP, but also was used as justification for not starting intensifying antihypertensive medication when home readings were lower [20, 21]. Finally, SMBP by women in the usual care arm may have reduced between-groups differences, limiting the evidence for the intervention’s effectiveness.
What helps (or hinders) SMBP in practice?
Qualitative work from linked studies showed that women were highly motivated and empowered by SMBP, reporting greater control, knowledge and reassurance. Interviews and ethnographic observations showed that good communication and effective partnerships between women and clinicians underpinned the use of SMBP, and may in turn have supported shared decision-making [22].
However, uncertainty remains around home-clinic differences and how this information could be translated into clinical decisions, with the qualitative work in the BUMP trials revealing that some clinicians had higher confidence in clinical readings (rather than home readings) when it came to taking action [15, 20].
Proteinuria self-testing in pregnancy
Linked to the BUMP programme, the Proteinuria Detection In Pregnancy (UDIP) diagnostic accuracy study compared the performance of proteinuria testing by women, midwives and automated colorimetric readers. It showed that pregnant women could detect dipstick proteinuria with similar accuracy to healthcare professionals, suggesting urine self-testing alongside BP self-monitoring would be feasible. Furthermore, the study also showed that automated colorimetric testing was not significantly different to performance by women or healthcare professionals. Although the accuracy of urine dipstick testing is not as high as desired in a diagnostic test, testing in the context of antenatal care, which includes repeated testing, is likely to be beneficial [13]. Furthermore, using protein testing in combination with home BP monitoring could allow better reassurance to both healthcare professionals and women that the important indicators of evolving pre-eclampsia are being monitored.
The cost-effectiveness of SMBP
Cost-effectiveness analyses of the BUMP 1&2 trials showed that SMBP had no additional cost over and above usual care [19]. The use of SMBP may be better value for money when used for women with pregnancy hypertension than for women at risk of hypertension, due to a greater risk of developing complications both during and following pregnancy in these women. These findings are in keeping with previous smaller cost-effectiveness studies in the Netherlands and the UK [23, 24]. A key issue is provision of validated monitors and in the future they might also be available for post-partum monitoring and beyond as this is often warranted [25,26,27]. During the COVID-19 pandemic, the National Health Service in England provided some BP monitors for those with hypertensive pregnancy (see below) and some hospitals may have a small number of monitors for loan [28]. However, for many in the UK, the only option for a pregnant woman is to purchase a monitor, which may result in exclusion of some groups.
Acceptability of SMBP in the BUMP trials
The SMBP studies described above had good uptake, and linked qualitative work has shown that SMBP is acceptable to higher risk and hypertensive pregnant women and their antenatal care teams [7]. A recent survey suggests that most UK obstetricians (96%) now consider SMBP to be part of current antenatal care [29]. While SMBP during pregnancy has been shown to be acceptable, safe, and no more expensive than usual care, the challenge now is to understand how it can be most effectively integrated into existing care pathways and used to make improvements to outcomes.
Could self-monitoring be used to guide tighter BP control?
The CHIPS international study of targeting diastolic BP as the intervention showed that tighter control of hypertension was associated with fewer severe hypertension episodes and is safe [30]. More recently, the CHAP study has shown that antihypertensive treatment of mild to moderate chronic hypertension improves pregnancy outcomes, without increased detection of babies small for gestational age [31]. There is a clear rationale for managing women’s blood pressure with tight control, targeting a diastolic BP of 85 mmHg. SMBP could support this change in practice towards earlier and more active management, but would require further work in ensuring that women and healthcare professionals understand and take appropriate action on self-monitored BP readings.
Lessons from rapid implementation of SMBP during the COVID-19 pandemic
The COVID-19 pandemic resulted in an urgent shift from clinic-based maternity care to greater remote monitoring, necessitating the rapid implementation of SMBP. In the UK, guidelines were quickly produced, recommending that SMBP was prioritised for women with hypertension or pre-eclampsia, those with risk factors, or those required to self-isolate [32]. Implementation was further supported by the provision of BP monitors by the National Health Service in England, validated for use in pregnancy. During this time, SMBP was predominantly used to provide additional BP monitoring for hypertensive or high-risk pregnant women, rather than replacing face-to-face visits for normal-risk women. Maternity units and women were positive about monitoring to reduce clinic visits and to give women more control and insight into their own BP. However, there were implementation challenges particularly around embedding SMBP into existing care pathways, interpreting readings and managing the provision of monitors [28]. This revealed a need for further research into appropriate care pathways and information and guidance around management of white coat or masked (high home BP readings, normal clinic readings) hypertension.
