Abstract
Automated cuff measured blood pressure (BP) is the global standard used for diagnosing hypertension, but there are concerns regarding the accuracy of the method. Individual variability in systolic BP (SBP) amplification from central (aorta) to peripheral (brachial) arteries could be related to the accuracy of cuff BP, but this has never been determined and was the aim of this study. Automated cuff BP and invasive brachial BP were recorded in 795 participants (74% male, aged 64 ± 11 years) receiving coronary angiography at five independent research sites (using seven different automated cuff BP devices). SBP amplification was recorded invasively by catheter and defined as brachial SBP minus aortic SBP. Compared with invasive brachial SBP, cuff SBP was significantly underestimated (130 ± 18 mmHg vs. 138 ± 22 mmHg, p < 0.001). The level of SBP amplification varied significantly among individuals (mean ± SD, 7.3 ± 9.1 mmHg) and was similar to level of difference between cuff and invasive brachial SBP (mean difference –7.6 ± 11.9 mmHg). SBP amplification explained most of the variance in accuracy of cuff SBP (R2 = 19%). The accuracy of cuff SBP was greatest among participants with the lowest SBP amplification (ptrend < 0.001). After cuff BP values were corrected for SBP amplification, there was a significant improvement in the mean difference from the intra-arterial standard (p < 0.0001) and in the accuracy of hypertension classification according to 2017 ACC/AHA guideline thresholds (p = 0.005). The level of SBP amplification is a critical factor associated with the accuracy of conventional automated cuff measured BP.

Similar content being viewed by others
Introduction
High blood pressure (BP) is the leading modifiable risk factor for cardiovascular disease and contributes to more than 10 million deaths annually [1]. Accurate BP measurement is a critical component of the healthcare pathway to enable correct identification of high BP, and consequent BP management to reduce risk for cardiovascular disease events [2]. International guidelines recommend that appropriately validated, upper arm cuff-based, automated BP measuring devices are used for clinical diagnosis and management [3]. Irrespective of the clinical value of automated cuff BP devices, accuracy concerns that could influence correct diagnosis in some people have been raised, specifically that the cuff BP does not accurately represent the true intra-arterial BP values [4]. Indeed, cuff systolic BP (SBP) systematically underestimates intra-arterial brachial SBP, whereas cuff diastolic BP (DBP) systematically overestimates intra-arterial brachial DBP (both by about 6 mmHg on average) [5]. Better understanding of the mechanisms underlying the inaccuracy of cuff BP could help towards refining accuracy and improving individual BP risk stratification.
The operating principles of standard automated (oscillometric) cuff devices are based on analysis of pressure (or volume) waveforms detected at the brachial artery. These waveforms are extracted from the cuff pressure (deflation) curve and processed to construct an oscillometric waveform envelope [6]. From this, the mean arterial pressure is identified from the maximal amplitude, and proprietary algorithms are employed to estimate SBP and DBP [7]. These brachial artery waveforms captured by cuff devices have characteristic morphology that are unique to each individual [8] but to our knowledge they are not considered in the proprietary algorithms used to estimate BP parameters. Recently we found that the arterial waveform morphology at the brachial artery was distinct among people with high level of central to brachial SBP amplification (e.g. >15 mmHg; relatively high amplitude, narrow systolic peak) compared to those with low SBP amplification (lower amplitude, broad systolic wave) [9]. These characteristic differences in waveform morphology could result in systematic bias in automated cuff BP measurement that is dependent on SBP amplification. If this were the case, we may expect to see a positive association between the magnitude of SBP amplification and the magnitude of error in cuff measured BP. The aim of this study was to determine the relationship between individual variability in SBP amplification and the accuracy of cuff BP compared with intra-arterial brachial BP. Given that different cuff BP devices have unique proprietary algorithms to estimate BP, we wanted to confirm findings in a large subject population across several models of cuff BP devices.
Methods
Study population
Participants were 795 patients undergoing coronary angiography who were recruited from five independent research sites using a variety of automated cuff BP devices. At each study site clinical characteristics, cuff BP, invasive brachial BP and invasive aortic BP measurements were recorded in accordance with available international standard guidelines [10] and then combined as a convenience sample. Interarm differences in cuff BP were recorded as a screening measure for eligibility, and only those subjects with no major interarm BP difference proceeded to the invasive measurements. This was as a quality control measure to rule out those with possible upper limb stenosis or hemodynamic abnormality that could influence accurate BP measurements. Further details on the study population, inclusion and exclusion criteria are provided in published studies [11,12,13,14,15] from the different research sites and are summarised in Supplementary Table 1. The current analysis includes data but is completely separate from our previous published paper [15]. Participants provided written, informed consent at each research site.
