Abstract
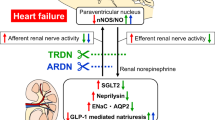
Excessive activation of the sympathetic nervous system is one of the pathophysiological hallmarks of hypertension and heart failure. Within the central nervous system, the paraventricular nucleus (PVN) of the hypothalamus and the rostral ventrolateral medulla in the brain stem play critical roles in the regulation of sympathetic outflow to peripheral organs. Information from the peripheral circulation, including serum concentrations of sodium and angiotensin II, is conveyed to the PVN via adjacent structures with a weak blood–brain barrier. In addition, signals from baroreceptors, chemoreceptors and cardiopulmonary receptors as well as afferent input via the renal nerves are all integrated at the level of the PVN. The brain renin-angiotensin system and the balance between nitric oxide and reactive oxygen species in these brain areas also determine the final sympathetic outflow. Additionally, brain inflammatory responses have been shown to modulate these processes. Renal denervation interrupts both the afferent inputs from the kidney to the PVN and the efferent outputs from the PVN to the kidney, resulting in the suppression of sympathetic outflow and eliciting beneficial effects on both hypertension and heart failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hering D, Schlaich M. The role of central nervous system mechanisms in resistant hypertension. Curr Hypertens Rep. 2015;17:58.
Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–21.
Kario K. Essential manual of 24 h blood pressure management: from morning to nocturnal hypertension. Chichester, West Sussex, UK; Malden, MA: John Wiley & Sons Inc.; 2015.
Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46.
Hirooka Y. Sympathetic activation in hypertension: importance of the central nervous system. Am J Hypertens. 2020;33:914–26.
Zheng H, Patel KP. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton Neurosci. 2017;204:57–64.
Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates RVLM-projecting PVN neurons. Am J Physiol Heart Circ Physiol. 2015;308:H1103–11.
Osborn JW, Foss JD. Renal nerves and long-term control of arterial pressure. Compr Physiol. 2017;7:263–320.
Xu B, Zheng H, Patel KP. Enhanced activation of RVLM-projecting PVN neurons in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H1700–11.
Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–77.
Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci. 2011;161:6–13.
Hirooka Y, Kishi T, Ito K, Sunagawa K. Potential clinical application of recently discovered brain mechanisms involved in hypertension. Hypertension 2013;62:995–1002.
Shinohara K, Liu X, Morgan DA, Davis DR, Sequeira-Lopez ML, Cassell MD, et al. Selective deletion of the brain-specific isoform of renin causes neurogenic hypertension. Hypertension 2016;68:1385–92.
Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, et al. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–82.
Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–8.
Mukerjee S, Gao H, Xu J, Sato R, Zsombok A, Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019;74:1181–91.
Llorens-Cortes C, Touyz RM. Evolution of a new class of antihypertensive drugs: targeting the brain renin-angiotensin system. Hypertension. 2020;75:6–15.
Sakai K, Hirooka Y, Matsuo I, Eshima K, Shigematsu H, Shimokawa H, et al. Overexpression of eNOS in NTS causes hypotension and bradycardia in vivo. Hypertension. 2000;36:1023–8.
Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901.
Li YF, Roy SK, Channon KM, Zucker IH, Patel KP. Effect of in vivo gene transfer of nNOS in the PVN on renal nerve discharge in rats. Am J Physiol Heart Circ Physiol. 2002;282:H594–601.
Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension. 2011;58:966–73.
McBryde FD, Liu BH, Roloff EV, Kasparov S, Paton JFR. Hypothalamic paraventricular nucleus neuronal nitric oxide synthase activity is a major determinant of renal sympathetic discharge in conscious Wistar rats. Exp Physiol. 2018;103:419–28.
Kishi T, Hirooka Y, Ito K, Sakai K, Shimokawa H, Takeshita A. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension. 2002;39:264–8.
Hirooka Y, Sakai K, Kishi T, Ito K, Shimokawa H, Takeshita A. Enhanced depressor response to endothelial nitric oxide synthase gene transfer into the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens Res. 2003;26:325–31.
Kishi T, Hirooka Y, Kimura Y, Sakai K, Ito K, Shimokawa H, et al. Overexpression of eNOS in RVLM improves impaired baroreflex control of heart rate in SHRSP. Rostral ventrolateral medulla. Stroke-prone spontaneously hypertensive rats. Hypertension. 2003;41:255–60.
Eshima K, Hirooka Y, Shigematsu H, Matsuo I, Koike G, Sakai K, et al. Angiotensin in the nucleus tractus solitarii contributes to neurogenic hypertension caused by chronic nitric oxide synthase inhibition. Hypertension. 2000;36:259–63.
Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–6.
Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004;109:2357–62.
Nishihara M, Hirooka Y, Matsukawa R, Kishi T, Sunagawa K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens. 2012;30:97–106.
