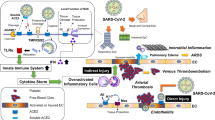
Abstract
The number of reported cases with coronavirus disease 2019 (COVID-19) has exceeded 620 million worldwide, still having a profound impact on people’s health and daily lives since its occurrence and outbreak in December 2019. From the early phase of the COVID-19 pandemic, there has been a concern that the rapid spread of this communicable disease can negatively influence non-communicable diseases. Accumulating data indicate that the restriction on the access to medical care, psychological distress, and life-style changes triggered by the pandemic have indeed affected blood pressure control in hypertensive patients. Since our previous report in 2020 that summarized the findings of the literature related to COVID-19 and hypertension, there has been a considerable progress in our understanding of the association between these two disorders; nonetheless, there are remaining challenges and emerging questions in the field. In this article, we aim to summarize the latest information on the impact of the pandemic on blood pressure control, the use of the renin-angiotensin system inhibitors in patients with COVID-19, and the blood pressure changes as one of the possible post-acute sequelae of COVID-19 (also known as long COVID). We also summarize the evidence of telemedicine and COVID-19 vaccination in hypertensive subjects, based on data available as of June 2022.

Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) infection, has been diagnosed in over 620 million individuals worldwide as of November 2022. From the initial stage of its outbreak, a serious concern has been raised that COVID-19 pandemic could worsen non-communicable diseases (NCDs) including hypertension [1, 2]. In our previous report, we have summarized the state of research on the influence of COVID-19 pandemic on hypertension and related diseases, based on the data available as of June 2020 [1]. Since then, there has been a rapid expansion of studies in several key areas, e.g., the impact of initial lockdowns on people’s health and blood pressure (BP) control, the use of the renin-angiotensin system (RAS) inhibitors in patients with COVID-19, and the association between hypertension and severe COVID-19. Moreover, among the points raised in future perspectives of the report [1], tremendous progress has been made in the development of COVID-19 vaccines, and there is also a growing need for the implementation of telemedicine. In addition, a new issue has emerged that COVID-19 affects the infected individuals even after an acute phase (referred to as “long COVID”). In this article, we aim to provide an updated information on these topics, based on data available as of June 2022. Given the significant biological and clinical differences among the SARS-CoV-2 variants, the study periods of the references are mentioned where relevant. For most of the studies, the data were taken before the spread of the Delta variant (around June 2021) and the Omicron variant (December 2021) [3]. Because of the space constraint, hypertension-related diseases (such as cardiovascular diseases) as post-acute sequelae of COVID-19 will be discussed in detail as a separate manuscript [4].
COVID-19 outbreak in Japan and its influence on hypertensive patients in early 2020
In Japan, the first case of COVID-19 was officially identified on 15 January 2020 and the number of confirmed cases increased to 15 by the end of that month. The Japanese government classified the disease as a “designated infectious disease” on 1 February 2020, which legally allowed the government to recommend hospitalization and to use public funds for the treatment of COVID-19. Three days later, COVID-19 cluster was identified among guests aboard the cruise ship Diamond Princess at Yokohama port [5]. The first death due to COVID-19 was reported in Kanagawa Prefecture on 13 February 2020. The number of the confirmed cases rapidly increased and reached 2000 by the end of March 2020. As a result, the Japanese government announced a state of emergency on 7 April 2020 to control the transmission of SARS-CoV-2; people were encouraged to self-isolate by staying at home and to reduce the person-to-person contact during daily activities.
Owing to the lack of effective vaccines and medications at the initial phase, social distancing, along with masking and hand disinfection, was highly recommended to reduce the risk of SARS-CoV-2 infection. Although these measures helped protect people from the viral infection, the literature suggests that the rapid spread of COVID-19 and the lockdowns disrupted medical supply chains and highly restricted the access to medical care [6]. One study involving nearly 800,000 subjects in 26 hospitals in Japan showed that the number of outpatient visits decreased by 22% in May 2020, and the outpatient prescription, including calcium channel blockers and other common medications, decreased by 20% in May 2020 [7].
The quarantine and behavioral restrictions also had negative impacts on the psychological wellness [8]. A report by the Ministry of Health, Labor and Welfare in Japan showed that the ratio of people with anxiety peaked in February to May 2020 (https://www.mhlw.go.jp/stf/newpage_18041.html). Negative or speculative information on COVID-19, as well as controversial expert opinions, was an additional factor that potentially promoted people’s anxiety (“headline stress disorder”) [9,10,11,12]. Patients with hypertension might have been particularly stressed, because the initial reports from Wuhan, China, indicated that subjects with hypertension had a high mortality rate due to COVID-19 [13]. In addition, there was also a concern that RAS inhibitors might negatively affect the clinical course of COVID-19, based on several experimental studies (these topics will be discussed in detail later in this article).
In addition, the COVID-19 pandemic has drastically influenced people’s life-style. During the lockdown in early 2020, altered physical activity and/or food consumption were reported in several studies [14], including those from UK [15], Poland [16], Spain [17], and China [18], which likely had a negative impact on body weight control [16, 17]. Several lines of evidence also indicate that the influences of COVID-19 restrictions on people’s daily activities were not necessarily uniform. According to a study performed in Turkey, those with high body mass index (BMI) consumed sweetened and carbonated drinks more than other participants [19]. In another study that was performed across several countries during the COVID-19 lockdown, there were regional differences in the impact on well-being [14]. Studies from Italy reported that a subpopulation of individuals was able to reorganize their life-style in response to the lockdown, increasing physical exercising and improving diet quality [20, 21].