Non-invasive (cuff-based) and invasive brachial BP difference
Cuff brachial BP was recorded using seven different commercially available automated cuff BP devices (Supplementary Table 1). Cuff BP was measured precisely simultaneous to invasive brachial BP in five studies (four published [11, 14, 15], one unpublished), and immediately prior to invasive brachial BP in two studies [12, 13]. Participants were excluded if there was an interarm difference >3 mmHg (in two published studies [11, 14]) or >5 mmHg (in four published studies [12, 13, 15], one unpublished). In all studies, only those subjects without significant interarm differences went on to have cuff BP and invasive BP recorded on opposite arms. A total of 166 participants were excluded on the basis of interarm BP differences. Non-invasive (cuff) SBP, DBP and pulse pressure (PP) difference (inaccuracy) was calculated as cuff minus invasive brachial SBP, DBP and PP.
Invasive (intra-arterial) SBP amplification
Details of intra-arterial BP collection procedures for each individual study are provided in previous publications [11,12,13,14,15] and are summarised in Supplementary Table 1. Briefly, a solid-state or fluid-filled catheter was advanced from the right radial artery access site and positioned in the ascending aorta within 1–5 cm of the aortic valve, with confirmation by fluoroscopy. In six of the seven studies [11,12,13, 15], intra-arterial BP was measured by positioning a catheter in the ascending aorta to capture invasive aortic BP waveforms and then pulled back to the mid-humeral level in the right brachial artery to record invasive brachial BP waveforms. In one study, a dual-sensor, solid-state catheter allowed simultaneous measurement of invasive aortic and brachial BP [14]. Invasive SBP, DBP and PP amplification were defined as invasive brachial minus invasive aortic SBP, DBP and PP.
Statistical analysis
Clinical characteristics and BP are presented as mean ± SD or n (%). Differences between continuous clinical characteristics and BP measures were assessed by t tests or one-way ANOVA with post hoc Tukey HSD test to quantify the statistical significance of any differences. Agreement between cuff and invasive brachial SBP was assessed by mean difference and SD of the mean difference and visualised by Bland Altman plots [16]. Pearson correlation and linear regression within Bland-Altman plots were used to determine the magnitude and direction of any proportional systematic bias (comparing correlation coefficients using Fisher’s z). The association between cuff and invasive brachial BP difference and invasive SBP amplification was assessed using univariable and multivariable linear regression adjusting for potential confounders including sex, age, height, coronary artery disease, heart rate, and mean invasive brachial arterial pressure. These variables were included in the adjusted models because they were associated or had suspected associations with the difference and invasive SBP amplification. An analysis for the use of antihypertensive medication was also conducted on data from a subgroup of participants with available data. The total number of participants was 795 for all analyses except the multiple regression, in which complete data was available for 755 participants (Supplementary Fig. 1). Since the average level of cuff SBP underestimation was similar to the average level of SBP amplification, we performed a cuff SBP correction to determine if this resulted in an improvement in the mean difference of cuff SBP from the invasive standard. The cuff SBP correction was performed by adding each individual’s level of invasive SBP amplification to their cuff SBP measure. BP stages were classified using brachial cuff BP, corrected brachial cuff BP and invasive brachial BP. Since brachial cuff measurements used to classify hypertension categories were corrected for invasive SBP amplification, invasive brachial BP was used as the reference standard. All classifications were according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines [3]. The concordance of BP classifications was assessed by comparing BP classification defined using cuff brachial BP with the one obtained using invasive brachial BP. Similar BP classification concordance was performed between corrected cuff BP and invasive brachial BP. These classification concordances were compared using kappa statistics, proportions of agreement and the two-proportion tests. Statistical analyses were performed using STATA version 17.0, p values ≤ 0.05 were considered statistically significant.
Results
Clinical characteristics
Clinical characteristics are outlined within Table 1 Participants were generally representative of patients undergoing coronary angiography, who were on average, of older age and higher body mass index, and more than half the population had hypertension based on cuff BP values according to the 2017 ACC/AHA guidelines. More than two thirds of participants reported taking at least one hypertensive medication.