These implementation studies are part of a wider emerging evidence base and health system interest in virtual care [33]. The rapid reconfiguration of antenatal services in response to the pandemic has highlighted a significant shift to remote provision, of which SMBP was an integral component [34,35,36]. A large UK study undertaken during the COVID-19 pandemic, highlighted the potential accessibility and efficiency advantages of remote antenatal care reported by women including saving time, stress, travel expenses and reducing time off work and/or childcare [7]. The study enabled the development of a maternity-specific framework of the domains of quality that appear most relevant to stakeholders in remote antenatal care: efficiency and timeliness; effectiveness; safety; accessibility; equity and inclusion; person-centredness and choice and continuity [37].
Future directions addressing the evidence gaps
Developing clinical pathways for SMBP as a system
The studies above have shown there remains a need for further evidence around antihypertensive treatment in pregnancy, including guidance on up-titration and optimal antihypertension medication in the context of SMBP, and the clinical significance of white coat (or masked) hypertension.
Successful implementation will require consideration of the shifts in workload between staff and pregnant women that SMBP will entail, as well as support for healthcare professionals to overcome barriers, such as long-held beliefs about accuracy of automated BP monitors in pregnancy and concerns about the foetal impact of tight BP control, despite recent evidence supporting this strategy [30, 31]. Trusted educational resources for both healthcare teams and pregnant people will be needed to support any such implementation. SMBP appears to work best with good relationships and communication between women and their health care professionals and this should be supported as an integral part of the intervention [22].
Self-monitoring for multiple indicators
The new development of hypertension and pre-eclampsia are a substantial concern through pregnancy, and antenatal care includes monitoring for a range of indicators of evolving disease. The UDIP study described above showed that self-testing for proteinuria could support remote care of women with hypertension, and indeed around half of maternity units surveyed during the pandemic were also offering proteinuria self-testing in selected groups [28]. Companies are beginning to develop kits specially design for home testing in pregnancy and some studies in pregnancy are including additional interventions such as home foetal monitoring through cardiotocography and symptom checklists [38, 39]. Robust evaluation of the clinical outcomes of such multi-faceted interventions is awaited.
Ensuring equity in research and implementation
The long-standing inequalities in maternity outcomes have been amplified and thrown into even sharper focus by the pandemic [40, 41]. Addressing the challenges of these inequalities requires a high-quality and inclusive evidence base [42]. Without this approach, there are risks that the introduction of new modes of antenatal care might compound the problems of marginalisation, disadvantage and clinical risk for women in some most at-risk groups [37, 42]. This evidence is currently lacking. While studies examining the use of remote monitoring and telehealth show promising results, as outlined above, they are not conclusive. Many have assessed only individual components of maternity care, are generally small scale and not reflective of diverse populations [43,44,45,46]. More generally, research focussed on digital health and access to care has remained very limited [47]. While we may well be witnessing a steady shift towards increasing inclusion of self-monitoring and remote care in antenatal care pathways, we need to focus on the potentially important implications that this shift has for access to care, and potentially on clinical outcomes [33].
Summary and conclusions
These large SMBP trials, alongside shifts in care pathways introduced during the COVID-19 pandemic, have served to test the implementation of SMBP, but a further organisational shift is needed to see definitive benefit. While pregnant women are willing and able to use and interpret SMBP readings, current care pathways need to evolve further to facilitate potential impact on clinical outcomes. System changes can often be hard, and implementation of this complex intervention is not straightforward. This body of research has shown that, both outside of pregnancy and now for the pregnant population, the intervention needs to be multi-faceted and system-wide, beyond handing out BP monitors, in order to change clinical outcomes [4, 10, 11].
