Abstract
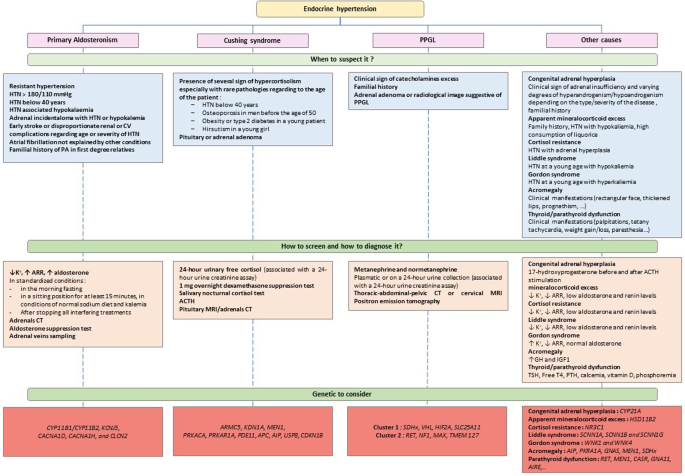
Hypertension (HTN) affects more than 30% of adults worldwide. It is the most frequent modifiable cardiovascular (CV) risk factor, and is responsible for more than 10 million death every year. Among patients with HTN, we usually distinguish secondary HTN, that is HTN due to an identified cause, and primary HTN, in which no underlying cause has been found. It is estimated that secondary hypertension represents between 5 and 15% of hypertensive patients [1]. Therefore, routine screening of patients for secondary HTN would be too costly and is not recommended. In addition to the presence of signs suggesting a specific secondary cause, screening is based on specific criteria. Identifying secondary HTN can be beneficial for patients in certain situations, because it may lead to specific treatments, and allow better control of blood pressure and sometimes even a cure. Besides, it is now known that secondary HTN are more associated with morbidity and mortality than primary HTN. The main causes of secondary HTN are endocrine and renovascular (mainly due to renal arteries abnormalities). The most frequent endocrine cause is primary aldosteronism, which diagnosis can lead to specific therapies. Pheochromocytoma and Cushing syndrome also are important causes, and can have serious complications. Other causes are less frequent and can be suspected on specific situations. In this article, we will describe the endocrine causes of HTN and discuss their treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245–54.
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37.
Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global Burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317:165–82.
de Freminville J-B, Amar L. How to explore an endocrine cause of hypertension. JCM. 2022;11:420.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–57.
Mancia Chairperson G, Kreutz Co-Chair R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. Published Online First: 21 June 2023. https://doi.org/10.1097/HJH.0000000000003480.
Braun LT, Vogel F, Reincke M. Long-term morbidity and mortality in patients with Cushing’s syndrome. J Neuroendocrinol. 2022;34:e13113.
Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50.
Januszewicz A, Mulatero P, Dobrowolski P, Monticone S, Van der Niepen P, Sarafidis P, et al. Cardiac phenotypes in secondary hypertension. J Am Coll Cardiol. 2022;80:1480–97.
Conn JW. Plasma renin activity in primary aldosteronism: importance in differential diagnosis and in research of essential hypertension. JAMA. 1964;190:222–5.
Conn JW, Cohen EL, Rovner DR, Nesbit RM. Normokalemic primary aldosteronism: a detectable cause of curable “Essential” hypertension. JAMA. 1965;193:200–6.
Gordon RD, Ziesak MD, Tunny TJ, Stowasser M, Klemm SA. Evidence That Primary Aldosteronism May Not Be Uncommon: 12% Incidence Among Antihypertensive Drug Trial Volunteers. Clin Exp Pharmacol Physiol. 1993;20:296–8.
Anderson GH, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609–15.
Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Rutherford JC. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharm Physiol. 1994;21:315–8.
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300.
Plouin P-F, Amar L, Chatellier G, on behalf of the COMETE-Conn Study Group. Trends in the prevalence of primary aldosteronism, aldosterone-producing adenomas, and surgically correctable aldosterone-dependent hypertension. Nephrol Dialysis Transplant. 2004;19:774–7.
Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies–a review of the current literature. Horm Metab Res. 2012;44:157–62.
Buffolo F, Monticone S, Burrello J, Tetti M, Veglio F, Williams TA, et al. Is primary Aldosteronism still largely unrecognized? Horm Metab Res. 2017;49:908–14.
Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–20.
Parasiliti-Caprino M, Lopez C, Prencipe N, Lucatello B, Settanni F, Giraudo G, et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J Hypertens. 2020;38:1841–8.
Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173:10–20.
Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021;9:876–92.
Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921–6.
Burrello J, Monticone S, Losano I, Cavaglià G, Buffolo F, Tetti M, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension. 2020;75:1025–33.
Mulatero P, Stowasser M, Loh K-C, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–50.
Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond). 2007;113:267–78.
Stowasser M, Sharman J, Leano R, Gordon RD, Ward G, Cowley D, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab. 2005;90:5070–6.
Chang Y-Y, Liao C-W, Tsai C-H, Chen C-W, Pan C-T, Chen Z-W, et al. Left ventricular dysfunction in patients with primary aldosteronism: a propensity score-matching follow-up study with tissue doppler imaging. J Am Heart Assoc. 2019;8:e013263.
Hung C-S, Chou C-H, Liao C-W, Lin Y-T, Wu X-M, Chang Y-Y, et al. Aldosterone induces tissue inhibitor of Metalloproteinases-1 expression and further contributes to collagen accumulation: from clinical to bench studies. Hypertension. 2016;67:1309–20.
Parksook WW, Williams GH. Aldosterone and cardiovascular diseases. Cardiovasc Res. 2022; cvac027.
van der Heijden CDCC, Smeets EMM, Aarntzen EHJG, Noz MP, Monajemi H, Kersten S, et al. Arterial wall inflammation and increased hematopoietic activity in patients with primary aldosteronism. J Clin Endocrinol Metab. 2020;105:e1967–80.
Demirkiran A, Everaars H, Elitok A, van de Ven PM, Smulders YM, Dreijerink KM, et al. Hypertension with primary aldosteronism is associated with increased carotid intima-media thickness and endothelial dysfunction. J Clin Hypertens (Greenwich). 2019;21:932–41.
Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, et al. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–8.
Monticone S, Sconfienza E, D’Ascenzo F, Buffolo F, Satoh F, Sechi LA, et al. Renal damage in primary aldosteronism: a systematic review and meta-analysis. J Hypertens. 2020;38:3–12.
Huby A-C, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation. 2015;132:2134–45.
Spyroglou A, Handgriff L, Müller L, Schwarzlmüller P, Parasiliti-Caprino M, Fuss CT, et al. The metabolic phenotype of patients with primary aldosteronism: impact of subtype and sex - a multicenter-study of 3566 Caucasian and Asian subjects. Eur J Endocrinol. 2022;187:361–72.
Wolley MJ, Pimenta E, Calhoun D, Gordon RD, Cowley D, Stowasser M. Treatment of primary aldosteronism is associated with a reduction in the severity of obstructive sleep apnoea. J Hum Hypertens. 2017;31:561–7.
Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014;63:20–31.
Savard S, Amar L, Plouin P-F, Steichen O. Cardiovascular complications associated with primary aldosteronism. Hypertension. 2013;62:331–6.
Milliez P, Girerd X, Plouin P-F, Blacher J, Safar ME, Mourad J-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–8.
Buffolo F, Tetti M, Mulatero P, Monticone S. Aldosterone as a mediator of cardiovascular damage. Hypertension. 2022;79:1899–911.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–9.
Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium: The multi-ethnic study of atherosclerosis. Hypertension. 2020;76:113–20.
Amar L, Baguet JP, Bardet S, Chaffanjon P, Chamontin B, Douillard C, et al. SFE/SFHTA/AFCE primary aldosteronism consensus: Introduction and handbook. Ann d’Endocrinologie. 2016;77:179–86.
Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro M-C, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the working group on endocrine hypertension of the european society of hypertension∗. J Hypertens. 2020;38:1919–28.
Seccia TM, Letizia C, Muiesan ML, Lerco S, Cesari M, Bisogni V, et al. Atrial fibrillation as presenting sign of primary aldosteronism: results of the Prospective Appraisal on the Prevalence of Primary Aldosteronism in Hypertensive (PAPPHY) Study. J Hypertens. 2020;38:332–9.
Mosso L, Carvajal C, González A, Barraza A, Avila F, Montero J, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161–5.
