Abstract
Prenatal hypoxia can affect vascular functions in young offspring. However, there is limited knowledge regarding whether and how prenatal hypoxia influences vascular functions in aged offspring. This study compared the effects of prenatal hypoxia on the mesenteric arteries (MA) between a young adult and aged offspring and investigated the underlying mechanisms. Pregnant rats were randomly divided into the control and prenatal hypoxia groups. The vascular functions and molecular levels were assessed in 5-month-old (5 M) or 20-month-old (20 M) offspring. Prenatal hypoxia decreased acetylcholine-mediated vascular relaxations in 20-M but not 5-M offspring. Sodium nitroprusside-mediated relaxation curves were not altered by prenatal hypoxia in 5- and 20-M offspring. Prenatal hypoxia enhanced the contractile responses caused by phenylephrine, phorbol 12,13-dibutyrate, and 5-hydroxytryptamine only in 5-M offspring. The endothelial NO synthase (eNOS) activities were decreased along with downregulated eNOS mRNA expression and phosphorylated eNOS/total eNOS protein expression in 20-M offspring with prenatal hypoxia. The NADPH oxidase (NOX) inhibitor apocynin and superoxide dismutase (SOD) mimetic tempol restored the acetylcholine-mediated weaker relaxations in 20-M offspring with prenatal hypoxia. Enzyme-linked immunosorbent and dihydroethidium assay showed that prenatal hypoxia enhanced oxidative stress in 20-M offspring. Transmission electron microscopy showed that prenatal hypoxia damaged mitochondrial structures in the MA endothelial cells of 20-M offspring. Increased NOX2 protein expression and decreased SOD3 expression were found in 20-M offspring. The results demonstrated that endothelial dysfunction induced by intrauterine hypoxia occurred with aging via enhanced oxidative stress and decreased nitric oxide activities in aged offspring.
Similar content being viewed by others
Introduction
Cardiovascular disease is one of the leading causes of global mortality. Accumulated evidence has shown that adverse in utero environments can increase the susceptibility to cardiovascular diseases in adult offspring [1, 2]. Intrauterine hypoxia, the most important and common stress in pregnancy, may arise under multiple conditions [3, 4], such as preeclampsia, placental insufficiency, and umbilical cord compression. Existing investigations have proven that prenatal hypoxia can lead to fetal intrauterine growth restriction [5, 6] and impair the cardiovascular structures and functions of adult offspring [7].
Recently, the hypothesis of the fetal origins of adult diseases (developmental origins of health and diseases) has been further developed, i.e., prenatal insults (as the first hit before birth) followed by postnatal factors as the “second hit after birth” can further increase the susceptibility to cardiovascular diseases [8]. Although only the first hit could induce health problems in offspring, postnatal second hits, such as aging, may be able to amplify cardiovascular diseases, e.g., hypertension and coronary heart diseases [9]. Aging can damage vascular structures and functions [10,11,12,13]. However, it has remained unknown whether aging would aggravate prenatal hypoxia-induced vascular injury.
It is well known that hypoxia initiates the centralization of the blood flow away from the peripheral circulation to important organs, such as the heart and brain [14, 15]. The decreased blood flow in the peripheral circulation may impair the development of peripheral organs. Small mesenteric arteries (MA), which are important peripheral resistance blood vessels, play an important role in regulating blood pressure. Endothelial cells and smooth muscle cells, the most dominant components of blood vessels, are essential for regulating vascular tone [15, 16]. It is well established that endogenous nitric oxide (NO), generated by endothelial NO synthase (eNOS), plays a crucial role in maintaining the vascular tone. Endogenous NO diffuses to smooth muscle cells and mediates vasodilation through the sGC–cGMP signaling pathway. Acetylcholine (ACh), as a stimulator for endogenous endothelial NO production, and sodium nitroprusside (SNP), as an exogenous NO donor for smooth muscle cells, have commonly been used in the determination of vascular dilatory functions and mechanisms.
Oxidative stress is an imbalance between the overproduction of reactive oxygen species (ROS) and the inactivation of anti-oxidative molecules [17,18,19]. ROS directly decrease NO production by inhibiting eNOS expression and cause eNOS uncoupling, leading to endothelial dysfunction [20, 21]. Mitochondria are the main source of ROS; if the mitochondria are damaged, ROS will accumulate [22]. Previous studies have shown that hypoxia or aging could potentiate oxidative stress in the aorta [6, 23]. Oxidative stress is involved in vascular dysfunction; however, it is unknown whether oxidative stress is implicated in the potential dysfunction of aged MA after exposure to prenatal hypoxia. Malondialdehyde (MDA, a product of lipid peroxidation) is the classical indicator in oxidative stress. Endogenous antioxidants, including superoxide dismutase (SOD), copper/zinc-SOD (Cu/Zn-SOD), and catalase (CAT), are markers with the ability to eliminate ROS [24,25,26]. These major markers of oxidative stress may contribute to vascular dysfunction in the circulation and local tissue.