What is needed now is a system response that addresses evidence gaps such as the addressing home-clinic BP difference, understanding the most effective antihypertensive mediation and titration schedules to use in pregnancy while adequately considering issues of equity in research and implementation.
References
Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. National Institute for Health and Clinical Excellence: Guidance. London 2010.
Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74.
Baral-Grant S, Haque MS, Nouwen A, Greenfield SM, McManus RJ. Self-monitoring of blood pressure in hypertension: a UK primary care survey. Int J Hypertens. 2012;2012:582068.
Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
Constanti M, Boffa R, Floyd CN, Wierzbicki AS, McManus RJ, Glover M. Options for the diagnosis of high blood pressure in primary care: a systematic review and economic model. J Hum Hypertens. 2020;35:455–61.
Hodgkinson JA, Tucker KL, Crawford C, Greenfield SM, Heneghan C, Hinton L, et al. Is self-monitoring of blood pressure in pregnancy safe and effective? BMJ. 2014;349:g6616.
Hinton L, Tucker KL, Greenfield SM, Hodgkinson JA, Mackillop L, McCourt C, et al. Blood pressure self-monitoring in pregnancy (BuMP) feasibility study; a qualitative analysis of women’s experiences of self-monitoring. BMC Preg Childbirth. 2017;17:427.
Tucker KL, Taylor KS, Crawford C, Hodgkinson JA, Bankhead C, Carver T, et al. Blood pressure self-monitoring in pregnancy: examining feasibility in a prospective cohort study. BMC Preg Childbirth. 2017;17:442.
Kalafat E, Benlioglu C, Thilaganathan B, Khalil A. Home blood pressure monitoring in the antenatal and postpartum period: a systematic review meta-analysis. Preg Hypertens. 2020;19:44–51.
Chappell LC, Tucker KL, Galal U, Yu LM, Campbell H, Rivero-Arias O, et al. Effect of self-monitoring of blood pressure on blood pressure control in pregnant individuals with chronic or gestational hypertension: the BUMP 2 randomized clinical trial. JAMA. 2022;327:1666–78.
Tucker KL, Mort S, Yu LM, Campbell H, Rivero-Arias O, Wilson HM, et al. Effect of self-monitoring of blood pressure on diagnosis of hypertension during higher-risk pregnancy: the BUMP 1 randomized clinical trial. JAMA. 2022;327:1656–65.
Tucker KL, Hodgkinson J, Wilson HM, Crawford C, Stevens R, Lay-Flurrie S, et al. Current prevalence of self-monitoring of blood pressure during pregnancy: the BUMP survey. J Hypertens. 2021;39:994–1001.
Jakubowski BE, Stevens R, Wilson H, Lavallee L, Brittain L, Crawford C, et al. Cross-sectional diagnostic accuracy study of self-testing for proteinuria during hypertensive pregnancies: the UDIP study. BJOG. 2022;129:2142–8.
Magee LA, von Dadelszen P, Chan S, Gafni A, Gruslin A, Helewa M, et al. Women’s views of their experiences in the CHIPS (Control of Hypertension in Pregnancy Study) pilot trial. Hypertens Preg. 2007;26:371–87.
Tucker KL, Bankhead C, Hodgkinson J, Roberts N, Stevens R, Heneghan C, et al. How do home and clinic blood pressure readings compare in pregnancy? Hypertension 2018;72:686–94.
Bowen L, Pealing L, Tucker K, McManus RJ, Chappell LC. Adherence with blood pressure self-monitoring in women with pregnancy hypertension, and comparisons to clinic readings: a secondary analysis of OPTIMUM-BP. Preg Hypertens. 2021;25:68–74.
Pealing LM, Tucker KL, Mackillop LH, Crawford C, Wilson H, Nickless A, et al. A randomised controlled trial of blood pressure self-monitoring in the management of hypertensive pregnancy. OPTIMUM-BP: a feasibility trial. Preg Hypertens. 2019;18:141–9.
Ross-McGill H, Hewison J, Hirst J, Dowswell T, Holt A, Brunskill P, et al. Antenatal home blood pressure monitoring: a pilot randomised controlled trial. BJOG. 2000;107:217–21.