Regarding the BP control in hypertensive patients, several studies reported the BP alteration during the initial phase of the COVID-19 pandemic. In Japan, Kobayashi et al. reported that the office BP significantly increased from 136.5 ± 17.5/78.2 ± 12.0 to 138.6 ± 18.6/79.0 ± 12.2 mmHg, whereas home BP significantly decreased from 128.2 ± 10.3/75.8 ± 8.8 to 126.9 ± 10.2/75.2 ± 9.0 mmHg after the announcement for the state of emergency, increasing the ratio of white coat hypertension [22]. These changes in BP were associated with the increase in chronic stress [22]. Analysis of a large annual health check-up data of Japanese individuals reported that systolic and diastolic BP were increased by ~1–2 and 0.5–1 mmHg, respectively, during the state of emergency [23]. Endo et al. analyzed glycemic and BP control in 176 Japanese patients with diabetes mellitus and reported that both systolic and diastolic BP did not change during the state of emergency, however they rose significantly afterwards [24]. According to a questionnaire survey conducted by a healthcare company on Japanese patients who were taking antihypertensive medications, ~90% of the respondents changed their day-to-day life-style, and 17.2% observed a change in their BP after the COVID-19 pandemic (https://www.healthcare.omron.co.jp/zeroevents/bloodpressure/topics/01.html).
In other countries, an annual health program in the United States reported the increase in BP that ranged from 1.1 to 2.5 mmHg for systolic BP and 0.1 to 0.5 mmHg for diastolic BP during the COVID-19 pandemic [25]. In another study, the mean systolic and diastolic BP increased from April–August 2019 to April–August 2020 (127.5 mmHg vs. 131.6 mmHg; p < 001, and 79.2 mmHg vs. 80.2 mmHg; p < 001, respectively) [26]. In a subpopulation of the participants of Strategy of Blood Pressure Intervention in Elderly Hypertensive Patients (STEP) study conducted in China [27], patients with an increased level of anxiety had a higher rate of uncontrolled BP and an increased risk of cardiovascular events (hazard ratio [HR], 2.47; 95% confidence interval [CI], 1.10–5.58; p = 0.03) [28]. These data indicate that life-style changes, psychological stress, and the limited access to medical care have influenced BP control in the early phase of COVID-19 pandemic, predisposing hypertensive subjects to increased risk of cardiovascular events. A future survey of the long-term influence of the pandemic is warranted.
Hypertension and the risk for severe COVID-19
In our previous article, we have concluded that there was no clear evidence supporting that hypertension is an independent risk factor for the worse outcome in COVID-19 [1]. The Centers for Disease Control and Prevention report alerts that patients with type 1 and type 2 diabetes, obesity of BMI ≥ 30 kg/m2, and chronic kidney disease (CKD) are at increased risk of severe COVID-19 (https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html). Regarding the association between hypertension and severe COVID-19 outcomes, however, the report states that the meta-analyses and systematic reviews thus far are still inconclusive. Similar limitation is noted in the scientific brief released from the World Health Organization (based on the literature published before January 2021) (https://apps.who.int/iris/handle/10665/341848). In the Phase IV Observational Study to Associate Hypertension and Hypertension Treatment to COVID-19 (SARS-RAS) of the Italian Society of Hypertension, Iaccarino and colleagues analyzed the outcomes of 1591 patients with COVID-19 in March to April 2020, among which hypertension was observed in 55% [29]. In the multivariate analysis of that study, diabetes, chronic obstructive pulmonary disease, and CKD were the independent predictors of mortality other than age; however, hypertension was not [29]. In a recent report from CAPACITY-COVID, a registry that included more than 9000 patients with confirmed or highly suspected SARS-CoV-2 infection in Europe between March 2020 and April 2021, hypertension was not independently associated with in-hospital mortality [30]. However, according to a meta-analysis that included 1558 COVID-19 patients from six retrospective studies conducted in 2020 [31], hypertension, as well as diabetes, chronic obstructive pulmonary disease (COPD), cardio- and cerebrovascular disease, was an independent risk factor for exacerbation of COVID-19. Among 803 COVID-19 patients with hypertension, significant predictors of heart failure were average in-hospital systolic BP and pulse pressure, and the standard deviations of systolic and diastolic BP were independently associated with in-hospital mortality and intensive care unit (ICU) admission [32]. Therefore, there is currently mixed evidence for an association between hypertension and severe COVID-19. It is possible that the quality of BP control, as well as the severity and duration of hypertension, can influence the clinical course of cardiovascular complications in COVID-19 [33]. Nonetheless, given the established role of hypertension as the principal risk factor for cardiovascular diseases, this association may not necessarily be limited to COVID-19. In addition, a large fluctuation in BP in COVID-19 patients with poor prognosis may simply reflect their critical conditions.
Hypertension and long COVID
Post-acute sequelae of COVID-19, also called long COVID, has increasingly been recognized worldwide [4]. In a real-time tracking based on a mobile application in April 2020, 558 out of 4182 (13.3%) COVID-19 patients in the UK reported symptoms that lasted for ≥ 28 days, including fatigue, headache, dyspnea, and anosmia [34]. These symptoms were more likely to occur with older age, higher BMI, and female sex [35]. In a multi-omic investigation of 309 COVID-19 patients [36], the frequently self-reported symptoms during convalescence (2–3 months after the initial diagnosis of COVID-19) were fatigue (52% of participants), cough (25%), and anosmia/dysgeusia (18%). The predictors for long COVID identified in that study were type 2 diabetes, SARS-CoV-2 RNAemia (circulating mRNA fragments of SARS-CoV-2), Epstein-Barr virus viremia, and specific auto-antibodies such as anti-interferon-α2 [36].