Substantial evidence has shown that chronic hypoxia during pregnancy could damage the endothelial function in the fetal aorta [6], adult cerebral arteries [7], and pulmonary arteries [27], via enhanced oxidative stress, altered endothelial nitric oxide synthase, and its regulatory proteins. Our laboratory reported that prenatal hypoxia potentiated phenylephrine-induced vascular responses in adult offspring MA [28]. However, there is very limited information regarding the influences of prenatal hypoxia on aged offspring MA. No comparisons have been performed on the influences of prenatal hypoxia on vascular functions between young and aged offspring. Therefore, the present study detected vascular contractile and dilation responses and measured the levels of oxidative stress in the circulation and local mesenteric arteries, with the aim to evaluate whether aging would aggravate the influence of prenatal hypoxia on the cardiovascular systems, as well as the underlying mechanisms related to oxidative stress.
Materials and methods
Animals
Sprague-Dawley rats were housed in a controlled environment at 22 ℃ with a 12-h light/dark cycle. One female rat mated with two male rats at 18:00. If deciduous vaginal mucus plugs were identified the next morning, the day was recorded as the first day of gestation. Pregnant rats were randomly divided into the control group (N = 15) and the prenatal hypoxia group (N = 14). During gestational days 5–21, the prenatal hypoxia group was maintained in the hypoxia cabin (10.5% oxygen) and the control group was raised in the normoxia cabin (21% oxygen). Hypoxia was achieved and maintained by the mixture of nitrogen gas and air, which was continuously monitored with an oxygen analyzer (Hangtian Pengcheng Instrument, Beijing, China). At gestational day 21, all pregnant rats were moved to the normoxia environment. After giving birth, the male offspring was raised and used at 5 months old (5 M) or 20 months old (20 M). All procedures performed were approved by the Institute Animal Welfare Committee and were in accordance with the Guide for the Institutional Animal Care and Use Committee of Soochow University.
Measurement of vascular functions in adult and aged offspring
Following the euthanasia of 5- and 20-M offspring, the third–fourth branches of the mesenteric arteries (MA) were dissected from connective tissues and cut into 2-mm long segments. The segments were then mounted on an M series Myograph System (Radnoti LLC, Covina, California, USA) in a chamber filled with HEPES-PSS solution (contained mmol/L: NaCl 141.85, KCl 4.7, MgSO4 1.7, EDTA 0.51, CaCl2·2H2O 2.79, KH2PO4 1.17, glucose 5.0, and HEPES 10.0, pH 7.4), bubbled with 95% O2 and 5% CO2 at 37 ℃. After equilibration for 30 min, the artery was stimulated with 120 mmol/L potassium chloride (KCl) to achieve the optimal resting tension and assess the vascular capability [7, 29]. In the adult and aged offspring MA, the accumulative concentration of acetylcholine (ACh, 10−9–10−4 mol/L) or sodium nitroprusside (SNP, 10−9–10−4 mol/L) was added following the application of 5-hydroxytryptamine (5-HT, 10−6 mol/L), which produced and maintained vasoconstrictions at a steady state for at least 15–20 min [30, 31]. Phenylephrine (PE, 10−9−10−4 mol/L), phorbol 12,13-dibutyrate (PDBu, protein kinase C agonist, 10−11–10−5 mol/L), or 5-HT (10−6 mol/L) was used to determine the contractile responses in the MA from both the adult and aged offspring [32, 33]. The relaxation responses caused by ACh or SNP were normalized by 5-HT-induced constriction platforms. The constriction was normalized by the maximum contraction in response to 120 mmol/L KCl. Signals were monitored and recorded using the Chart 7 PowerLab system (AD Instrument, Australia).
Functions of endothelial NOS and oxidative stress in aged offspring MA
In the aged offspring, the MA was contracted with accumulated concentrations of PE (10−9–10−4 mol/L) with or without Nω-nitro-L-arginine methyl ester (L-NAME, an inhibitor of NOS, 10−4 mol/L) incubation for 30 min [34]. Dose-dependent ACh was applied following the 5-HT-induced constriction platform in the presence or absence of L-NAME, tempol (SOD analog, 10−4 mol/L), or apocynin (NOX inhibitor, 10−5 mol/L) incubation for 30 min [35].
Transmission electron microscopy analysis
The MA of the aged offspring were isolated and cut into 1-mm3 sections and then immediately fixed with 2.5% glutaraldehyde at 4 ℃; after washing with polydimethyl salar buffer, the specimens were fixed with 1% osmium tetroxide, dehydrated in graded concentrations of ethanol (50, 70, 90, and 100%), and then embedded in Epon 812. Ultrathin sections were selected for double staining with uranyl acetate and lead citrate and were observed using a transmission electron microscope at Fudan University (Shanghai, China).
Enzyme-linked immunosorbent assay
The contents of ROS, MDA, SOD, Cu/Zn-SOD, and CAT in the blood plasma and mesenteric arteries of the aged offspring were measured via enzyme-linked immunosorbent assay (ELISA) using commercially available kits by Huaying Biochemical Corporation (Beijing, China). All experiments were processed and analyzed in a blind manner.
Detection of vascular superoxide anion (O2 −)
Fresh MA were immediately embedded in Tissue-Tek OCT compound (Sakura Finetek Japan, Tokyo, Japan) and snap-frozen; the fixed frozen samples were cut into 10-mm-thick sections with a cryostat at −20 ℃ and placed on glass slides. Dihydroethidium (DHE, 2 μmol/L, Sigma-Aldrich) was applied to each tissue section [36, 37], and the slides were subsequently incubated at 37 ℃ in the dark for 30 min. Fluorescence was visualized under a fluorescent laser scanning microscope (Nikon Eclipse 80i, Japan).