McManus RJ, Chappell L, Tucker K, Hinton L, Rivero-Arias O, Allen J, et al. Optimising the monitoring and management of raised blood pressure during pregnancy: The BUMP Research Programme. NIHR Programme Grant Report; 2023.
Chisholm A, Tucker KL, Crawford C, Green M, Greenfield S, Hodgkinson J, et al. Self-monitoring blood pressure in pregnancy: evaluation of health professional experiences of the BUMP trials. BMC Preg Childbirth - Submitted for publication. 2023.
Chisholm A, Tucker KL, Crawford C, Green M, Greenfield S, Hodgkinson J, et al. Self-monitoring blood pressure in pregnancy: evaluation of pregnant people’s experiences of the BUMP trials. BMC Preg Childbirth - Submitted for publication. 2023.
Pealing L, Tucker KL, Fletcher B, Lawley E, Chappell LC, McManus RJ, et al. Perceptions and experiences of blood pressure self-monitoring during hypertensive pregnancy: A qualitative analysis of women’s and clinicians’ experiences in the OPTIMUM-BP trial. Preg Hypertens. 2022;30:113–23.
Lanssens D, Vandenberk T, Smeets CJ, De Canniere H, Vonck S, Claessens J, et al. Prenatal remote monitoring of women with gestational hypertensive diseases: cost analysis. J Med Internet Res. 2018;20:e102.
Xydopoulos G, Perry H, Sheehan E, Thilaganathan B, Fordham R, Khalil A. Home blood-pressure monitoring in a hypertensive pregnant population: cost-minimization study. Ultrasound Obstet Gynecol. 2019;53:496–502.
Cairns AE, Tucker KL, Crawford C, McManus RJ, Powell J. Implementing self-management: a mixed methods study of women’s experiences of a postpartum hypertension intervention (SNAP-HT). Trials. 2020;21:508.
Cairns AE, Tucker KL, Leeson P, Mackillop LH, Santos M, Velardo C, et al. Self-management of postnatal hypertension: the SNAP-HT Trial. Hypertension. 2018;72:425–32.
Giorgione V, Jansen G, Kitt J, Ghossein-Doha C, Leeson P, Thilaganathan B. Peripartum and long-term maternal cardiovascular health after preeclampsia. Hypertension. 2023;80:231–41.
Wilson H, Tucker KL, Chisholm A, Hodgkinson J, Lavallee L, Mackillop L, et al. Self-monitoring of blood pressure in pregnancy: a mixed methods evaluation of a national roll-out in the context of a pandemic. Preg Hypertens. 2022;30:7–12.
Fletcher B, Chappell LC, Lavallee L, Wilson HM, Stevens R, Mackillop L, et al. Changes to management of hypertension in pregnancy, and attitudes to self-management: An online survey of obstetricians, before and following the first wave of the COVID-19 pandemic. Preg Hypertens. 2021;26:54–61.
Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–17.
Tita AT, Szychowski JM, Boggess K, Dugoff L, Sibai B, Lawrence K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781–92.
RCOG. Royal College of Obstetricians and Gynaecologists guidlines—self-monitoring of blood pressure in pregnancy https://www.rcog.org.uk/globalassets/documents/guidelines/2020-03-30-self-monitoring-of-blood-pressure-in-pregnancy.pdf2020
Herzer KR, Pronovost PJ. Ensuring quality in the era of virtual care. JAMA 2021;325:429–30.
Jardine J, Relph S, Magee LA, von Dadelszen P, Morris E, Ross-Davie M, et al. Maternity services in the UK during the coronavirus disease 2019 pandemic: a national survey of modifications to standard care. BJOG. 2021;128:880–9.
Coxon K, Turienzo CF, Kweekel L, Goodarzi B, Brigante L, Simon A, et al. The impact of the coronavirus (COVID-19) pandemic on maternity care in Europe. Midwifery. 2020;88:102779.
Davis-Floyd R, Gutschow K, Schwartz DA. Pregnancy, birth and the COVID-19 pandemic in the United States. Med Anthropol. 2020;39:413–27.