Evidence regarding the impact of pre-existing hypertension on long COVID is still insufficient, e.g., hypertension was not investigated [35] nor selected as a predictor of long COVID [36]. Among 222 Saudi Arabian patients hospitalized with COVID-19 between May and July 2020, persistent symptoms (after a median of 122 days of discharge) were present in 56.3% of patients, among which most frequent ones were shortness of breath (40.1%), cough (27.5%), and fatigue (29.7%) (Table 1) [37]. After multivariable adjustment, pre-existing hypertension was associated with an increased risk of new or persistent symptoms (HR, 1.73; 95% CI, 1.09–2.74) along with female sex and length of hospital stay. Although 28.8% of patients did not return to baseline (before SARS-CoV-2 infection) health status, pre-existing hypertension was not a significant predictor of the non-return (HR, 1.69; 95% CI, 0.68–4.16). In another study from Norway, a total of 312 patients diagnosed as COVID-19 during February to April 2020 were followed for symptoms at 6 months [38]. In that study, 60.6% of patients had persistent symptoms, including fatigue, difficulty concentrating, disturbed smell/taste, memory problems, and dyspnea. In a regression analysis, being female, pre-existing lung disease, COVID-19 severity, and increased convalescent antibody titers were the independent predictors of the increased fatigue score. Although hypertension was associated with the fatigue score in a univariate analysis, this association was not significant after adjustment [38].
Is hypertension a post-acute sequela of COVID-19?
Emerging evidence indicate that cardiovascular complications can occur as a post-acute sequela of COVID-19 [4]. Using national healthcare databases from the US Department of Veterans Affairs, Xie et al. built a cohort of 153,760 individuals with positive for COVID-19 testing between March 2020 and January 2021 (Table 1) [39]. By comparing this database with two sets of control cohorts including 5,637,647 and 5,859,411 individuals, COVID-19 survivors were shown to have the increased risk of cardiovascular diseases, including stroke, transient ischemic attack, ischemic heart disease, pericarditis, myocarditis, heart failure, dysrhythmia, and thromboembolic disease, independently of pre-existing hypertension and other cardiovascular risk factors.
Besides these cardiovascular complications, several reports suggest that COVID-19 survivors can have an elevated BP at a post-acute phase [40]. Based on the above-mentioned US Department of Veterans Affairs national healthcare databases, the same group [39] estimated a 6-month incident sequela in COVID-19 patients who survived for at least the first 30 days after the diagnosis (Table 1) [41]. Compared with 4,990,835 non-infected controls, 73,435 COVID-19 survivors had excess burdens of hypertension (15.18 incident diagnoses per 1000 patients with COVID-19), as well as obesity (9.53) and diabetes (8.23). In addition to oral hypoglycemic agents and insulin, the introduction of antihypertensive drugs (beta-blockers, calcium channel blockers, and thiazide diuretics) was also increased.
Cohen et al. performed a retrospective cohort study to evaluate the risk of persistent clinical sequelae among 87,337 adults aged ≥ 65 years during the post-acute phase of COVID-19 (diagnosed before April 2020) (Table 1) [42]. In that study, 32% required medical attention for at least one new or persistent clinical sequela, which was 11% higher than the non-infected comparison group. As well as respiratory failure (risk difference, 7.55; 95% CI, 7.18–8.01), fatigue (5.66; 5.03–6.27), kidney injury (2.59; 2.03–3.12), and cardiac rhythm disorders (2.19; 1.76–2.57), the increased risk of hypertension (4.43; 2.27–6.37) was observed in the study participants. The same investigator group reported similar findings among 193,113 COVID-19 patients aged 18–65 years (Table 1) [43], i.e., 14% of the COVID-19 patients required medical attention because of aftereffects, which was 5% higher than the control group. The risk for developing hypertension among COVID-19 patients was 81% higher (95% CI, 10–196%) than that in the matched control group.
The underlying mechanisms of the BP elevation after recovery from COVID-19 are unclear [40]. A retrospective case series of nine patients with COVID-19 under a prolonged intensive care reported that these patients developed hyperreninemia, along with hypernatremia, hyperchloremia, and reduced glomerular filtration rate [44]. Although the duration of these changes is unclear, these changes may in part contribute to BP elevation after the recovery from COVID-19. The development of de novo hypertension or the deterioration of BP control needs to be monitored at a post-acute phase.
Current evidence on ACE inhibitors and ARBs in patients with COVID-19
As summarized in our previous article [1], several retrospective studies from different countries [45,46,47,48,49,50] and meta-analyses [51,52,53,54,55] provided evidence for continuing treatment with RAS inhibitors in hypertensive patients with COVID-19 in the early phase of the pandemic. Although these initial studies were conducted in the United States, China, and Europe, several reports from Japan are currently available. In a retrospective, multicenter, observational study from Kanagawa, Japan [56], ACEIs and ARBs were prescribed in 15% of a total of 151 patients hospitalized with COVID-19 between February and May 2020. In this study, indices for the severity of COVID-19 such as in-hospital death and ICU admission were not different between ACEI/ARB group and non-ACEI/ARB group. The study found that the occurrence of new-onset or worsening mental confusion was significantly lower in ACEI/ARB group in crude analysis and after adjustment with age, sex, and diabetes [56]. COVID-19 registry Japan (COVIREGI-JP) is a nationwide registry that includes hospitalized patients with a positive SARS-CoV-2 test [57]. Using the data from COVIREGI-JP as of November 2020, the study by Yoshihara et al. analyzed the factors associated with the increased risk of primary outcomes, which were in-hospital death, ventilator support, extracorporeal membrane oxygen support, and ICU admission. Although the study found that aging, male sex, COPD, severe renal impairment, and diabetes mellitus were associated with the increased primary outcomes, no associations were found with the use of ACEIs and ARBs [58]. In addition, several recent meta-analysis of observational studies reported the safety or the potential benefit of RAS inhibitors in patients with hypertension [59]. In a meta-analysis of 30 studies (published before May 2020) comprising 10,434 adult patients with COVID-19, including nine clinical studies from China, patients treated with RAS inhibitors showed a reduced risk of severe/death outcomes especially in Asia [60].