Real-time PCR
Total RNA was extracted from MA tissue using Trizol reagents (Takara, Japan). The purity and integrity of the RNA were determined with Nanodrop and agarose gel electrophoresis. RNA was reverse transcribed using a Revert Aid First-Strand cDNA Synthesis Kit (Takara). The gene primer sequences (Sangon Biotech, Shanghai, China) were shown in Table 1, and the targets had similar PCR efficiencies with the endogenous control. Real-time PCR was performed with the SYBR Green Supermix Taq Kit (Takara) and analyzed via an iCycler, MyiQ two Color Real-Time PCR Detection System (Bio-Rad). The 2−∆∆Ct method was used to comparatively quantify the mRNA level.
Western blot analysis
The protein abundances of eNOS, phosphorylated eNOS at serine 1177 (p-eNOS), NOX1, NOX2, NOX4, SOD1, SOD2, and SOD3 in the MA were measured with western blot normalized to β-actin. The primary antibodies included eNOS (1:200), p-eNOS (1:200), NOX1 (1:200), NOX2 (1:200), NOX4 (1:200), SOD1 (1:100), SOD2 (1:100), and SOD3 (1:100) (Santa Cruz Biotech, California, USA). Horseradish peroxidase-coupled rabbit anti-goat secondary antibody (1:2000) was used for eNOS, p-eNOS, NOX2, SOD1, and SOD2, and goat anti-rabbit secondary antibody (1:2000) was used for the other proteins. The immunoreactive bands were visualized using the Tanon imaging system (Tanon Science & Technology Co., Ltd.). Imaging signals were digitized and analyzed, and the ratio of the band intensity to β-actin was subsequently obtained to quantify the relative protein expression.
Data analysis
Data were expressed as the mean ± SEM. Significance was determined using the Mann–Whitney U test or two-way repeated measures ANOVA analysis followed by the Bonferroni post hoc test. GraphPad Prism 5 was used to analyze the concentration-response curves for vascular responses. *p < 0.05; **p < 0.01; ***p < 0.001. N indicates the number of pregnant rats in each group, and n indicates the count of the offspring obtained from a pregnancy.
Results
Measurement of vascular dilation in 5- and 20-M offspring
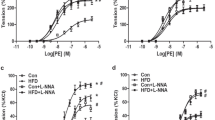
In 5-M offspring MA, prenatal hypoxia had no significant influence on the vasodilation induced by ACh (Fig. 1a). However, compared with the control 20-M offspring, the ACh-mediated vascular dilation was altered in the prenatal hypoxia 20-M offspring MA (Fig. 1c), and the logEC50 values were significantly augmented in the prenatal hypoxia 20-M offspring compared with the control 20-M offspring (Table 2). Consistent with 5-M offspring (Fig. 1b), prenatal hypoxia did not impact the SNP-caused relaxation curves in 20-M offspring (Fig. 1d). SNP-elicited dilatory responses in the control 20-M offspring (Emax: 71.64 ± 2.60%) were weaker than those in the control 5-M offspring (Emax: 92.46 ± 2.74%) (Table 2). Prenatal hypoxia only changed the Emax of the SNP-induced relaxation in 5-M offspring and not in the aged offspring (Table 2). N = 8, n = 2–3, and total number = 17–24 per group.
Dilation responses in mesenteric arteries of 5- and 20-M offspring. Acetylcholine (ACh)-mediated relaxation in control and prenatal hypoxia 5-M offspring (a) and 20-M offspring (c). b Sodium nitroprusside (SNP)-mediated relaxation in the control and prenatal hypoxia 5-M offspring (b) and 20-M offspring (d). *p < 0.05; ***p < 0.001. N = 8, n = 2–3, and total number = 17–24 per group
Measurements of vasoconstriction in 5- and 20-M offspring
Prenatal hypoxia did not influence the KCl-induced vascular tension in 5- or 20-M offspring (Figs. 2a–e). In 5-M offspring MA, the PE, 5-HT, or PDBu (PKC agonist)-mediated constrictions were increased by prenatal hypoxia (Fig. 2b–d). However, the three agonist-mediated contractile responses were similar between the control 20-M and prenatal hypoxia 20-M offspring (Fig. 2f–h), which were similar to those in the prenatal hypoxia 5-M offspring. The logEC50 and Emax were shown in Table 2. N = 5, n = 2, and total number = 10 per group.
