Abstract
The recent discovery of mechanosensitive ion channels has promoted mechanobiological research in the field of hypertension and nephrology. We previously reported Piezo2 expression in mouse mesangial and juxtaglomerular renin-producing cells, and its modulation by dehydration. This study aimed to investigate how Piezo2 expression is altered in hypertensive nephropathy. The effects of the nonsteroidal mineralocorticoid receptor blocker, esaxerenone, were also analyzed. Four-week-old Dahl salt-sensitive rats were randomly assigned to three groups: rats fed a 0.3% NaCl diet (DSN), rats fed a high 8% NaCl diet (DSH), and rats fed a high salt diet supplemented with esaxerenone (DSH + E). After six weeks, DSH rats developed hypertension, albuminuria, glomerular and vascular injuries, and perivascular fibrosis. Esaxerenone effectively decreased blood pressure and ameliorated renal damage. In DSN rats, Piezo2 was expressed in Pdgfrb-positive mesangial and Ren1-positive cells. Piezo2 expression in these cells was enhanced in DSH rats. Moreover, Piezo2-positive cells accumulated in the adventitial layer of intrarenal small arteries and arterioles in DSH rats. These cells were positive for Pdgfrb, Col1a1, and Col3a1, but negative for Acta2 (αSMA), indicating that they were perivascular mesenchymal cells different from myofibroblasts. Piezo2 upregulation was reversed by esaxerenone treatment. Furthermore, Piezo2 inhibition by siRNA in the cultured mesangial cells resulted in upregulation of Tgfb1 expression. Cyclic stretch also upregulated Tgfb1 in both transfections of control siRNA and Piezo2 siRNA. Our findings suggest that Piezo2 may have a contributory role in modulating the pathogenesis of hypertensive nephrosclerosis and have also highlighted the therapeutic effects of esaxerenone on salt-induced hypertensive nephropathy.

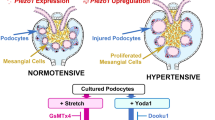
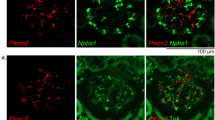
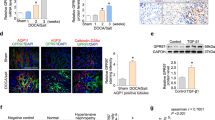
Mechanochannel Piezo2 is known to be expressed in the mouse mesangial cells and juxtaglomerular renin-producing cells, and this was confirmed in normotensive Dahl-S rats. In salt-induced hypertensive Dahl-S rats, Piezo2 upregulation was observed in the mesangial cells, renin cells, and notably, perivascular mesenchymal cells, suggesting its involvement in kidney fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N. Engl J Med. 1988;318:1657–66.
Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–7.
Dworkin LD, Hostetter TH, Rennke HG, Brenner BM. Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest. 1984;73:1448–61.
Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–9.
Kimura G, Brenner BM. The renal basis for salt sensitivity in hypertension. In Laragh JH, Brenner BM (eds), Hypertension, pathophysiology, diagnosis, and management. 2nd edn. Raven Press: New York, NY, USA, 1995, 1569–88.
Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–93.
Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–43.
Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:50–67.
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60.
Szczot M, Nickolls AR, Lam RM, Chesler AT. The form and function of PIEZO2. Annu Rev Biochem. 2021;90:507–34.
Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, et al. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 2018;362:464–7.
Mochida Y, Ochiai K, Nagase T, Nonomura K, Akimoto Y, Fukuhara H, et al. Piezo2 expression and its alteration by mechanical forces in mouse mesangial cells and renin-producing cells. Sci Rep. 2022;12:4197. https://doi.org/10.1038/s41598-022-07987-7.
Arai K, Tsuruoka H, Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharm. 2015;769:266–73.
Li L, Guan Y, Kobori H, Morishita A, Kobara H, Masaki T, et al. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens Res. 2019;42:769–78.
Olson JL, Wilson SK, Heptinstall RH. Relation of glomerular injury to preglomerular resistance in experimental hypertension. Kidney Int. 1986;29:849–57.
Hirakata M, Kaname S, Chung UG, Joki N, Hori Y, Noda M, et al. Tyrosine kinase dependent expression of TGF-beta induced by stretch in mesangial cells. Kidney Int. 1997;51:1028–36.
Moon JY, Tanimoto M, Gohda T, Hagiwara S, Yamazaki T, Ohara I, et al. Attenuating effect of angiotensin-(1-7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-A(y)/Ta mice. Am J Physiol Ren Physiol. 2011;300:F1271–82.
Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17:3438–46.
Shibota M, Nagaoka A, Shino A, Fujita T. Renin-angiotensin system in stroke-prone spontaneously hypertensive rats. Am J Physiol. 1979;236:H409–16.
Yasuda T, Kondo S, Homma T, Harris RC. Regulation of extracellular matrix by mechanical stress in rat glomerular mesangial cells. J Clin Invest. 1996;98:1991–2000.
Gao F, Wang DH. Impairment in function and expression of transient receptor potential vanilloid type 4 in Dahl salt-sensitive rats: significance and mechanism. Hypertension. 2010;55:1018–25.
Zhou Y, Castonguay P, Sidhom EH, Clark AR, Dvela-Levitt M, Kim S, et al. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science. 2017;358:1332–6.