Several randomized controlled trials have also been conducted to evaluate whether continuing or discontinuing RAS inhibitors affect outcomes in patients with COVID-19. In the Blockers of Angiotensin Receptor and Angiotensin-Converting Enzyme inhibitors suspension in hospitalized patients with coronavirus infection (BRACE CORONA) study, the largest investigator-initiated, multicenter, registry-based randomized trial that completed in July 2020, there was no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue (334 patients) vs. continue RAS inhibitors (325 patients) among 659 patients hospitalized with mild to moderate COVID-19 and who were taking these medications before hospital admission [61]. In a subgroup analysis according to disease severity, patients with a moderate severity who continued RAS inhibitors had more days alive and out of hospital through 30 days than those who discontinued these drugs [62]. Both in the Randomized Elimination or ProLongation of ACEIs and ARBs in COronaVIrus Disease 2019 (REPLACE COVID) trial, a prospective, randomized, open-label trial done at 20 large referral hospitals in seven countries worldwide [63], and the Stopping ACE-inhibitors in Covid-19 (ACEI-COVID) trial, a multicenter, randomized, controlled, open-label trial [64], discontinuation of RAS inhibitors did not significantly affect the severity of COVID-19 and the primary outcome measure of these studies.
Concerns that RAS inhibitors might affect the clinical course of COVID-19 were mainly derived from several experimental data suggesting that the expression of angiotensin converting enzyme 2 (ACE2), which serves as the cell entry receptor for SARS-CoV-2, was upregulated by these agents; nonetheless, a recent systematic review on 88 articles involving 168 experiments on this issue has found that ACE2 upregulation by RAS inhibitors was a rare event, rather than the common consequence [65]. Given these clinical and experimental evidence, RAS inhibitors should be continued in patients admitted to hospital with COVID-19 unless there is a distinct medical contraindication to ongoing therapy, which is consistent with the previous statements of the international societies [66,67,68,69] and information from the Japanese Society of Hypertension [70].
COVID-19 vaccines in patients with hypertension
One of the fundamental progresses that have been made since the beginning of the current pandemic is the development of COVID-19 vaccines, which include mRNA vaccines (i.e., mRNA-1273 and BNT162b2), adenovirus vector vaccine (AZD1222), and recombinant protein vaccine (NVX-CoV2373) [71,72,73,74]. As of June 2022, the above four vaccines have been approved in Japan. High immunogenicity of the mRNA vaccine (BNT162b2) was accompanied with increased antibody production, and the high antibody responses were also confirmed in Japan [75, 76].
Currently, evidence is mixed regarding the immune reactions to the COVID-19 vaccination in hypertensive patients [77,78,79]. In a study from Israel that investigated the antibody titers 1–2 weeks after the second dose of BNT162b2 vaccination, multivariate linear regression analysis found that hypertension was associated with lower SARS-CoV-2 receptor-binding domain IgG levels (assessed using reagents provided by Beckman-Coulter) but higher neutralization titers (SARS-CoV-2 pseudo-virus neutralization assay) [77]. In another study from Italy, hypertension was independently associated with lower antibody titers 3 weeks after two doses of BNT162b2 inoculation (the titer was assessed using Elecsys anti-SARS-CoV-2, Roche) [78]. Finally, in a study from Greece, the antibody titers (evaluated using Elecsys anti-SARS-CoV-2 S, Roche) were not statistically different from non-hypertensives at 3 months after the second dose of BNT162b2 vaccination [79]. In other populations, the antibody titer is reported to be relatively low in patients with diabetes mellitus and in elderly people [79,80,81,82].
The overall incidence of hypertension after mRNA vaccination (mRNA-1273 and BNT162b2) was reported to be less than 1% [73, 74]. Headache, malaise, fever, and pain (muscle or joint) are frequently observed after vaccination, which may indirectly affect BP. The increase in BP after mRNA vaccination (BNT162b2) has been reported in a case series of nine patients with stage III hypertension [83]. In that study, the median age was 73 years, seven women and two men, and all but one patient had a history of hypertension [83]. In another study, six subjects showed an average increase in home BP by 10 mmHg or more during the first 5 days after the first dose of mRNA vaccine [84]. The short interval between vaccination and BP increase seems to be consistent with the response to pain or physical stress associated with vaccination. In Japan, a recent study reported the incidence of adverse reactions to the COVID-19 vaccine (mRNA-1273, BNT162b2) among pregnant women [85]. According to that study, 4840 (73.6%) were vaccinated twice, and 557 (8.5%) were vaccinated once. Among the study participants, increased BP has been observed only in five subjects (0.09%) after the first dose and seven subjects (0.14%) after the second dose. Although more evidence would be necessary, sustained BP increase after COVID-19 vaccination seems to be a rare event.
Myocarditis has been recognized as a rare but important complication of COVID-19 mRNA (BNT162b2 and mRNA-1273) vaccination [86]. Adolescent males are known to have the increased risk of this complication [86]. Patients with myocarditis typically present with chest pain a few days after the mRNA vaccination and have elevated cardiac troponin levels with ST elevation by electrocardiogram. Although the underlying mechanisms are not entirely clear, the hyper-immunoreaction induced by the vaccine has been proposed.
According to the surveillance by Israeli Ministry of Health [87], the number of cases with myocarditis was 117 during 149 million person-days of follow-up in those who received two doses of BNT162b2 vaccine, as compared with 98 cases during 296 million person-days of follow-up in the unvaccinated group (the rate ratio of 2.35; 95% CI, 1.10–5.02). The rate ratio was highest in male recipient between the ages of 16 and 19 years (8.96; 95% CI, 4.50–17.83) and was lowest in those with 30 years or older (1.00; 95% CI, 0.61–1.64 for male and 0.82; 95% CI, 0.33–2.02 for female) [87]. Predominant occurrence of myocarditis in adolescent males following COVID-19 mRNA vaccination has been consistently reported in other studies [88, 89]. A comparison of BNT162b2 and mRNA-1273 vaccines indicated that the incidence of myocarditis is higher in the latter, particularly after the second dose [88, 90]. Although less frequent, the inoculation of adenovirus-vectored vaccine (AZD1222) can also trigger myocarditis [90, 91].