Hinton L, Dakin FH, Kuberska K, Boydell N, Willars J, Draycott T, et al. Quality framework for remote antenatal care: qualitative study with women, healthcare professionals and system-level stakeholders. BMJ Qual Saf. 2022. https://doi.org/10.1136/bmjqs-2021-014329. Epub ahead of print.
Bekker NM. Home-telemonitoring versus hospital care in complicated pregnancies: the randomised controlled HoTeL trial. Lancet Digit Health. 2023;5:e116–e124.
Burke AE, Thaler KM, Geva M, Adiri Y. Feasibility and acceptability of home use of a smartphone-based urine testing application among women in prenatal care. Am J Obstet Gynecol. 2019;221:527–8.
Knight M, Bunch K, Tuffnell D, Patel R, Shakespeare J, Kotnis R, et al. Saving Lives, Improving Mothers’ Care Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2017-19. on behalf of MBRRACE-UK National Perinatal Epidemiology Unit, University of Oxford; 2021.
Draper ES, Gallimore ID, Smith LK, Matthews RJ, Fenton AC, Kurinczuk JJ, et al. on behalf of the MBRRACE-UK Collaboration. MBRRACE-UK Perinatal Mortality Surveillance Report, UK perinatal deaths for births from January to December 2020. Leicester: The Infant Mortality and Morbidity Studies, Department of Health Sciences, University of Leicester; 2022.
Hinton L, Kuberska K, Dakin FH. A qualitative study of the dynamics of access to remote antenatal care through the lens of candidacy. J Health Serv Res Policy. 2023;28:222–32.
Alves DS, Times VC, da Silva EMA, Melo PSA, Novaes MA. Advances in obstetric telemonitoring: a systematic review. Int J Med Inf. 2020;134:104004.
van den Heuvel JF, Groenhof TK, Veerbeek JH, van Solinge WW, Lely AT, Franx A, et al. eHealth as the next-generation perinatal care: an overview of the literature. J Med Internet Res. 2018;20:e202.
Ming WK, Mackillop LH, Farmer AJ, Loerup L, Bartlett K, Levy JC, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290.
Butler Tobah YS, LeBlanc A, Branda ME, Inselman JW, Morris MA, Ridgeway JL, et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221:638.e1–e8.
Mackintosh N, Gong QS, Hadjiconstantinou M, Verdezoto N. Digital mediation of candidacy in maternity care: managing boundaries between physiology and pathology. Soc Sci Med. 2021;285:114299.
Acknowledgements
This paper summarises a programme of work that would not have been possible without the support of participating patients and health care professionals. We thank Lucy Curtin for administrative support.
Funding
This work was funded by a National Institute for Health Research (NIHR) Programme grant for applied research (RP-PG-1209-10051) and NIHR professorships awarded to Dr McManus (NIHR-RP-R2-12-015) and Dr. Chappell (NIHR -RP-2014-05-019). Drs. McManus and Tucker received funding from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research Oxford and the Thames Valley (ARC-OxTV). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. LH was based in The Healthcare Improvement Studies Institute (THIS Institute), University of Cambridge from December 2019 - May 2023. THIS Institute is supported by the Health Foundation, an independent charity committed to bringing about better health and healthcare for people in the UK.
Author information
Authors and Affiliations
Contributions
RM, LC, KT and LH conceived the studies described and gained the funding. MG provided support throughout around patient and public involvement and interpretation of research findings through the charity Action on Pre-eclampsia. The first draft of the article was written by KT and subsequently edited and approved by all co-authors. All authors have read, provided critical revision and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
RM has received BP monitors for research use from Omron and is working with them to develop a telemonitoring system for use in primary care. The BUMP+ app was adapted for commercial use by Sensyne. RM receives no personal payment for such work. The remaining authors have no disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tucker, K.L., Hinton, L., Green, M. et al. Using self-monitoring to detect and manage raised blood pressure and pre-eclampsia during pregnancy: the BUMP research programme and its impact. Hypertens Res 47, 714–720 (2024). https://doi.org/10.1038/s41440-023-01474-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01474-w