Nishihara M, Hirooka Y, Kishi T, Sunagawa K. Different role of oxidative stress in paraventricular nucleus and rostral ventrolateral medulla in cardiovascular regulation in awake spontaneously hypertensive rats. J Hypertens. 2012;30:1758–65.
Li C, Li Y, Zhao Z, Lv Y, Gu B, Zhao L. Aerobic exercise regulates synaptic transmission and reactive oxygen species production in the paraventricular nucleus of spontaneously hypertensive rats. Brain Res. 2019;1712:82–92.
Ahmari N, Santisteban MM, Miller DR, Geis NM, Larkin R, Redler T, et al. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. Am J Physiol Heart Circ Physiol. 2019;317:H279–89.
Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, et al. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66:309–16.
Waki H, Hendy EB, Hindmarch CC, Gouraud S, Toward M, Kasparov S, et al. Excessive leukotriene B4 in nucleus tractus solitarii is prohypertensive in spontaneously hypertensive rats. Hypertension. 2013;61:194–201.
Marvar PJ, Hendy EB, Cruise TD, Walas D, DeCicco D, Vadigepalli R, et al. Systemic leukotriene B4 receptor antagonism lowers arterial blood pressure and improves autonomic function in the spontaneously hypertensive rat. J Physiol. 2016;594:5975–89.
Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51.
Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55.
Kandzari DE, Mahfoud F, Bhatt DL, Bohm M, Weber MA, Townsend RR, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76:1410–7.
Oparil S, Sripairojthikoon W, Wyss JM. The renal afferent nerves in the pathogenesis of hypertension. Can J Physiol Pharm. 1987;65:1548–58.
Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–22.
Zheng H, Katsurada K, Liu X, Knuepfer MM, Patel KP. Specific afferent renal denervation prevents reduction in neuronal nitric oxide synthase within the paraventricular nucleus in rats with chronic heart failure. Hypertension. 2018;72:667–75.
Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension. 2016;68:1415–23.
Veiga AC, Milanez MIO, Ferreira GR, Lopes NR, Santos CP, De Angelis K, et al. Selective afferent renal denervation mitigates renal and splanchnic sympathetic nerve overactivity and renal function in chronic kidney disease-induced hypertension. J Hypertens. 2020;38:765–73.
Lopes NR, Milanez MIO, Martins BS, Veiga AC, Ferreira GR, Gomes GN, et al. Afferent innervation of the ischemic kidney contributes to renal dysfunction in renovascular hypertensive rats. Pflug Arch. 2020;472:325–34.
Milanez MIO, Veiga AC, Martins BS, Pontes RB, Bergamaschi CT, Campos RR, et al. Renal sensory activity regulates the gamma-aminobutyric acidergic inputs to the paraventricular nucleus of the hypothalamus in goldblatt hypertension. Front Physiol. 2020;11:601237.
Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol. 2016;310:R262–7.
Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin ii-induced hypertension. Circ Res. 2015;117:547–57.
Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst. 1983;8:273–85.
Kuo DC, Nadelhaft I, Hisamitsu T, de Groat WC. Segmental distribution and central projections of renal afferent fibers in the cat studied by transganglionic transport of horseradish peroxidase. J Comp Neurol. 1983;216:162–74.
Wyss JM, Donovan MK. A direct projection from the kidney to the brainstem. Brain Res. 1984;298:130–4.
Weiss ML, Chowdhury SI, Patel KP, Kenney MJ, Huang J. Neural circuitry of the kidney: NO-containing neurons. Brain Res. 2001;919:269–82.
Katsurada K, Nandi SS, Zheng H, Liu X, Sharma NM, Patel KP. GLP-1 mediated diuresis and natriuresis are blunted in heart failure and restored by selective afferent renal denervation. Cardiovascular Diabetol. 2020;19:57.
Patel KP, Xu B, Liu X, Sharma NM, Zheng H. Renal denervation improves exaggerated sympathoexcitation in rats with heart failure: a role for neuronal nitric oxide synthase in the paraventricular nucleus. Hypertension. 2016;68:175–84.
Ramchandra R, Hood SG, May CN. Central exogenous nitric oxide decreases cardiac sympathetic drive and improves baroreflex control of heart rate in ovine heart failure. Am J Physiol Regul Integr Comp Physiol. 2014;307:R271–80.
Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M, Francis J. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res Cardiol. 2011;106:273–86.
Zheng H, Liu X, Katsurada K, Patel KP. Renal denervation improves sodium excretion in rats with chronic heart failure: effects on expression of renal ENaC and AQP2. Am J Physiol Heart Circ Physiol. 2019;317:H958–68.
Sharp TE 3rd, Polhemus DJ, Li Z, Spaletra P, Jenkins JS, Reilly JP, et al. Renal denervation prevents heart failure progression via inhibition of the renin-angiotensin system. J Am Coll Cardiol. 2018;72:2609–21.