The estimation for the exact incidence of myocarditis following mRNA vaccination is challenging, owing to the variable severity and the lack of systematic evaluation. However, a meta-analysis indicated that myocarditis occurs 11 cases per one million recipients with COVID-19 mRNA vaccines [92]. According to a recent review, 354 cases of myocarditis 0–7 days after vaccination was reported after 164 million doses of mRNA vaccine [93]. In comparison, the risk of myocarditis after the SARS-CoV-2 infection seems much higher, which is estimated to be 1500 cases per million [93]. A recent meta-analysis demonstrated that the relative risk of myocarditis was more than seven times higher in SARS-CoV-2 infection than COVID-19 vaccination [90]. Therefore, the benefit of COVID-19 vaccination seems to outweigh the low risk of myocarditis, and COVID-19 vaccination is currently recommended for everyone ≥12 years of age.
Vaccine-induced thrombotic thrombocytopenia (VITT) has been reported following the inoculation with the adenovirus vector vaccine AZD1222 predominantly in females [94]. Studies have revealed that antibodies to platelet factor 4 (PF4) that were unrelated to the use of heparin were tested positive for these cases, suggesting a possible mechanism of VITT [95]. VITT and positive testing for antibodies against PF4 have not been reported in individuals immunized with BNT162b2 and mRNA-1273 vaccines [96]. Further studies are needed to better identify VITT’s pathophysiological mechanisms and genetic, demographic, or clinical predisposition.
Telemedicine and the management of NCDs in the era of COVID-19
The importance of telemedicine has been increasingly recognized in the face of the prolonged pandemic. In an analysis of serial cross-sectional data from the US National Disease and Therapeutic Index audit between January 2018 and June 2020 [97], telemedicine visits increased from 1.2% in 2018 to 19.5% in 2020. In sharp contrast, office-based visits decreased by 50% in the second quarter of 2020 compared with the same period in 2018–2019. In hypertension, self-measured home BP monitoring is the preferred method for BP management, and many home BP devices are capable of transmitting BP data, making it easy to apply telemedicine in this field. In Japan, although there were not many opportunities for telemedicine in a usual state, telemonitoring using self-measured BP was widely introduced at the time of the Great East Japan Earthquake in 2011 [98, 99]. In other countries, a number of clinical trials for telemedicine using self-measured home BP have been performed, and many meta-analysis have already been published [100]. Most of these studies have shown that the addition of telemedicine, i.e., telecommunication between patients and health care providers based on self-measured home BP, is more effective in BP control than the management of hypertension based on home BP measurement only. Nonetheless, several limitations of telemedicine also need to be noted. In the above-mentioned study [97], the increase in telemedicine visits and the decrease in office visits were associated with the reduced BP assessment (50% reduction in the second quarter of 2020). The number of new treatment visits for hypertension was also reduced by 39%. The same group also reported that the rapid increase in telemedicine during the early phase of COVID-19 pandemic was followed by a re-bound in office visits, although telemedicine still accounted for 20% of care [101]. The authors noted that nearly two-thirds of telemedicine visits were used for established patients [101]. Very recent ISH position paper has summarized important barriers that need to be overcome, including limited physical examination, unvalidated home BP monitoring device, and digital divide (e.g., low digital literacy and limited internet access) [102].
Unfortunately, in Japan, we found only a few studies that addressed the efficacy of telemedicine over conventional medical care during the COVID-19 pandemic. In the retrospective analysis by Onishi et al., the authors compared the efficacy of telemedicine (appointment by telephone) with clinic visit on HbA1c levels in outpatients with diabetes mellitus in 2020 [103]. The application of telemedicine was dependent on patients’ health status, living areas, and attending physicians’ decision. In the propensity score analysis involving 618 pairs with pre-HbA1c levels ≥ 7%, clinic visit group had a significantly better post-HbA1c levels than telemedicine group (7.4% vs. 7.5%; p = 0.023), again suggesting a room for the improvement in quality of telemedicine [103]. In high-risk situations, e.g., natural disasters and pandemic, BP telemonitoring has been shown to be a useful approach to minimize the disruption of medical care for the chronically ill patients [98, 104]. However, if the telemedicine is to be applied in a normal situation, a more rigorous assessment of its efficacy will be necessary, particularly the long-term BP control.
As for the cost of telemedicine, telecommunication among healthcare providers (such as exchanging electronic images for diagnostic purposes) is reported to be less expensive, whereas it has been debated whether telemedicine between patients and healthcare providers can reduce costs compared with the usual face-to-face medical care [105]. A recent study showed that an app for hypertension is cost-effective compared to usual care [106].
Conclusions and perspectives
In this article, we summarized the updated information regarding COVID-19 and hypertension, based on the literature available after the publication of our previous review [1]. Evidence indicates that the initial COVID-19 pandemic has indeed affected BP control in hypertensive patients. A long-term influence, as well as its impact on cardiovascular outcomes, needs to be evaluated in future studies. Thus far, several randomized controlled trials have provided evidence that RAS inhibitors should be continued unless there is contraindication. The evidence regarding the association between hypertension and COVID-19 severity is mixed. Well-designed clinical trials are necessary to clarify the significance of hypertension in COVID-19, including the risk of BP elevation at a post-acute phase.
Overall, evidence supports that COVID-19 vaccination to hypertensive patients is safe and induces appropriate immune responses. Several studies indicate that hypertension is associated with a lower antibody production; however, the timing and methods for antibody titer assessment are variable among studies. Although telemedicine reduces physical contacts and can overcome the restrictions on access to medical care in the face of the prolonged pandemic, there are also remaining challenges in BP telemonitoring. These include the validation of home BP devices, overcoming digital divide, and the build-up of evidence for a long-term BP control.