Constrictions in mesenteric arteries of 5- and 20-M offspring. Potassium chloride (KCl)-mediated constrictions in 5-M (a) and 20-M offspring (e). The constriction responses caused by 5-hydroxytryptamine (5-HT, 10−6 mol/L) in 5-M (b) and 20-M offspring (f). Phenylephrine (PE)-induced contractions in 5-M (c) and 20-M offspring (g). Contractile responses to accumulated concentrations of phorbol 12,13-dibutyrate (PDBu) in 5-M (d) and 20-M offspring (h). *p < 0.05; ***p < 0.001. N = 5, n = 2, and total number = 10 per group
Function of eNOS and oxidative stress in ACh-mediated relaxation in 20-M offspring MA
The endothelial NOS inhibitor L-NAME significantly decreased the ACh-mediated vasodilation in the control 20-M group (Fig. 3a), but not in the prenatal hypoxia 20-M group (Fig. 3b). In addition, the PE-induced contractile responses were significantly enhanced by L-NAME in the control 20-M offspring (Fig. 3c) (PE alone: logEC50: −5.77 ± 0.10, Emax: 178.50 ± 7.69%; PE + L-NAME: logEC50: −6.22 ± 0.08, Emax: 211.70 ± 6.61%), while L-NAME showed limited effects on the PE-induced vessel contraction in the prenatal hypoxia 20-M group (Fig. 3d).
L-NAME, tempol, and apocynin in regulating ACh-induced vasodilation in 20-M offspring MA. a–b Acetylcholine (ACh)-mediated vasodilation with or without Nω-nitro-L-arginine methyl ester (L-NAME, 10−4 mol/L) in the control and prenatal hypoxia offspring. c–d The dose-dependent PE (10−9–10−4 mol/L) induced vascular contraction in the presence or absence of L-NAME in mesenteric arteries of 20-M offspring. e–f The effect of tempol (the SOD analog) on ACh-induced relaxation in the control and prenatal hypoxia groups. g–h The effect of apocynin (NOX inhibitor) on ACh-induced dilation in 20-M offspring group. *p < 0.05; ***p < 0.001, N = 4, n = 2–3, and total number = 8–12 per group
Tempol (SOD analog) and apocynin (NOX inhibitor) restored the ACh-induced vascular relaxation in the prenatal hypoxia 20-M offspring, but not in the control 20-M offspring (Fig. 3e–h). The logEC50 values of the ACh-mediated relaxation with tempol or apocynin, respectively, shifted to the left in the prenatal hypoxia 20-M offspring (Table 3). N = 4, n = 2–3, and total number = 8–12 per group.
Ultrastructures of the MA endothelium in 20-M offspring
In the control 20-M offspring, the mitochondrial membrane structure was complete, and the mitochondrial cristae were arranged relatively clearly (Fig. 4a). However, vacuolization in the mitochondria, breaks, and disappearance of the mitochondrial cristae were observed in the aged prenatal hypoxia offspring (Fig. 4b).
Anti-oxidative stress indicators and oxidative stress markers in plasma and MA of 20-M offspring
In 20-M offspring, oxidative indicators (ROS and its metabolite MDA) and anti-oxidative factors (SOD, Cu/Zn-SOD, and CAT) were measured in the plasma and MA. The concentrations of SOD and CAT were significantly reduced in the prenatal hypoxia plasma (Fig. 5a), while the levels of ROS, Cu/Zn-SOD, and MDA showed no significant differences in the plasma between the two groups. Compared with the control, the levels of ROS and MDA were augmented, whereas SOD and CAT were decreased in the prenatal hypoxia MA (Fig. 5b). N = 5, n = 2–3, and total number = 10–14 per group. DHE fluorescence showed that a superoxide radical (O2−) was significantly increased in the MA of prenatal hypoxia compared with the control (Fig. 5c–d). N = 5, n = 1–2, and total number = 5–8 per group.
Oxidative stress levels in plasma and mesenteric arteries in 20-M offspring. Concentrations of superoxide dismutase (SOD), copper/zinc-SOD (Cu/Zn-SOD), catalase (CAT) reactive oxygen species (ROS), and malondialdehyde (MDA) in plasma (a) and mesenteric arteries (b). *p < 0.05; **p < 0.01, N = 5, n = 2–3, and total number = 10–14 per group. The immunosignal of O2− in mesenteric arteries of the control (c) and prenatal hypoxia groups (d). N = 5, n = 1–2, and total number = 5–8 per group
Relative mRNA and protein expression in 20-M offspring MA
As shown in Fig. 6a, the relative mRNA expression of eNOS was remarkably decreased in the prenatal hypoxia 20-M offspring, compared with the control 20-M offspring. The protein expression ratio of p-eNOS to eNOS was also reduced in the prenatal hypoxia 20-M offspring. The mRNA expression of NOX2 showed a trend of increasing in the prenatal hypoxia group (p = 0.0687) (Fig. 6b). No significant differences were identified in the NOX1 and NOX4 mRNA expressions between the control and prenatal hypoxia groups. Among NOX proteins, only the NOX1 protein abundance was significantly augmented in prenatal hypoxia (Fig. 6b). The mRNA and protein expression of SOD3 were substantially lower in the prenatal hypoxia 20-M group than in the control 20-M group (Fig. 6c). No significant differences were observed in the SOD1 and SOD2 expression between the two groups (Fig. 6c). N = 5, n = 2–3, and total number = 10–13 per group.