BP measurements
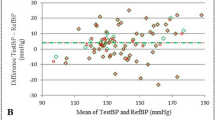
Cuff and invasive BP measurements are outlined within Table 2. Cuff-measured SBP significantly underestimated invasive brachial SBP (130 ± 18 mmHg vs. 138 ± 22 mmHg, p < 0.001), whereas cuff DBP significantly overestimated invasive brachial DBP (76 ± 11 mmHg vs. 69 ± 10 mmHg, p < 0.001). Bland-Altman plots revealed significant bias for cuff SBP to overestimate invasive brachial SBP at lower BP levels but underestimate invasive brachial SBP at higher BP levels (Fig. 1). The slope of the bias was significantly attenuated after correcting cuff SBP for the corresponding level of SBP amplification for each individual (r = −0.27 vs. r = −0.09, z = 5.25, p < 0.0001). There was also a significant improvement in the mean difference, but not standard deviation, between cuff SBP and invasive brachial SBP when cuff SBP was corrected with SBP amplification (−7.6 ± 11.9 mmHg vs. −0.3 ± 11.4 mmHg, p < 0.001). Although there was wide individual variability in SBP amplification (mean ± SD, 7.3 ± 9.1 mmHg), the mean SBP amplification was similar to the mean difference between cuff SBP and invasive brachial SBP (−7.6 ± 11.9 mmHg). Analysis of cuff and invasive BP measurement was also conducted for each of the seven devices across all the study sites (Supplementary Table 2). Findings were broadly similar with the pooled analysis except for the device used by Ding et al. [12], where an underestimation (instead of overestimation) of cuff DBP was observed.
Bland-Altman plots of differences between cuff SBP (top) and cuff SBP corrected with SBP amplification (bottom) and invasive brachial SBP. Dashed lines represent the lines of best fit. Solid lines are mean difference ± 2 SDs. Bland-Altman plots show wide scatter and evidence of systematic bias for greater underestimation of invasive brachial SBP with increasing level of BP, but the slope of this association was significantly attenuated when cuff SBP was corrected by adding individual corresponding SBP amplification (r = −0.27 vs. r = −0.09, z = 5.25, p < 0.0001). There was a significant improvement in the mean difference between standard cuff and corrected cuff SBP from invasive brachial SBP (−7.6 ± 11.9 mmHg vs. −0.30 ± 11.4 mmHg, p < 0.001)
Relationship of BP accuracy with SBP amplification
Fig. 2 presents differences between cuff and invasive brachial SBP, DBP, and PP by invasive SBP amplification quintiles. There was a significant trend towards greater underestimation of cuff SBP across quintiles of SBP amplification (ptrend < 0.001). The accuracy of cuff SBP was greatest among participants with the lowest SBP amplification. The difference between cuff and invasive brachial SBP was significantly associated with SBP amplification, even after controlling for multiple potential confounders (β[95%CI]:–0.52[–0.60 to –0.44], p < 0.001) and SBP amplification explained most of the variance in accuracy of cuff SBP (R2 = 19%, Table 3). Participant characteristics, cuff, invasive brachial and aortic BP data used in the adjusted models were compared in Supplementary Table 3. There were no significant differences between the missing (n = 40) and the complete (n = 755) datasets for the proportion of male participants and coronary artery disease, mean age, invasive SBP amplification, cuff and invasive brachial difference (p > 0.065, all). Results were unchanged when adjusted for the use of antihypertensive medication (Supplementary Table 4). Supplementary Table 5 presents participants clinical characteristics and BP measures across quintiles of invasive SBP amplification. Age, height, coronary artery disease, heart rate, invasive brachial SBP, PP, mean arterial pressure, invasive aortic SBP, DBP and mean arterial pressure were significantly different across these quintiles (ptrend ≤ 0.023 for all).
Bar plots (mean, SE) cuff minus invasive brachial systolic blood pressure (SBP, top), diastolic (DBP, middle) and pulse pressure (PP, bottom) by invasive SBP amplification quintiles. There was a stepwise increase in mean differences between cuff and invasive brachial SBP, PP for each of elevated invasive SBP quintile (ptrend < 0.001) whilst there was a slight decrease for DBP (p = 0.04)
BP classification concordance
Concordance of 2017 ACC/AHA BP classification according to cuff and invasive brachial BP is presented in Table 4. Without correction for SBP amplification, there was fair agreement (Cohen κ, 0.42, and 57.4% concordance) between cuff and invasive brachial BP across BP classification thresholds. After cuff BP values were corrected for SBP amplification, there was an improvement in the accuracy of hypertension classification (Cohen κ, 0.49, and 63.7% concordance, p for two proportion test = 0.005). There were similar patterns of concordance when the 2020 International Society of Hypertension and 2018 European Society of Hypertension/European Society of Cardiology classification guidelines were applied (Supplementary Tables 6, 7 respectively).