Fu Y, Wan P, Zhang J, Li X, Xing J, Zou Y, et al. Targeting mechanosensitive Piezo1 alleviated renal fibrosis through p38MAPK-YAP pathway. Front Cell Dev Biol. 2021;9:741060. https://doi.org/10.3389/fcell.2021.741060.
Zhao X, Kong Y, Liang B, Xu J, Lin Y, Zhou N, et al. Mechanosensitive Piezo1 channels mediate renal fibrosis. JCI Insight. 2022;7:e152330. https://doi.org/10.1172/jci.insight.152330.
He Y, Deng B, Liu S, Luo S, Ning Y, Pan X, et al. Myeloid Piezo1 deletion protects renal fibrosis by restraining macrophage infiltration and activation. Hypertension. 2022;79:918–31.
Sakai T, Kriz W. The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl). 1987;176:373–86.
Di Carlo SE, Peduto L. The perivascular origin of pathological fibroblasts. J Clin Invest. 2018;128:54–63.
Minatoguchi S, Saito S, Furuhashi K, Sawa Y, Okazaki M, Shimamura Y, et al. A novel renal perivascular mesenchymal cell subset gives rise to fibroblasts distinct from classic myofibroblasts. Sci Rep. 2022;12:5389. https://doi.org/10.1038/s41598-022-09331-5.
Shaw I, Rider S, Mullins J, Hughes J, Péault B. Pericytes in the renal vasculature: roles in health and disease. Nat Rev Nephrol. 2018;14:521–34.
Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9.
Gerarduzzi C, Di Battista JA. Myofibroblast repair mechanisms post-inflammatory response: a fibrotic perspective. Inflamm Res. 2017;66:451–65.
Schimmel K, Ichimura K, Reddy S, Haddad F, Spiekerkoetter E. Cardiac fibrosis in the pressure overloaded left and right ventricle as a therapeutic target. Front Cardiovasc Med. 2022;9:886553. https://doi.org/10.3389/fcvm.2022.886553.
Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161–80.
Black LM, Lever JM, Agarwal A. Renal inflammation and fibrosis: a double-edged sword. J Histochem Cytochem. 2019;67:663–81.
Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66.
Kuppe C, Ibrahim MM, Kranz J, Zhang X, Ziegler S, Perales-Patón J, et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589:281–6.
Weller RO. Vascular pathology in hypertension. Age Ageing. 1979;8:99–103.
Correa-Rotter R, Hostetter TH, Manivel JC, Rosenberg ME. Renin expression in renal ablation. Hypertension. 1992;20:483–90.
Konda R, Orikasa S, Sakai K, Ota S, Kimura N. The distribution of renin containing cells in scarred kidneys. J Urol. 1996;156:1450–4.
Faraggiana T, Venkataseshan VS, Inagami T, Churg J. Immunohistochemical localization of renin in end-stage kidneys. Am J Kidney Dis. 1988;12:194–9.
Kintscher U, Bakris GL, Kolkhof P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br J Pharm. 2022;179:3220–34.
Tezuka Y, Ito S. The time to reconsider mineralocorticoid receptor blocking strategy: arrival of nonsteroidal mineralocorticoid receptor blockers. Curr Hypertens Rep. 2022;24:215–24.
Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: Role of oxidative stress. Hypertension. 2007;50:877–83.
Ennis IL, Pérez NG. Cardiac mineralocorticoid receptor and the Na+/H+ exchanger: spilling the beans. Front Cardiovasc Med. 2020;7:614279. https://doi.org/10.3389/fcvm.2020.614279.
Chambers L, Dorrance AM. Regulation of ion channels in the microcirculation by mineralocorticoid receptor activation. Curr Top Membr. 2020;85:151–85.
Cosgun ZC, Sternak M, Fels B, Bar A, Kwiatkowski G, Pacia MZ, et al. Rapid shear stress-dependent ENaC membrane insertion is mediated by the endothelial glycocalyx and the mineralocorticoid receptor. Cell Mol Life Sci. 2022;79:235. https://doi.org/10.1007/s00018-022-04260-y.
Acknowledgements
We thank Ms. Kaori Mikami for her technical support.
Funding
This work was supported in part by JSPS KAKENHI Grant Number JP17K09736 and JP20K08616, and by Japan Agency for Medical Research and Development (18gm5810019h9903).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Esaxerenone was provided by Daiichi Sankyo Co., LTD., Tokyo, Japan.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ochiai, K., Mochida, Y., Nagase, T. et al. Upregulation of Piezo2 in the mesangial, renin, and perivascular mesenchymal cells of the kidney of Dahl salt-sensitive hypertensive rats and its reversal by esaxerenone. Hypertens Res 46, 1234–1246 (2023). https://doi.org/10.1038/s41440-023-01219-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01219-9
Keywords
This article is cited by
-
Roles of the mechanosensitive ion channel Piezo1 in the renal podocyte injury of experimental hypertensive nephropathy
Hypertension Research (2024)
-
Mechanical stress is involved in mechanism of hypertensive nephropathy
Hypertension Research (2023)