mRNA and protein expression in 20-M offspring. a The mRNA expression of endothelial NO synthase (eNOS) and the protein expression ratio of phosphorylated eNOS to total eNOS. The mRNA and protein expression of NOX1, NOX2, and NOX4 (b) and SOD1, SOD2, and SOD3 (c). The molecular weights for each blot were shown, *p < 0.05; **p < 0.01, N = 5, n = 2–3, and total number = 10–13 per group
Discussion
The present study demonstrated that (1) after exposure to chronic hypoxia during pregnancy, endothelial injury in vasodilation was found in the MA of the aged offspring. Smooth muscle-dependent vascular relaxation by SNP was not significantly affected by prenatal hypoxia in the MA of the young and aged offspring. (2) Prenatal hypoxia increased the vascular constrictions of the MA in the young offspring, but not in the aged offspring. In the aged prenatal hypoxia offspring, the dysfunction of the endothelium-dependent relaxation was due to decreased eNOS (p-eNOS) activities and increased oxidative stress in the MA, which were intimately associated with decreased NO production and bioavailability. Together, the data suggested that aging promoted the prenatal hypoxia-initiated vascular dysfunction; in turn, prenatal hypoxia accelerated vascular aging in offspring.
Mesenteric arteries, particularly the third and fourth levels, provide a significant source of vascular resistance and play a vital role in regulating blood pressure [38]. The origin of hypertension includes endothelial disorders [39]. Previous studies have demonstrated that ACh-induced relaxations in the MA were affected by chronic hypoxia during late pregnancy (gestational days 15–21) [40]. The present study demonstrated that prenatal hypoxia did not show significant changes in endothelium-dependent vascular relaxation in the adult male offspring. However, the aging process as an additional negative stress promoted endothelial damage in the MA of aged offspring exposed to prenatal hypoxia. This finding indicates that prenatal hypoxia might cause subtle changes in the endothelium, and the potential injury by prenatal hypoxia could be aggravated by postnatal factors, such as aging.
As a classical vasodilator, ACh could induce vascular relaxation by binding the M3 receptor and activating NO production in the endothelium. The released NO acts on the sGC–cGMP signaling pathway in smooth muscle cells, evoking vascular dilation [41]. In the present study, the exogenous NO donor SNP-mediated smooth muscle relaxation showed no significant differences between the control and prenatal hypoxia offspring, which suggests that the ACh-mediated vasodilatory dysfunction was not due to the NO downstream signaling pathway in smooth muscle cells; it was primarily from the reduction of NO production or bioavailability. Nitric oxide production mainly occurs via NOS in the endothelium [42]. L-NAME had no effects on ACh-induced relaxation, which suggests that ACh-induced relaxation was not mainly through eNOS-mediated NO production in 20-M offspring MA following prenatal hypoxia, associated with a reduced protein expression of p-eNOS. Compared with the control aged offspring, eNOS was significantly impaired by prenatal hypoxia, leading to endothelial dysfunction in 20-M offspring.
In addition to eNOS, NO production is also regulated by oxidative stress through increased eNOS uncoupling [43]. Numerous studies have proven that chronic hypoxia or aging could result in oxidative stress [5, 23]. The present study also found ultrastructural changes in the MA, particularly the damaged mitochondria in vascular endothelial cells following prenatal hypoxia. It is well known that mitochondrial injury is closely related to ROS production [44, 45]. Dihydroethidium assay showed that the production of O2− (an important type of ROS) was increased in the prenatal hypoxia group, which suggests that altered mitochondria might promote increased ROS in the endothelium. Furthermore, ELISA analysis demonstrated that an imbalance of the reduced antioxidants and higher oxidative markers existed in the plasma and the local mesenteric vessels. The enhanced oxidative stress was associated with the inferior eNOS functionality in the aged prenatal hypoxia offspring. Previous studies have shown that prenatal hypoxia caused oxidative stress and impaired vascular functions [46, 47]. The present study was the first investigation to demonstrate increased oxidative stress in both the circulation and small-resistance mesenteric arteries in prenatal hypoxia aged offspring. The clinical significance of this finding is as follows: for individuals suffering from prenatal hypoxia, special attention should be paid to the potential increases in systemic and vascular oxidative stress levels for the early prevention of vascular diseases before and during aging.
Reactive oxygen species derived from NOX could rapidly react with NO to form the stable peroxynitrite anion (ONOO−), resulting in a decline of NO bioavailability. Previous studies have suggested that NOX1 caused a self-perpetuating cycle of superoxide production to impair endothelium-dependent relaxation [48, 49]. In the measurement of MA dilation, apocynin (NOX inhibitor) enhanced ACh-mediated relaxation in the prenatal hypoxia group, but not in the control. The NOX1 protein expression was higher in the prenatal hypoxia aged group. The data suggest that prenatal hypoxia resulted in the upregulation of NOX1 protein expression via enhancing oxidative stress, which led to a declined endothelial vasodilation in the aged MA.