Discussion
The key novel finding of this study was that the individual level of SBP amplification was significantly associated with the accuracy of cuff measured SBP. This was confirmed in independent study samples and across several different automated cuff BP measurement devices, each using unique proprietary algorithms to estimate BP. The accuracy of cuff measured SBP was highest among individuals with the lowest levels of SBP amplification, and cuff SBP progressively underestimated intra-arterial brachial SBP as SBP amplification increased. Multiple regression analysis identified SBP amplification as the factor explaining most of the variance in the accuracy of cuff measured SBP compared with intra-arterial brachial SBP. To our knowledge, this is the first study to report such findings, which provide insight on a key factor relevant the accuracy of conventional upper arm automated cuff BP methods.
The observed relationship between cuff accuracy and SBP amplification could be explained by the automated cuff measurement method itself, which has similar operating principles between devices (albeit having different algorithms) and employs analytical processes that are largely unchanged for decades [17,18,19]. A variety of propriety algorithms can be applied (e.g. using fixed-ratio coefficients) to estimate SBP and DBP at specific fractions of the envelope peak. These BPs are designed to copy the BP values recorded by manual auscultation [20, 21]. A critical factor relating to automated cuff BP compared with auscultatory BP is that individual differences in the shape of the waveform envelope result in different levels of accuracy of the estimated BP values [22,23,24]. Alongside this, we have observed phenotypic differences in the brachial arterial waveform shapes [9] (and consequent estimation of mean arterial pressure) [25] between individuals with low- compared to high- SBP amplification. It is possible these phenotypic waveform differences associated with SBP amplification, are influencing cuff accuracy via variability in the waveform envelope shape and consequent error in cuff SBP estimations. Such a problem could be rectified through development of BP estimation algorithms that are individualised based on pressure waveform characteristics associated with SBP amplification [26]. However, to date there are no mechanistic studies to determine whether the waveform envelope is influenced by BP amplification and arterial waveform shapes. Other factors potentially influencing the findings could include such things as local tissue properties under the cuff, the location of the cuff on the upper arm and cuff inflation or deflation rates.
This study identified a systematic error in the accuracy of cuff BP (compared with intra-arterial brachial BP) associated with SBP amplification and this has direct implications for accurate assessment of the true risk related to BP (the actual intra-arterial BP values). With respect to non-invasive cuff BP measurement, even small BP errors at the individual level can have large consequences on correct hypertension classification, prevalence and control [27]. An underestimation of 4/2 (SBP/DBP) mmHg corresponds to lowering hypertension prevalence but increasing hypertension control estimates by more than 5% respectively [28]. A small lowering in SBP (e.g. 2 mmHg) also correlates to about 7 to 10% reduction in ischemic heart disease and stroke mortality [29]. Given the consistency of our principal findings across seven separate automated BP measurement devices, the results may be applied to advance the individual level accuracy of standard cuff BP measurement compared with intra-arterial values, and thus achieve greater precision in cardiovascular risk stratification and treatment. However, the relative clinical value of invasive BP at either the central aorta or brachial artery has yet to be determined in large scale datasets, and cuff BP remains the clinical standard. As a point of interest, cuff SBP was the same average value as intra-arterial central aortic SBP, and explains the often-reported lack of SBP amplification between invasive central SBP and cuff SBP.