Maiolino G, Rossitto G, Bisogni V, Cesari M, Seccia TM, Plebani M, et al. Quantitative value of aldosterone‐renin ratio for detection of aldosterone‐producing adenoma: the Aldosterone‐Renin Ratio for Primary Aldosteronism (AQUARR) Study. J Am Heart Assoc. 2017;6:e005574.
Reznik Y, Amar L, Tabarin A. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 3: Confirmatory testing. Ann Endocrinol (Paris). 2016;77:202–7.
Baron S, Amar L, Faucon A-L, Blanchard A, Baffalie L, Faucard C, et al. Criteria for diagnosing primary aldosteronism on the basis of liquid chromatography-tandem mass spectrometry determinations of plasma aldosterone concentration. J Hypertens. 2018; 36. https://doi.org/10.1097/HJH.0000000000001735.
Funder JW. Primary aldosteronism. Hypertension. 2019;74:458–66.
Douillard C, Houillier P, Nussberger J, Girerd X. SFE/SFHTA/AFCE Consensus on Primary Aldosteronism, part 2: First diagnostic steps. Ann Endocrinol (Paris). 2016;77:192–201.
Aldea ML, Barallat J, Martín MA, Rosas I, Pastor MC, Granada ML. Sodium interference in the determination of urinary aldosterone. Clin Biochem. 2016;49:295–7.
Lin DC, Raizman JE, Holmes DT, Don-Wauchope AC, Yip PM. Evaluation of a chemiluminescent immunoassay for urinary aldosterone on the DiaSorin LIAISON automated platform against RIA and LC-MS/MS. Clin Chem Lab Med. 2017;55:e181–3.
Alnazer RM, Veldhuizen GP, Kroon AA, de Leeuw PW. The effect of medication on the aldosterone-to-renin ratio. A critical review of the literature. J Clin Hypertens (Greenwich). 2021;23:208–14.
Veldhuizen GP, Alnazer RM, de Leeuw PW, Kroon AA. The Effects of Verapamil, Hydralazine, and Doxazosin on Renin, Aldosterone, and the Ratio Thereof. Cardiovasc Drugs Ther. Published Online First: 13 September 2021. https://doi.org/10.1007/s10557-021-07262-3.
Beeftink MMA, van der Sande NGC, Bots ML, Doevendans PA, Blankestijn PJ, Visseren FLJ, et al. Safety of Temporary Discontinuation of Antihypertensive Medication in Patients With Difficult-to-Control Hypertension. Hypertension. 2017;69:927–32.
Zennaro M-C, Boulkroun S, Fernandes-Rosa FL. Pathogenesis and treatment of primary aldosteronism. Nat Rev Endocrinol. 2020;16:578–89.
Boulkroun S, Fernandes-Rosa FL, Zennaro M-C. Old and new genes in primary aldosteronism. Best Pr Res Clin Endocrinol Metab. 2020;34:101375.
Mulatero P, Sechi LA, Williams TA, Lenders JWM, Reincke M, Satoh F, et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension ∗. J Hypertens. 2020;38:1929–36.
Bardet S, Chamontin B, Douillard C, Pagny J-Y, Hernigou A, Joffre F, et al. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 4: Subtype diagnosis. Ann Endocrinol (Paris). 2016;77:208–13.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Kempers MJE, Lenders JWM, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus ARMM, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–37.
Rossi GP, Crimì F, Rossitto G, Amar L, Azizi M, Riester A, et al. Feasibility of imaging-guided adrenalectomy in young patients with primary aldosteronism. Hypertension. 2022;79:187–95.
Schloetelburg W, Ebert I, Petritsch B, Weng AM, Dischinger U, Kircher S, et al. Adrenal wash-out CT: moderate diagnostic value in distinguishing benign from malignant adrenal masses. Eur J Endocrinol. 2021;186:183–93.
Gao Y, Ding J, Cui Y, Li T, Sun H, Zhao D, et al. Functional nodules in primary aldosteronism: identification of CXCR4 expression with 68Ga-pentixafor PET/CT. Eur Radio. 2023;33:996–1003.
Heinze B, Fuss CT, Mulatero P, Beuschlein F, Reincke M, Mustafa M, et al. Targeting CXCR4 (CXC Chemokine Receptor Type 4) for Molecular Imaging of Aldosterone-Producing Adenoma. Hypertension. 2018;71:317–25.
Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, et al. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension. 2016;67:139–45.
De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75:1034–44.