Finally, a majority of the studies cited in this article are based on data obtained before the spread of the Delta and Omicron variants. Updated information would be necessary regarding how the newer variant strains of SARS-CoV-2, as well as vaccination status, affect the clinical course of hypertensive patients and BP control.
References
Shibata S, Arima H, Asayama K, Hoshide S, Ichihara A, Ishimitsu T, et al. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertens Res. 2020;43:1028–46.
Itoh H. A new normal for hypertension medicine with coronavirus disease-2019 (COVID-19): proposal from the president of the Japanese Society of Hypertension. Hypertens Res. 2020;43:857–8.
Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761.
Matsumoto C, Shibata S, Kishi T, Morimoto S, Mogi M, Yamamoto Y, et al. Long COVID and Hypertension-Related Disorders: A Report from the Japanese Society of Hypertension Project Team on COVID-19. Hypertens Res. in press.
Takeuchi I. COVID-19 first stage in Japan—how we treat ‘Diamond Princess Cruise Ship’ with 3700 passengers? Acute Med Surg. 2020;7:e506.
Skeete J, Connell K, Ordunez P, DiPette DJ. Approaches to the management of hypertension in resource-limited settings: strategies to overcome the hypertension crisis in the post-COVID era. Integr Blood Press Control. 2020;13:125–33.
Yamaguchi S, Okada A, Sunaga S, Ikeda Kurakawa K, Yamauchi T, Nangaku M, et al. Impact of COVID-19 pandemic on healthcare service use for non-COVID-19 patients in Japan: retrospective cohort study. BMJ Open. 2022;12:e060390.
Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–20.
Talevi D, Socci V, Carai M, Carnaghi G, Faleri S, Trebbi E, et al. Mental health outcomes of the CoVID-19 pandemic. Riv Psichiatr. 2020;55:137–44.
Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531–42.
Dong M, Zheng J. Letter to the editor: Headline stress disorder caused by Netnews during the outbreak of COVID-19. Health Expect. 2020;23:259–60.
Bagus P, Pena-Ramos JA, Sanchez-Bayon A. COVID-19 and the political economy of mass hysteria. Int J Environ Res Public Health. 2021;18:1376.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42.
Faulkner J, O’Brien WJ, McGrane B, Wadsworth D, Batten J, Askew CD, et al. Physical activity, mental health and well-being of adults during initial COVID-19 containment strategies: a multi-country cross-sectional analysis. J Sci Med Sport. 2021;24:320–6.
Robinson E, Boyland E, Chisholm A, Harrold J, Maloney NG, Marty L, et al. Obesity, eating behavior and physical activity during COVID-19 lockdown: a study of UK adults. Appetite. 2021;156:104853.
Sidor A, Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12:1657.
Martinez-de-Quel O, Suarez-Iglesias D, Lopez-Flores M, Perez CA. Physical activity, dietary habits and sleep quality before and during COVID-19 lockdown: a longitudinal study. Appetite. 2021;158:105019.
Ding D, Cheng M, Del Pozo Cruz B, Lin T, Sun S, Zhang L, et al. How COVID-19 lockdown and reopening affected daily steps: evidence based on 164,630 person-days of prospectively collected data from Shanghai, China. Int J Behav Nutr Phys Act. 2021;18:40.
Barcin-Guzeldere HK, Devrim-Lanpir A. The association between body mass index, emotional eating and perceived stress during COVID-19 partial quarantine in healthy adults. Public Health Nutr. 2022;25:43–50.
Cancello R, Soranna D, Zambra G, Zambon A, Invitti C. Determinants of the lifestyle changes during COVID-19 pandemic in the residents of northern Italy. Int J Environ Res Public Health. 2020;17:6287.
Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attina A, Cinelli G, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18:229.
Kobayashi K, Chin K, Umezawa S, Ito S, Yamamoto H, Nakano S, et al. Influence of stress induced by the first announced state of emergency due to coronavirus disease 2019 on outpatient blood pressure management in Japan. Hypertens Res. 2022;45:675–85.
Satoh M, Murakami T, Obara T, Metoki H. Time-series analysis of blood pressure changes after the guideline update in 2019 and the coronavirus disease pandemic in 2020 using Japanese longitudinal data. Hypertens Res. 2022;45:1408–17.
Endo K, Miki T, Itoh T, Kubo H, Ito R, Ohno K, et al. Impact of the COVID-19 pandemic on glycemic control and blood pressure control in patients with diabetes in Japan. Intern Med. 2022;61:37–48.
Laffin LJ, Kaufman HW, Chen Z, Niles JK, Arellano AR, Bare LA, et al. Rise in blood pressure observed among US adults during the COVID-19 pandemic. Circulation. 2022;145:235–7.
Shah NP, Clare RM, Chiswell K, Navar AM, Shah BR, Peterson ED. Trends of blood pressure control in the U.S. during the COVID-19 pandemic. Am Heart J. 2022;247:15–23.
Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385:1268–79.
Zhang S, Zhong Y, Wang L, Yin X, Li Y, Liu Y, et al. Anxiety, home blood pressure monitoring, and cardiovascular events among older hypertension patients during the COVID-19 pandemic. Hypertens Res. 2022;45:856–65.
Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M, et al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension. 2020;76:366–72.
McFarlane E, Linschoten M, Asselbergs FW, Lacy PS, Jedrzejewski D, Williams B, et al. The impact of pre-existing hypertension and its treatment on outcomes in patients admitted to hospital with COVID-19. Hypertens Res. 2022;45:834–45.
Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12:6049–57.
Ran J, Song Y, Zhuang Z, Han L, Zhao S, Cao P, et al. Blood pressure control and adverse outcomes of COVID-19 infection in patients with concomitant hypertension in Wuhan, China. Hypertens Res. 2020;43:1267–76.