There are several study strengths, including a large population sample with high-quality intra-arterial measured brachial BP as the reference standard to confirm BP accuracy, as well as intra-arterial measurement of SBP amplification. Additionally, the findings were confirmed across five independent research sites, using seven independent automated BP devices. Potential limitations include a relatively homogenous clinical study sample comprising people with an indication for coronary angiography who are mostly older men and with multiple risk factors for cardiovascular disease. The results may therefore have limited generalisability beyond those with similar characteristics, although collecting invasive BP from healthy individuals without indication for coronary angiography is unethical. Although data were recorded according to guidelines [10], this is a convenience sample from several study centres and data collection protocols were not standardized across the study sites. The dynamic response of the fluid-filled catheter systems used to record pressure waveforms was assessed and confirmed to be in an appropriate range at four of the five study sites [12, 13, 15]. The findings from the study site that did not assess the dynamic response were in accordance with the pooled results from all studies. Effort was made to maintain the transducer at heart level throughout the research procedure at each site using fluid-filled catheters. However, a standardized protocol was not used to identify the phlebostatic axis and this could have led to hydrostatic errors. As an observational study, unmeasured confounding cannot be ruled out and causal inference of SBP amplification on cuff BP accuracy would need to be confirmed in an experimental design. Although correcting cuff SBP with SBP amplification improved the mean difference between cuff and invasive brachial SBP, the standard deviation remained similar, indicating no improvement in the precision (variance) of BP measurements after correction. This study is unable to determine the origin of the lack of change in variance, for example whether it is an intrinsic measurement issue or the added variance of the distribution of BP in the cohort. This question will need to be resolved to improve BP measurement precision within individuals. Finally, the accuracy of cuff BP was compared with invasive brachial BP because both measures are recorded at the same arterial site and cuff BP is the clinical standard. However, invasive central aortic BP may have stronger concordance with clinical outcomes than invasive brachial BP and cuff BP, but this needs to be determined in future studies.
In conclusion, this study found a significant association between the accuracy of conventional cuff measured BP (as it pertains to invasive brachial BP) and the magnitude of SBP amplification between the aorta and the site of cuff measurement – the brachial artery. Greatest cuff accuracy was associated with lowest SBP amplification, whereas cuff inaccuracy was related to higher SBP amplification. Enhancing the accuracy of BP measurement in clinical practice and research is an urgent and ongoing priority for major organisations worldwide [30, 31], and the findings from this study may ultimately be applied towards this goal.
References
Campbell NRC, Schutte AE, Varghese CV, Ordunez P, Zhang XH, Khan T, et al. Sao Paulo call to action for the prevention and control of high blood pressure: 2020. J Clin Hypertens (Greenwich). 2019;21:1744–52.
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on high blood pressure research. Hypertension. 2005;45:142–61.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–e115.
Sharman JE, Marwick TH. Accuracy of blood pressure monitoring devices: a critical need for improvement that could resolve discrepancy in hypertension guidelines. J Hum Hypertens. 2019;33:89–93.
Picone DS, Schultz MG, Otahal P, Aakhus S, Al-Jumaily AM, Black JA, et al. Accuracy of cuff-measured blood pressure: systematic reviews and meta-analyses. J Am Coll Cardiol. 2017;70:572–86.
Alpert BS, Quinn D, Gallick D. Oscillometric blood pressure: a review for clinicians. J Am Soc Hypertens. 2014;8:930–8.
Sharman JE, Tan I, Stergiou GS, Lombardi C, Saladini F, Butlin M, et al. Automated ‘oscillometric’ blood pressure measuring devices: how they work and what they measure. J Hum Hypertens. 2023;37:93–100.
Jílek JO, Stork M. Pulsations in the blood pressure cuff: oscillations or arterial pulses? Int J Biol Biomed Eng. 2012;6:35–42.
Picone DS, Schultz MG, Peng X, Black JA, Dwyer N, Roberts-Thomson P, et al. Discovery of new blood pressure phenotypes and relation to accuracy of cuff devices used in daily clinical practice. Hypertension. 2018;71:1239–47.
Sharman JE, Avolio AP, Baulmann J, Benetos A, Blacher J, Blizzard CL, et al. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017;38:2805–12.
Cheng HM, Wang KL, Chen YH, Lin SJ, Chen LC, Sung SH, et al. Estimation of central systolic blood pressure using an oscillometric blood pressure monitor. Hypertens Res. 2010;33:592–9.
Ding F-H, Li Y, Zhang R-Y, Zhang Q, Wang J-G. Comparison of the SphygmoCor and Omron devices in the estimation of pressure amplification against the invasive catheter measurement. J Hypertens. 2013;31:86–93.
Kowalski C, Yang K, Charron T, Doucet M, Hatem R, Kouz R, et al. Inaccuracy of brachial blood pressure and its potential impact on treatment and aortic blood pressure estimation. J Hypertens. 2021;39:2370–8.
Lin MM, Cheng HM, Sung SH, Liao CF, Chen YH, Huang PH, et al. Estimation of central aortic systolic pressure from the second systolic peak of the peripheral upper limb pulse depends on central aortic pressure waveform morphology. J Hypertens. 2012;30:581–6.