Le Floch E, Cosentino T, Larsen CK, Beuschlein F, Reincke M, Amar L, et al. Identification of risk loci for primary aldosteronism in genome-wide association studies. Nat Commun. 2022;13:5198.
Steichen O, Zinzindohoué F, Plouin P-F, Amar L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm Metab Res. 2012;44:221–7.
Williams TA, Gong S, Tsurutani Y, Tezuka Y, Thuzar M, Burrello J, et al. Adrenal surgery for bilateral primary aldosteronism: an international retrospective cohort study. Lancet Diabetes Endocrinol. 2022;10:769–71.
Lim PO, Jung RT, MacDonald TM. Raised aldosterone to renin ratio predicts antihypertensive efficacy of spironolactone: a prospective cohort follow-up study. Br J Clin Pharm. 1999;48:756–60.
Parthasarathy HK, Ménard J, White WB, Young WF, Williams GH, Williams B, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29:980–90.
Fagart J, Hillisch A, Huyet J, Bärfacker L, Fay M, Pleiss U, et al. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem. 2010;285:29932–40.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in Type 2 diabetes. N. Engl J Med. 2020;383:2219–29.
Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and Type 2 diabetes. N. Engl J Med. 2021;385:2252–63.
Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474–84.
Bogman K, Schwab D, Delporte M-L, Palermo G, Amrein K, Mohr S, et al. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone Synthase (CYP11B2). Hypertension. 2017;69:189–96.
Azizi M, L Amar L, Menard J. Aldosterone synthase inhibition in humans. Nephrol, dialysis, Transpl.: official publication of the European Dialysis and Transplant Association - European Renal Association 2013; 28. https://doi.org/10.1093/ndt/gfs388.
Freeman MW, Halvorsen Y-D, Marshall W, Pater M, Isaacsohn J, Pearce C, et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388:395–405.
Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. <B>Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan</B>. Hypertens Res. 2004;27:193–202.
Lenders JWM, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75.
Amar L, Servais A, Gimenez-Roqueplo A-P, Zinzindohoue F, Chatellier G, Plouin P-F. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90:2110–6.
Giavarini A, Chedid A, Bobrie G, Plouin P-F, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99:1438–44.
Zelinka T, Petrák O, Turková H, Holaj R, Štrauch B, Kršek M, et al. High Incidence of Cardiovascular Complications in Pheochromocytoma. Horm Metab Res. 2012;44:379–84.
Dobrowolski P, Januszewicz A, Klisiewicz A, Gosk-Przybyłek M, Pęczkowska M, Kabat M, et al. Left ventricular structural and functional alterations in patients with pheochromocytoma/paraganglioma before and after surgery. JACC Cardiovasc Imaging. 2020;13:2498–509.
Robertson V, Poli F, Hobson B, Saratzis A, Ross Naylor A. A systematic review and meta-analysis of the presentation and surgical management of patients with carotid body tumours. Eur J Vasc Endovasc Surg. 2019;57:477–86.
Lenders JWM, Duh Q-Y, Eisenhofer G, Gimenez-Roqueplo A-P, Grebe SKG, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42.
Eisenhofer G, Peitzsch M, Kaden D, Langton K, Mangelis A, Pamporaki C, et al. Reference intervals for LC-MS/MS measurements of plasma free, urinary free and urinary acid-hydrolyzed deconjugated normetanephrine, metanephrine and methoxytyramine. Clin Chim Acta. 2019;490:46–54.
Därr R, Kuhn M, Bode C, Bornstein SR, Pacak K, Lenders JWM, et al. Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: a systematic review. Endocrine. 2017;56:495–503.
Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo A-P, Grossman AB, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pr Endocrinol Metab. 2007;3:92–102.
Boyle JG, Davidson DF, Perry CG, Connell JMC. Comparison of diagnostic accuracy of urinary free metanephrines, vanillyl mandelic Acid, and catecholamines and plasma catecholamines for diagnosis of pheochromocytoma. J Clin Endocrinol Metab. 2007;92:4602–8.
Pamporaki C, Prejbisz A, Małecki R, Pistrosch F, Peitzsch M, Bishoff S, et al. Optimized procedures for testing plasma metanephrines in patients on hemodialysis. Sci Rep. 2021;11:14706.
Därr R, Pamporaki C, Peitzsch M, Miehle K, Prejbisz A, Peczkowska M, et al. Biochemical diagnosis of phaeochromocytoma using plasma-free normetanephrine, metanephrine and methoxytyramine: importance of supine sampling under fasting conditions. Clin Endocrinol (Oxf). 2014;80:478–86.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041.