Zheng H, Liu X, Sharma NM, Patel KP. Renal denervation improves cardiac function in rats with chronic heart failure: Effects on expression of beta-adrenoceptors. Am J Physiol Heart Circ Physiol. 2016;311:H337–46.
Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81.
Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha-Singh K, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–9.
Esler MD, Bohm M, Sievert H, Rump CL, Schmieder RE, Krum H, et al. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J. 2014;35:1752–9.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Lobo MD, et al. Six-month results of treatment-blinded medication titration for hypertension control following randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO trial. Circulation. 2019;139:2542–53.
Azizi M, Daemen J, Lobo MD, Mahfoud F, Sharp ASP, Schmieder RE, et al. 12-month results from the unblinded phase of the RADIANCE-HTN SOLO trial of ultrasound renal denervation. JACC Cardiovasc Inter. 2020;13:2922–33.
Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–86.
Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–8.
Zazgornik J, Biesenbach G, Janko O, Gross C, Mair R, Brucke P, et al. Bilateral nephrectomy: the best, but often overlooked, treatment for refractory hypertension in hemodialysis patients. Am J Hypertens. 1998;11:1364–70.
Fatouleh RH, Hammam E, Lundblad LC, Macey PM, McKenzie DK, Henderson LA, et al. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin. 2014;6:275–83.
Shantha GP, Pancholy SB. Effect of renal sympathetic denervation on apnea-hypopnea index in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2015;19:29–34.
Kario K, Hettrick DA, Prejbisz A, Januszewicz A. Obstructive sleep apnea-induced neurogenic nocturnal hypertension: a potential role of renal denervation? Hypertension. 2021;77:1047–60.
Kario K, Ikemoto T, Kuwabara M, Ishiyama H, Saito K, Hoshide S. Catheter-based renal denervation reduces hypoxia-triggered nocturnal blood pressure peak in obstructive sleep apnea syndrome. J Clin Hypertens. 2016;18:707–9.
Hoshide S, Kario K, Hoshide Y, Umeda Y, Hashimoto T, Kunii O, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003;16:434–8.
Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–6.
Hoshide S, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: The Japan Morning Surge-Home Blood Pressure Study. Hypertension. 2016;68:54–61.
Kario K, Kanegae H, Tomitani N, Okawara Y, Fujiwara T, Yano Y, et al. Nighttime blood pressure measured by home blood pressure monitoring as an independent predictor of cardiovascular events in general practice. Hypertension. 2019;73:1240–8.
Kario K, Hoshide S, Mizuno H, Kabutoya T, Nishizawa M, Yoshida T, et al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020;142:1810–20.
Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021;77:2909–19.
Dorr O, Liebetrau C, Mollmann H, Gaede L, Troidl C, Haidner V, et al. Brain-derived neurotrophic factor as a marker for immediate assessment of the success of renal sympathetic denervation. J Am Coll Cardiol. 2015;65:1151–3.
Dorr O, Liebetrau C, Mollmann H, Gaede L, Troidl C, Rixe J, et al. Soluble fms-like tyrosine kinase-1 and endothelial adhesion molecules (intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1) as predictive markers for blood pressure reduction after renal sympathetic denervation. Hypertension. 2014;63:984–90.
Funding
This work was supported in part by National Institutes of Health grants R56 HL124104, P01 HL62222, and R01 DK114663; by an endowed McIntyre Professorship (to KPP); and by a Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad (to KKatsurada).
Author information
Authors and Affiliations
Contributions
KKatsurada, YO, YI, KPP and KKario conducted the conception and design of the study. KKatsurada and KPP performed the study including experimental data collection and analyses. KKatsurada drafted the manuscript. KKatsurada, YO, YI, KPP and KKario edited and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kazuomi Kario MD PhD, received speaker fees and worked as a consultant to JIMRO Co. Ltd., Medtronic Co. Inc. and Terumo Co. Inc. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katsurada, K., Ogoyama, Y., Imai, Y. et al. Renal denervation based on experimental rationale. Hypertens Res 44, 1385–1394 (2021). https://doi.org/10.1038/s41440-021-00746-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-021-00746-7
Keywords
This article is cited by
-
The role of afferent renal nerves in regulating sympathetic outflow via central nervous system mechanisms
Hypertension Research (2024)
-
ATRAP in the paraventricular nucleus of the hypothalamus as another key player in the control of sympathetic outflow
Hypertension Research (2024)
-
Renal denervation improves cardiac function independently of afterload and restores myocardial norepinephrine levels in a rodent heart failure model
Hypertension Research (2024)
-
Contribution of afferent renal nerve signals to acute and chronic blood pressure regulation in stroke-prone spontaneously hypertensive rats
Hypertension Research (2023)
-
Renal denervation: basic and clinical evidence
Hypertension Research (2022)