Yamazaki O, Shibata S. Severe COVID-19 and preexisting hypertension: a matter of age? Hypertens Res. 2022;45:1523–5.
Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037–40.
Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–31.
Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–95.e820.
Tleyjeh IM, Saddik B, AlSwaidan N, AlAnazi A, Ramakrishnan RK, Alhazmi D, et al. Prevalence and predictors of Post-Acute COVID-19 Syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLoS ONE. 2021;16:e0260568.
Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–13.
Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–90.
Saeed S, Tadic M, Larsen TH, Grassi G, Mancia G. Coronavirus disease 2019 and cardiovascular complications: focused clinical review. J Hypertens. 2021;39:1282–92.
Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–64.
Cohen K, Ren S, Heath K, Dasmariñas MC, Jubilo KG, Guo Y, et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2022;376:e068414.
Daugherty SE, Guo Y, Heath K, Dasmarinas MC, Jubilo KG, Samranvedhya J, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373:n1098.
Hultstrom M, von Seth M, Frithiof R. Hyperreninemia and low total body water may contribute to acute kidney injury in COVID-19 patients in intensive care. J Hypertens. 2020;38:1613–4.
de Abajo FJ, Rodriguez-Martin S, Lerma V, Mejia-Abril G, Aguilar M, Garcia-Luque A, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–14.
Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–30.
Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382:2431–40.
Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–8.
Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertension. 2020;76:51–8.
Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–81.
Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2020;20:571–90.
Kerneis M, Ferrante A, Guedeney P, Vicaut E, Montalescot G. Severe acute respiratory syndrome coronavirus 2 and renin-angiotensin system blockers: a review and pooled analysis. Arch Cardiovasc Dis. 2020;113:797–810.
Koshy AN, Murphy AC, Farouque O, Ramchand J, Burrell LM, Yudi MB. Renin-angiotensin system inhibition and risk of infection and mortality in COVID-19: a systematic review and meta-analysis. Intern Med J. 2020;50:1468–74.
Patoulias D, Katsimardou A, Stavropoulos K, Imprialos K, Kalogirou MS, Doumas M. Renin-angiotensin system inhibitors and COVID-19: a systematic review and meta-analysis. Evidence for significant geographical disparities. Curr Hypertens Rep. 2020;22:90.
Pranata R, Permana H, Huang I, Lim MA, Soetedjo NNM, Supriyadi R, et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:983–90.
Matsuzawa Y, Ogawa H, Kimura K, Konishi M, Kirigaya J, Fukui K, et al. Renin-angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020;43:1257–66.
Matsunaga N, Hayakawa K, Terada M, Ohtsu H, Asai Y, Tsuzuki S, et al. Clinical epidemiology of hospitalized patients with coronavirus disease 2019 (COVID-19) in Japan: report of the COVID-19 registry Japan. Clin Infect Dis. 2021;73:e3677–89.
Yoshihara F, Ohtsu H, Nakai M, Tsuzuki S, Hayakawa K, Terada M, et al. Renin-angiotensin system blocker and the COVID-19 aggravation in patients with hypertension, diabetes, renal failure, cerebro-cardiovascular disease, or pulmonary disease: report by the COVID-19 registry Japan. J Cardiol. 2022;80:292–7.
Lee MMY, Docherty KF, Sattar N, Mehta N, Kalra A, Nowacki AS, et al. Renin-angiotensin system blockers, risk of SARS-CoV-2 infection and outcomes from CoViD-19: systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2022;8:165–78.
Xie Q, Tang S, Li Y. The divergent protective effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on clinical outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Ann Palliat Med. 2022;11:1253–63.
Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Dos Santos TM, Mazza L, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–64.
Macedo AVS, de Barros ESPGM, de Paula TC, Moll-Bernardes RJ, Mendonca Dos Santos T, Mazza L, et al. Discontinuing vs continuing ACEIs and ARBs in hospitalized patients with COVID-19 according to disease severity: insights from the BRACE CORONA trial. Am Heart J. 2022;249:86–97.
Cohen JB, Hanff TC, William P, Sweitzer N, Rosado-Santander NR, Medina C, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–84.
Bauer A, Schreinlechner M, Sappler N, Dolejsi T, Tilg H, Aulinger BA, et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir Med. 2021;9:863–72.
Kai H, Kai M, Niiyama H, Okina N, Sasaki M, Maeda T, et al. Overexpression of angiotensin-converting enzyme 2 by renin-angiotensin system inhibitors. Truth or myth? A systematic review of animal studies. Hypertens Res. 2021;44:955–68.
International Society of Hypertension. A statement from the International Society of Hypertension on COVID-19. https://ish-world.com/a-statement-from-the-international-society-of-hypertension-on-covid-19/
European Society of Hypertension. Statement of the European Society of Hypertension (ESH) on hypertension, renin-angiotensin system (RAS) blockers and COVID-19. https://www.eshonline.org/esh-content/uploads/2020/06/Statement-ESH-on-Hypertension-RAS-Blockers-and-COVID-19-Update-April-15-2020.pdf
ESC Council on Hypertension. Position statement of the ESC Council on hypertension on ACE-inhibitors and angiotensin receptor blockers. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
Statement from the American Heart Association. Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician. https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician
Japanese Society of Hypertension. Information of COVID-19. https://www.jpnsh.jp/corona.html
Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–32.
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–15.
Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383:2427–38.
Yamashita K, Suzuki A, Takebayashi S, Toguchi A, Ogitani K, Niizeki N, et al. Differential dynamics of humoral and cell-mediated immunity with three doses of BNT162b2 SARS-CoV-2 vaccine in healthcare workers in Japan: a prospective cohort study. Vaccines. 2022;10:1050.
Fujigaki H, Yamamoto Y, Koseki T, Banno S, Ando T, Ito H, et al. Antibody responses to BNT162b2 vaccination in Japan: monitoring vaccine efficacy by measuring IgG antibodies against the receptor-binding domain of SARS-CoV-2. Microbiol Spectr. 2022;10:e0118121.
Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999–1009.
Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022;38:e3465.
Parthymou A, Habeos EE, Habeos GI, Deligakis A, Livieratos E, Marangos M, et al. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: a longitudinal observational cohort study in western Greece. BMJ Open. 2022;12:e057084.
Sourij C, Tripolt NJ, Aziz F, Aberer F, Forstner P, Obermayer AM, et al. Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: the prospective COVAC-DM cohort study. Diabetes Obes Metab. 2022;24:849–58.
Papadokostaki E, Tentolouris A, Anastasiou IA, Psichogiou M, Iliaki E, Eleftheriadou I, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in people with diabetes: a prospective observational study. Vaccines. 2022;10:382.
Terpos E, Trougakos IP, Karalis V, Ntanasis-Stathopoulos I, Gumeni S, Apostolakou F, et al. Kinetics of anti-SARS-CoV-2 antibody responses 3 months post complete vaccination with BNT162b2; a prospective study in 283 health workers. Cells. 2021;10:1942.
Meylan S, Livio F, Foerster M, Genoud PJ, Marguet F, Wuerzner G, et al. Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hypertension. 2021;77:e56–7.
Zappa M, Verdecchia P, Spanevello A, Visca D, Angeli F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur J Intern Med. 2021;90:111–3.
Komine-Aizawa S, Haruyama Y, Deguchi M, Hayakawa S, Kawana K, Kobashi G, et al. The vaccination status and adverse effects of COVID-19 vaccine among pregnant women in Japan in 2021. J Obstet Gynaecol Res. 2022;48:1561–9.
Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–84.
Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–9.
Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–40.
Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–9.
Voleti N, Reddy SP, Ssentongo P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:951314.
Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410–22.
Wang M, Wen W, Zhou M, Wang C, Feng ZH. Meta-analysis of risk of myocarditis after messenger RNA COVID-19 vaccine. Am J Cardiol. 2022;167:155–7.
Task Force for the Management of C-otESoC. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1-epidemiology, pathophysiology, and diagnosis. Eur Heart J. 2022;43:1033–58.
Bilotta C, Perrone G, Adelfio V, Spatola GF, Uzzo ML, Argo A, et al. COVID-19 vaccine-related thrombosis: a systematic review and exploratory analysis. Front Immunol. 2021;12:729251.
Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–11.
Aleem A, Nadeem AJ. Coronavirus (COVID-19) vaccine-induced immune thrombotic thrombocytopenia (VITT). In: StatPearls. Treasure Island (FL); 2022. https://www.ncbi.nlm.nih.gov/pubmed/34033367.
Alexander GC, Tajanlangit M, Heyward J, Mansour O, Qato DM, Stafford RS. Use and content of primary care office-based vs telemedicine care visits during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3:e2021476.
Kario K, Nishizawa M, Hoshide S, Shimpo M, Ishibashi Y, Kunii O, et al. Development of a disaster cardiovascular prevention network. Lancet. 2011;378:1125–7.
Nishizawa M, Hoshide S, Okawara Y, Matsuo T, Kario K. Strict blood pressure control achieved using an ICT-based home blood pressure monitoring system in a catastrophically damaged area after a disaster. J Clin Hypertens. 2017;19:26–9.
Omboni S, McManus RJ, Bosworth HB, Chappell LC, Green BB, Kario K, et al. Evidence and recommendations on the use of telemedicine for the management of arterial hypertension: an international expert position paper. Hypertension. 2020;76:1368–83.
Cortez C, Mansour O, Qato DM, Stafford RS, Alexander GC. Changes in short-term, long-term, and preventive care delivery in US office-based and telemedicine visits during the COVID-19 pandemic. JAMA Health Forum. 2021;2:e211529.
Khan NA, Stergiou GS, Omboni S, Kario K, Renna N, Chapman N, et al. Virtual management of hypertension: lessons from the COVID-19 pandemic-International Society of Hypertension position paper endorsed by the World Hypertension League and European Society of Hypertension. J Hypertens. 2022;40:1435–48.
Onishi Y, Ichihashi R, Yoshida Y, Tahara T, Kikuchi T, Kobori T, et al. Substitution of telemedicine for clinic visit during the COVID-19 pandemic of 2020: Comparison of telemedicine and clinic visit. J Diabetes Investig. 2022;13:1617–25.
Wang H, Yuan X, Wang J, Sun C, Wang G. Telemedicine maybe an effective solution for management of chronic disease during the COVID-19 epidemic. Prim Health Care Res Dev. 2021;22:e48.
Akiyama M, Yoo BK. A systematic review of the economic evaluation of telemedicine in Japan. J Prev Med Public Health. 2016;49:183–96.
Nomura A, Tanigawa T, Kario K, Igarashi A. Cost-effectiveness of digital therapeutics for essential hypertension. Hypertens Res. 2022:45:1538–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Department of Health Development and Medicine in Osaka University is an endowed department supported by Anges, Daicel, and FunPep.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shibata, S., Kobayashi, K., Tanaka, M. et al. COVID-19 pandemic and hypertension: an updated report from the Japanese Society of Hypertension project team on COVID-19. Hypertens Res 46, 589–600 (2023). https://doi.org/10.1038/s41440-022-01134-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01134-5
Keywords
This article is cited by
-
2023 update and perspectives
Hypertension Research (2024)
-
What impacts do the new ESH 2023 guidelines have on the management of hypertension in Japan?
Hypertension Research (2023)
-
Long COVID and hypertension-related disorders: a report from the Japanese Society of Hypertension Project Team on COVID-19
Hypertension Research (2023)
-
Hypertension and severe COVID-19
Hypertension Research (2023)
-
Reply to: Hypertension and severe COVID-19
Hypertension Research (2023)