Under normal physiological conditions, an abundance of SOD in the circulation and tissue can eliminate ROS to avoid oxidative damage. Superoxide dismutase is the main antioxidant enzyme that protects cells and tissues from ROS, through converting superoxide anion into H2O2. Pretreatment with tempol (membrane-permeable SOD analog) restored ACh-mediated weaker vasodilation in the aged prenatal hypoxia group, which indicates that the degradation of SOD functions had a considerable effect on the programming of the MA dysfunction in the aged prenatal hypoxia offspring. A recent report indicated that SOD3 plays an important role in maintaining oxidative homeostasis [50]. In the present study, both the mRNA and protein expression of SOD3 were markedly reduced in the prenatal hypoxia group compared with the control, further manifesting a reduction in antioxidant defenses with decreased SOD. Although several studies have shown that prenatal hypoxia could enhance oxidative stress and cause increased vascular contractions in the fetus and adult offspring [6, 51], to the best of our knowledge, this study was the first investigation to indicate that excessive oxidative stress might contribute to prenatal hypoxia-decreased vasodilation in aged offspring and explains that the underlying mechanism of the decreased NO bioavailability was due to disabled nitric oxide synthase and excessive oxidative stress. The upregulation of NOX1 and the downregulation of SOD3 enhanced the production of ROS and might ultimately reduce the NO bioactivity, thus causing endothelial dysfunction. Importantly, antioxidant therapy, including the inhibition of NOX and the use of an SOD analog, might be interesting to weaken prenatal hypoxia-mediated endothelial insults in aged offspring.
One conclusion could be drawn that prenatal hypoxia did not affect SNP-mediated vascular relaxation, no matter in adult offspring or aged offspring, which indicates that hypoxia during pregnancy did not influence nitric oxide donor-mediated vasodilation in smooth muscle cells. However, acetylcholine-mediated dilation was significantly affected by prenatal hypoxia in the aged offspring instead of the younger offspring. These findings might be due to proper self-regulation in the mesenteric arterial endothelium of the younger offspring, while insufficient to regulate the endothelial vasodilation in the aged mesenteric artery. Moreover, functional alterations of eNOS and NADPH might decrease the production or bioavailability of NO, resulting in endothelial dysfunction in aged offspring following prenatal hypoxia.
In addition to the determination of vasodilation, the present study also assessed the vascular constriction responses caused by PE, 5-HT, and PDBu. Angiotensin II plays an important role in the regulation of vascular tension and is associated with oxidative stress [37]. In our colleague’s study, angiotensin II-induced vasoconstrictions in mesenteric arteries [52] were significantly weaker than those induced by other vasomotor agonists, i.e., 5-HT and PE. Compared with the control, prenatal hypoxia significantly increased MA vasoconstrictions in the adult offspring, consistent with the results of a recent report [53]. Nevertheless, the difference in vasoconstrictions by prenatal hypoxia disappeared in aged offspring. One speculation was that aged blood vessels tend to become rigid, thus affecting the vascular constrictions observed. During aging, arterial stiffness of the MA might occur and deserves further investigation.
Conclusions
Aging, as a postnatal factor, could promote prenatal hypoxia-induced endothelial dysfunction in the MA, associated with mitochondrial injury, via the pathways of weaker eNOS and increased oxidative stress. In turn, prenatal hypoxia speeded up vascular aging via vascular dysfunctions. The NOX inhibitor or SOD analog showed a protective effect against ROS-mediated vascular injury in aged offspring. The data provided new information to further understand the impact of prenatal hypoxia during the aging process and provided new insights into the development of novel preventive approaches in dealing with the fetal origins of cardiovascular diseases.
Limitations
Epigenetic factors (e.g., DNA methylation or histone acetylation) may affect fetal development in utero, as the underlying mechanisms for prenatal hypoxia-programmed cardiovascular problems, which deserve further investigations. In addition, Nrf2-regulated antioxidant protein production could be related to the increased oxidative stress, which is critical in understanding the mechanisms. Furthermore, the measurement of the arterial stiffness of the MA, as well as the NO production and bioavailability should be valuable in providing a mechanistic explanation. These limitations should be considered in future studies.
References
Eleftheriades M, Creatsas G, Nicolaides K. Fetal growth restriction and postnatal development. Ann N Y Acad Sci. 2006;1092:319–30.
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41.
Hemker SL, Sims-Lucas S, Ho J. Role of hypoxia during nephrogenesis. Pediatr Nephrol. 2016;31:1571–7.
Salman S, Brown ST, Nurse CA. Chronic nicotine induces hypoxia inducible factor-2α in perinatal rat adrenal chromaffin cells: role in transcriptional upregulation of KATP channel subunit Kir6.2. Am J Physiol Cell Physiol. 2012;302:C1531–1538.
Chen L, Zadi ZH, Zhang J, Scharf SM, Pae EK. Intermittent hypoxia in utero damages postnatal growth and cardiovascular function in rats. J Appl Physiol. 2018;124:821–30.
Zhu X, Gao Q, Tu Q, Zhong Y, Zhu D, Mao C, et al. Prenatal hypoxia enhanced angiotensin II-mediated vasoconstriction via increased oxidative signaling in fetal rats. Reprod Toxicol. 2016;60:21–28.
Tang J, Li N, Chen X, Gao Q, Zhou X, Zhang Y, et al. Prenatal hypoxia induced dysfunction in cerebral arteries of offspring rats. J Am Heart Assoc. 2017;6:e006630.
Rueda-Clausen CF, Morton JS, Dolinsky VW, Dyck JR, Davidge ST. Synergistic effects of prenatal hypoxia and postnatal high-fat diet in the development of cardiovascular pathology in young rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R418–426.
Yang D, Yang K, Yang M. Circular RNA in aging and age-related diseases. Adv Exp Med Biol. 2018;1086:17–35.
Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93:583–604.