Lenders JWM, Kerstens MN, Amar L, Prejbisz A, Robledo M, Taieb D, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020;38:1443–56.
Plouin P-F, Gimenez-Roqueplo A-P. Pheochromocytomas and secreting paragangliomas. Orphanet J Rare Dis. 2006;1:49.
Timmers HJLM, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–67.
Taïeb D, Hicks RJ, Hindié E, Guillet BA, Avram A, Ghedini P, et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2019;46:2112–37.
Timmers HJLM, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104:700–8.
Amar L, Lussey-Lepoutre C, Lenders JWM, Djadi-Prat J, Plouin P-F, Steichen O. Management of endocrine disease: Recurrence or new tumors after complete resection of pheochromocytomas and paragangliomas: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175:R135–45.
Favier J, Amar L, Gimenez-Roqueplo A-P. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11:101–11.
Burnichon N, Buffet A, Gimenez-Roqueplo A-P. Pheochromocytoma and paraganglioma: molecular testing and personalized medicine. Curr Opin Oncol. 2016;28:5–10.
Tian J, Bao Z, Yuan Y, Fang D, Zhan Y, Wang T, et al. The duration of preoperative administration of single α-Receptor Blocker Phenoxybenzamine before Adrenalectomy for Pheochromocytoma: 18 Years of Clinical Experience from Nationwide High-Volume Center. Biomed Res Int. 2019;2019:2613137.
Castinetti F, De Freminville JB, Guerin C, Cornu E, Sarlon G, Amar L. Controversies about the systematic preoperative pharmacological treatment before pheochromocytoma or paraganglioma surgery. Eur J Endocrinol. 2022;186:D17–24.
Gruber LM, Jasim S, Ducharme-Smith A, Weingarten T, Young WF, Bancos I. The Role for Metyrosine in the Treatment of Patients With Pheochromocytoma and Paraganglioma. J Clin Endocrinol Metab. 2021;106:e2393–2401.
Wengander S, Trimpou P, Papakokkinou E, Ragnarsson O. The incidence of endogenous Cushing’s syndrome in the modern era. Clin Endocrinol. 2019;91:263–70.
Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BMK, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4:611–29.
Graversen D, Vestergaard P, Stochholm K, Gravholt CH, Jørgensen JOL. Mortality in Cushing’s syndrome: a systematic review and meta-analysis. Eur J Intern Med. 2012;23:278–82.
Deutschbein T, Reimondo G, Dalmazi GD, Bancos I, Patrova J, Vassiliadi DA, et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022;10:499–508.
Cicala MV, Mantero F. Hypertension in Cushing’s syndrome: from pathogenesis to treatment. Neuroendocrinology. 2010;92:44–9.
Frey FJ, Odermatt A, Frey BM. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens. 2004;13:451–8.
Lugat A, Lasolle H, François M, Benhenda N, Bricaire L, Cornu E, et al. Pneumocystis pneumonia in patients with Cushing’s syndrome: a French multicenter retrospective study. Ann Endocrinol (Paris). 2022;S0003-4266:00836–8.
Van Zaane B, Nur E, Squizzato A, Dekkers OM, Twickler MTB, Fliers E, et al. Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94:2743–50.
Tabarin A, Assié G, Barat P, Bonnet F, Bonneville JF, Borson-Chazot F, et al. Consensus statement by the French Society of Endocrinology (SFE) and French Society of Pediatric Endocrinology & Diabetology (SFEDP) on diagnosis of Cushing’s syndrome. Ann d’Endocrinologie. 2022;83:119–41.
Nieman, Biller BMK LK, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526–40.
Braun LT, Vogel F, Zopp S, Marchant Seiter T, Rubinstein G, Berr CM, et al. Whom should we screen for Cushing syndrome? The Endocrine Society Practice Guideline Recommendations 2008 revisited. J Clin Endocrinol Metab. 2022;107:e3723–30.
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9:847–75.
Galm BP, Qiao N, Klibanski A, Biller BMK, Tritos NA. Accuracy of laboratory tests for the diagnosis of Cushing syndrome. J Clin Endocrinol Metab. 2020;105:2081–94.
Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, et al. The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165:383–92.
Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367:1605–17.