Bui TV, Picone DS, Schultz MG, Armstrong MK, Peng X, Black JA, et al. Comparison between cuff-based and invasive systolic blood pressure amplification. J Hypertens. 2022;40:2037–44.
Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60.
Celler BG, Argha A, Le PN, Ambikairajah E. Novel methods of testing and calibration of oscillometric blood pressure monitors. PLoS One. 2018;13:e0201123.
Ramsey M 3rd. Blood pressure monitoring: automated oscillometric devices. J Clin Monit. 1991;7:56–67.
Posey JA, Geddes LA, Williams H, Moore AG. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure. Cardiovasc Res Cent Bull. 1969;8:15–25.
Forouzanfar M, Dajani HR, Groza VZ, Bolic M, Rajan S, Batkin I. Oscillometric blood pressure estimation: past, present, and future. IEEE Rev Biomed Eng. 2015;8:44–63.
Chandrasekhar A, Yavarimanesh M, Hahn JO, Sung SH, Chen CH, Cheng HM, et al. Formulas to explain popular oscillometric blood pressure estimation algorithms. Front Physiol. 2019;10:1415.
Amoore JN, Vacher E, Murray IC, Mieke S, King ST, Smith FE, et al. Effect of the shapes of the oscillometric pulse amplitude envelopes and their characteristic ratios on the differences between auscultatory and oscillometric blood pressure measurements. Blood Press Monit. 2007;12:297–305.
Alvarez MA, Padwal R, Ringrose J, Jalali A, Hiebert W. Optimum waveform envelopes and amplitude ratios in oscillometric blood pressure estimation. Blood Press Monit. 2021;26:53–9.
Amoore JN, Lemesre Y, Murray IC, Mieke S, King ST, Smith FE, et al. Automatic blood pressure measurement: the oscillometric waveform shape is a potential contributor to differences between oscillometric and auscultatory pressure measurements. J Hypertens. 2008;26:35–43.
Schultz MG, Picone DS, Armstrong MK, Black JA, Dwyer N, Roberts-Thomson P, et al. The influence of SBP amplification on the accuracy of form-factor-derived mean arterial pressure. J Hypertens. 2020;38:1033–9.
Liu J, Cheng HM, Chen CH, Sung SH, Moslehpour M, Hahn JO, et al. Patient-specific oscillometric blood pressure measurement. IEEE Trans Biomed Eng. 2016;63:1220–8.
Padwal R, Campbell NRC, Schutte AE, Olsen MH, Delles C, Etyang A, et al. Optimizing observer performance of clinic blood pressure measurement: a position statement from the Lancet Commission on Hypertension Group. J Hypertens. 2019;37:1737–45.
Campbell NRC, Padwal R, Picone DS, Su H, Sharman JE. The impact of small to moderate inaccuracies in assessing blood pressure on hypertension prevalence and control rates. J Clin Hypertens (Greenwich). 2020;22:939–42.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388:2665–712.
John O, Campbell NRC, Brady TM, Farrell M, Varghese C, Velazquez Berumen A, et al. The 2020 “WHO technical specifications for automated non-invasive blood pressure measuring devices with cuff”. Hypertension. 2021;77:806–12.
Acknowledgements
The authors wish to thank all staff from the Royal Hobart Hospital Cardiology Department and Cardiac Catheterization Laboratory for their generous assistance in facilitating this study.
Funding
This work is supported by Royal Hobart Hospital Research Foundation grants (references 19-202 and 21-006) and was supported by a Vanguard Grant from the National Heart Foundation of Australia (reference 101836). DSP is supported by a Postdoctoral Fellowship (reference 104774) from the National Heart Foundation of Australia. MGS is supported by a Future Leadership Fellowship (reference 102553) from the National Heart Foundation of Australia. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CHC declares that Microlife Co. Ltd. and National Yang-Ming University have signed a contract for transfer of the non-invasive central blood pressure technique. JW reports having received grants from Novartis and Omron, and lecture and consulting fees from Novartis, Omron, Servier and Viatris. JES is principal investigator of a National Health and Medical Research Council partnership grant (S0026615) that includes a medical technology company that manufactures a central blood pressure monitor. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bui, T.V., Picone, D.S., Schultz, M.G. et al. Accuracy of cuff blood pressure and systolic blood pressure amplification. Hypertens Res 46, 1961–1969 (2023). https://doi.org/10.1038/s41440-023-01311-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01311-0