Laurent S. Defining vascular aging and cardiovascular risk. J Hypertens. 2012;30(Suppl):S3–8.
Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–66.
Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–57.
Baschat AA, Gembruch U, Reiss I, Gortner L, Diedrich K. Demonstration of fetal coronary blood flow by Doppler ultrasound in relation to arterial and venous flow velocity waveforms and perinatal outcome–the ‘heart-sparing effect’. Ultrasound Obstet & Gynecol: Off J Int Soc Ultrasound Obstet Gynecol. 1997;9:162–72.
Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–81.
Neuman NA, Ma S, Schnitzler GR, Zhu Y, Lagna G, Hata A. The four-and-a-half LIM domain protein 2 regulates vascular smooth muscle phenotype and vascular tone. J Biol Chem. 2009;284:13202–12.
Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5.
Dasuri K, Zhang L, Keller JN. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic Biol & Med. 2013;62:170–85.
Wang X, Wu Q, Liu A, Anadón A, Rodríguez JL, Martínez-Larrañaga MR, et al. Paracetamol: overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds in vivo and in vitro. Drug Metab Rev. 2017;49:395–437.
Risbano MG, Gladwin MT. Therapeutics targeting of dysregulated redox equilibrium and endothelial dysfunction. Handb Exp Pharmacol. 2013;218:315–49.
Xu C, Tang F, Lu M, Yang J, Han R, Mei M, et al. Astragaloside IV improves the isoproterenol-induced vascular dysfunction via attenuating eNOS uncoupling-mediated oxidative stress and inhibiting ROS-NF-κB pathways. Int Immunopharmacol. 2016;33:119–27.
Pan P, Wang X, Liu D. The potential mechanism of mitochondrial dysfunction in septic cardiomyopathy. J Int Med Res. 2018:46:2157–69.
Olgar Y, Degirmenci S, Durak A, Billur D, Can B, Kayki-Mutlu G, et al. Aging related functional and structural changes in the heart and aorta: MitoTEMPO improves aged-cardiovascular performance. Exp Gerontol. 2018;110:172–81.
Bombardini T, Costantino MF, Sicari R, Ciampi Q, Pratali L, Picano E. End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. Biomed Res Int. 2013;2013:235194.
Rochette L, Lorin J, Zeller M, Guilland JC, Lorgis L, Cottin Y, et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther. 2013;140:239–57.
Bonetta R. Potential therapeutic applications of MnSODs and SOD-mimetics. Chemistry. 2018;24:5032–41.
Liu J, Gao Y, Negash S, Longo LD, Raj JU. Long-term effects of prenatal hypoxia on endothelium-dependent relaxation responses in pulmonary arteries of adult sheep. Am J Physiol Lung Cell Mol Physiol. 2009;296:L547–554.
Liu B, Liu Y, Shi R, Feng X, Li X, Zhang W, et al. Chronic prenatal hypoxia down-regulated BK channel Β1 subunits in mesenteric artery smooth muscle cells of the offspring. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem, Pharmacol. 2018;45:1603–16.
Yuan TY, Yan Y, Wu YJ, Xu XN, Li L, Jiao XZ, et al. Vasodilatory effect of a novel Rho-kinase inhibitor, DL0805-2, on the rat mesenteric artery and its potential mechanisms. Cardiovasc Drugs Ther. 2014;28:415–24.
Grapow MT, Kern T, Reineke DC, Brett W, Bernet F, Rueter F, et al. Improved endothelial function after a modified harvesting technique of the internal thoracic artery. Eur J Cardio-Thorac Surg: Off J Eur Assoc Cardio-Thorac Surg. 2003;23:956–60; discussion 960–51.
Okudan N, Nurullahoglu Atalik KE, Gokbel H, Canbilen A, Kara I. Alpha lipoic acid treatment improved endothelium-dependent relaxation in diabetic rat aorta. Yakugaku zasshi: J Pharm Soc Jpn. 2011;131:739–44.
Kim SY, Park JT, Park JK, Lee JS, Choi JC. Aging impairs vasodilatory responses in rats. Korean J Anesthesiol. 2011;61:506–10.
Li W, Lv J, Wu J, Zhou X, Jiang L, Zhu X, et al. Maternal high-salt diet altered PKC/MLC20 pathway and increased ANG II receptor-mediated vasoconstriction in adult male rat offspring. Mol Nutr Food Res. 2016;60:1684–94.
Cao C, Edwards A, Sendeski M, Lee-Kwon W, Cui L, Cai CY, et al. Intrinsic nitric oxide and superoxide production regulates descending vasa recta contraction. Am J Physiol Ren Physiol. 2010;299:F1056–1064.
Heylen E, Huang A, Sun D, Kaley G. Nitric oxide-mediated dilation of arterioles to intraluminal administration of aldosterone. J Cardiovasc Pharmacol. 2009;54:535–42.
Yzydorczyk C, Comte B, Cambonie G, Lavoie JC, Germain N, Ting Shun Y, et al. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension. 2008;52:889–95.
Yzydorczyk C, Gobeil F Jr, Cambonie G, Lahaie I, Lê NL, Samarani S, et al. Exaggerated vasomotor response to ANG II in rats with fetal programming of hypertension associated with exposure to a low-protein diet during gestation. Am J Physiol Regul, Integr Comp Physiol. 2006;291:R1060–1068.