Steffensen C, Bak AM, Rubeck KZ, Jørgensen JOL. Epidemiology of Cushing’s syndrome. NEN. 2010;92:1–5.
Aresta C, Favero V, Morelli V, Giovanelli L, Parazzoli C, Falchetti A, et al. Cardiovascular complications of mild autonomous cortisol secretion. Best Pract Res Clin Endocrinol Metab. 2021;35:101494.
Hakami OA, Ahmed S, Karavitaki N. Epidemiology and mortality of Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. 2021;35:101521.
Rubinstein G, Osswald A, Braun LT, Vogel F, Kroiss M, Pilz S, et al. The role of adrenal venous sampling (AVS) in primary bilateral macronodular adrenocortical hyperplasia (PBMAH): a study of 16 patients. Endocrine. 2022;76:434–45.
Bertherat J, Bourdeau I, Bouys L, Chasseloup F, Kamenický P, Lacroix A. Clinical, pathophysiologic, genetic, and therapeutic progress in primary bilateral macronodular adrenal hyperplasia. Endocr Rev. 2023;44:567–628.
Theodoropoulou M, Reincke M. Genetics of Cushing’s disease: from the lab to clinical practice. Pituitary. 2022;25:689–92.
Fleseriu M, Biller BMK. Treatment of Cushing’s syndrome with osilodrostat: practical applications of recent studies with case examples. Pituitary. 2022;25:795–809.
Brue T, Rahabi H, Barry A, Barlier A, Bertherat J, Borson-Chazot F, et al. Position statement on the diagnosis and management of acromegaly: the French National Diagnosis and Treatment Protocol (NDTP). Annales d’Endocrinologie 2023;S0003426623006868.
Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM, et al. Acromegaly. Nat Rev Dis Prim. 2019;5:20.
Maione L, Chanson P. National acromegaly registries. Best Pract Res Clin Endocrinol Metab. 2019;33:101264.
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, et al. A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab. 2020;105:e937–46.
González B, Vargas G, De Los Monteros ALE, Mendoza V, Mercado M. Persistence of diabetes and hypertension after multimodal treatment of acromegaly. J Clin Endocrinol Metab. 2018;103:2369–75.
Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89:667–74.
Garby L, Caron P, Claustrat F, Chanson P, Tabarin A, Rohmer V, et al. Clinical Characteristics and Outcome of Acromegaly Induced by Ectopic Secretion of Growth Hormone-Releasing Hormone (GHRH): A French Nationwide Series of 21 Cases. J Clin Endocrinol Metab. 2012;97:2093–104.
Gadelha MR, Kasuki L, Korbonits M. The genetic background of acromegaly. Pituitary. 2017;20:10–21.
Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B, et al. Multidisciplinary management of acromegaly: A consensus. Rev Endocr Metab Disord. 2020;21:667–78.
El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390:2194–210.
Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. A summary of the endocrine society clinical practice guidelines on congenital adrenal hyperplasia due to Steroid 21-Hydroxylase deficiency. Int J Pediatr Endocrinol. 2010;2010:494173.
Nicolaides NC, Roberts ML, Kino T, Braatvedt G, Hurt DE, Katsantoni E, et al. A Novel point mutation of the human glucocorticoid receptor gene causes primary generalized glucocorticoid resistance through impaired interaction with the LXXLL Motif of the p160 coactivators: dissociation of the transactivating and transreppressive activities. J Clin Endocrinol Metab. 2014;99:E902–7.
Vitellius G, Lombes M. Genetics in endocrinology: glucocorticoid resistance syndrome. Eur J Endocrinol. 2020;182:R15–27.
Jeunemaitre X, Bassilana F, Persu A, Dumont C, Champigny G, Lazdunski M, et al. Genotype-phenotype analysis of a newly discovered family with Liddle’s syndrome. J Hypertens. 1997;15:1091–1100.
Garovic VD, Hilliard AA, Turner ST. Monogenic forms of low-renin hypertension. Nat Clin Pr Nephrol. 2006;2:624–30.
Young WF Jr, Calhoun DA, Lenders JWM, Stowasser M, Textor SC. Screening for endocrine hypertension: an endocrine society scientific statement. Endocr Rev. 2017;38:103–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Freminville, JB., Amar, L., Azizi, M. et al. Endocrine causes of hypertension: literature review and practical approach. Hypertens Res 46, 2679–2692 (2023). https://doi.org/10.1038/s41440-023-01461-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01461-1