Spiranec K, Chen W, Werner F, Nikolaev VO, Naruke T, Koch F, et al. Endothelial C-type natriuretic peptide acts on pericytes to regulate microcirculatory flow and blood pressure. Circulation. 2018;138:494–508.
Yzydorczyk C, Armengaud JB, Peyter AC, Chehade H, Cachat F, Juvet C, et al. Endothelial dysfunction in individuals born after fetal growth restriction: cardiovascular and renal consequences and preventive approaches. J Dev Orig Health Dis. 2017;8:448–64.
Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol. 2005;565(Pt 1):125–35.
Tangsucharit P, Takatori S, Zamami Y, Goda M, Pakdeechote P, Kawasaki H, et al. Muscarinic acetylcholine receptor M1 and M3 subtypes mediate acetylcholine-induced endothelium-independent vasodilatation in rat mesenteric arteries. J Pharmacol Sci. 2016;130:24–32.
Chang ML, Chang JS, Yu WY, Cheah KP, Li JS, Cheng HW, et al. Polygonum viviparum L. induces vasorelaxation in the rat thoracic aorta via activation of nitric oxide synthase in endothelial cells. BMC Complement Altern Med. 2014;14:150.
Joshi S, Kar S, Kavdia M. Computational analysis of interactions of oxidative stress and tetrahydrobiopterin reveals instability in eNOS coupling. Microvasc Res. 2017;114:114–28.
Miao Y, Zhou J, Zhao M, Liu J, Sun L, Yu X, et al. Acetylcholine attenuates hypoxia/ reoxygenation-induced mitochondrial and cytosolic ROS formation in H9c2 cells via M2 acetylcholine receptor. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem, Pharmacol. 2013;31:189–98.
Quoilin C, Mouithys-Mickalad A, Lecart S, Fontaine-Aupart MP, Hoebeke M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim Biophys Acta. 2014;1837:1790–1800.
Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS ONE. 2012;7:e31017.
Sartori C, Rexhaj E, Rimoldi SF, Allemann Y, Scherrer U. Fetal programming of cardiovascular disease: new causes and underlying mechanisms. Rev Med Suisse. 2012;8:1718–24. 1716
Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–679.
Troiano JA, Potje SR, Graton ME, Cavalari P, Pereira AA, Vale GT, et al. Decreased reactive oxygen species production and NOX1, NOX2, NOX4 expressions contribute to hyporeactivity to phenylephrine in aortas of pregnant SHR. Life Sci. 2016;144:178–84.
Karlsson K, Marklund SL. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988;255:223–8.
Tang J, Zhu Z, Xia S, Li N, Chen N, Gao Q, et al. Chronic hypoxia in pregnancy affected vascular tone of renal interlobar arteries in the offspring. Sci Rep. 2015;5:9723.
Wu C, Li J, Bo L, Gao Q, Zhu Z, Li D, et al. High-sucrose diets in pregnancy alter angiotensin II-mediated pressor response and microvessel tone via the PKC/Cav1.2 pathway in rat offspring. Hypertens Res: Off J Jpn Soc Hypertens. 2014;37:818–23.
Liu B, Shi R, Li X, Liu Y, Feng X, Chen X, et al. Downregulation of L-type voltage-gated Ca(2+), voltage-gated K(+), and large-conductance Ca(2+)-activated K(+) channels in vascular myocytes from salt-loading offspring rats exposed to prenatal hypoxia. J Am Heart Assoc. 2018;7:e008148.
Acknowledgements
XC and JT wrote the manuscript. ZX revised the manuscript. XC and JT raised the rats, performed the vascular tension measurements, and prepared Figs. 1–3. LQ and XF performed ELISA and ultrastructural tests and prepared Figs. 4–5. MZ and TX performed the DHE. QG, PZ, YL, and HS detected the mRNA and protein expression and prepared Fig. 6. All of the authors reviewed the manuscript.
Funding
Supported by the NSFC (81771592, 81320108006, and 81370719) and the Jiangsu Key Discipline/Laboratory (Fetology) Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, X., Qi, L., Fan, X. et al. Prenatal hypoxia affected endothelium-dependent vasodilation in mesenteric arteries of aged offspring via increased oxidative stress. Hypertens Res 42, 863–875 (2019). https://doi.org/10.1038/s41440-018-0181-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0181-7
Keywords
This article is cited by
-
Perinatal hypoxia weakens anticontractile influence of NO in rat arteries during early postnatal period
Pediatric Research (2024)
-
In vitro fertilization with frozen embryo transfer increased histamine-mediated contractile sensitivity via PKCβ in human umbilical vein
Reproductive Biology and Endocrinology (2023)
-
In utero hypoxia attenuated acetylcholine-mediated vasodilatation via CHRM3/p-NOS3 in fetal sheep MCA: role of ROS/ERK1/2
Hypertension Research (2022)
-
Melatonin ameliorates hypertension in hypertensive pregnant mice and suppresses the hypertension-induced decrease in Ca2+-activated K+ channels in uterine arteries
Hypertension Research (2021)
-
Management of oxidative stress and inflammation in cardiovascular diseases: mechanisms and challenges
Environmental Science and Pollution Research (2021)