Abstract
Fibromuscular dysplasia is a heterogeneous group of systemic, noninflammatory, and nonatherosclerotic diseases of the vascular wall. It is the second-most common abnormality of the renal artery. Although hypertension is the most common presenting symptom, other symptoms, such as pulsatile tinnitus, stroke, chest pain, or abdominal discomfort, may result from other affected vascular beds. Revascularization of the renal artery appears to be effective at lowering blood pressure in many patients with renal artery fibromuscular dysplasia. For a long time, the intrarenal pathophysiological changes and mechanisms leading to hypertension had hardly been studied in patients with renal artery fibromuscular dysplasia. Recent data, however, has provided more insight into the effects of renal artery fibromuscular dysplasia on the intrarenal microvasculature and the intra-renal renin-angiotensin system in these patients. Moreover, these data have changed our view of the pathophysiological mechanisms leading to hypertension in patients with renal artery fibromuscular dysplasia. In this review, we will discuss recent clinical and scientific developments regarding renal artery fibromuscular dysplasia with an emphasis on its effects on the kidney.
Similar content being viewed by others

Introduction
Fibromuscular dysplasia (FMD) is a heterogeneous group of non-inflammatory, non-atherosclerotic diseases of the vascular wall that lead to dissection, aneurysm, or stenosis of medium-sized arteries [1, 2]. After atherosclerotic renal artery stenosis, FMD is the second-most common abnormality of the renal artery [1, 2]. Although FMD is often considered to be a rare disease, several screening studies among potential kidney donors found a prevalence of 2.3–6.6% in the general population [3,4,5,6,7,8], suggesting that FMD is frequently overlooked in clinical practice. However, over the last few years, FMD has drawn increasing attention by clinicians and researchers, leading to a growing number of diagnoses [9] and the start of several research initiatives [10, 11]. In this review, we will discuss some recent clinical and scientific developments regarding renal artery FMD with an emphasis on its effects on the kidney.
Epidemiology
Renal artery FMD is presumably not as rare as often assumed: the estimated prevalence of renal artery FMD among potential kidney donors is 2.3–6.6% [3,4,5,6,7,8]. Furthermore, the assumption that renal artery FMD is a disease of young women requires revision: most patients are diagnosed in their fifth or sixth decade of life [4, 9, 11, 12], and FMD diagnoses have been made in octogenarians as well [11, 13]. Moreover, even though the US FMD Registry reported that 91% of FMD patients are female [11], the number of male patients in other large studies varies ranges from 19 to 23% [9, 12, 14, 15].
Over the past years, it has become more and more clear that FMD is not a local vascular abnormality of the renal arteries, but, in fact, a systemic vascular disease that can result in a variety of vascular problems. First, FMD has been described in almost every medium-sized human artery, including cervical, visceral, iliac, and upper limb arteries [16,17,18,19,20,21,22,23,24,25,26]. Several patient registries have already suggested that FMD is present in multiple vascular beds in at least 30% of patients but that number was considered to be an underestimate as diagnostic imaging was performed in case of symptoms only [10, 11]. Indeed, the recently published ARCADIA study [27] with systematic computed tomography angiography (CTA) or magnetic resonance angiography (MRA) of other vascular beds in all patients with symptomatic renal or cervical artery FMD demonstrated that multisite involvement is higher than expected: 48.0% of patients have dysplastic stenosis in at least one other vascular bed. Moreover, if aneurysms and dissections are also counted, multisite involvement was present in 66.1% of cases. The latter finding is supported by previous patient registries, demonstrating the high prevalence of arterial dissections (25.7%) and aneurysms (overall 21.7%, intracranial aneurysms 4.6–12.9%) [28,29,30,31] in other vascular beds. It has also become clear that the coronary arteries can be involved in FMD, and they most often present as a spontaneous coronary artery dissection or coronary tortuosity [32, 33]. Systematic MRA or CTA analyses of other vascular beds revealed that 50–86% of patients with spontaneous coronary artery dissection have FMD in at least one extracoronary artery [34,35,36,37,38]. Furthermore, thickness and distensibility of the radial and carotid artery are increased in patients with FMD [39]. These data strongly suggest that FMD is a systemic vasculopathy that can result in a variety of vascular problems. While recent studies have demonstrated several associations with genetic, hormonal, and environmental factors [40,41,42,43,44,45], the cause of these vascular abnormalities is still unknown.
Classification and histopathology
In the past, various histological classification systems have been proposed, but for clinical use, an angiographic classification system has been adopted to differentiate between the different subtypes of FMD [2, 15]. Multifocal FMD is the most common type in adults and is defined as the presence of at least two stenoses in a vessel segment, typically presenting with a string-of-beads appearance (Fig. 1a). Histologically, the medial layer of the vessel wall is affected in these patients [46]. Deposition of disorganized collagen in the zones of degenerating elastic fibrils leads to the formation of fibromuscular ridges and webs. Alternating areas of thick and thin medial fibroplasia result in the typical string-of-beads appearance [47]. Furthermore, weakening of the vascular wall occurs, making it more vulnerable to dissection, tortuosity, kinking, and aneurysm formation [45, 48]. Unifocal (also known as focal) FMD can present as either a solitary (<1 cm in length) or tubular stenosis (>1 cm in length) [15] (Fig. 1b) and is caused by intimal or adventitial fibroplasia, respectively [46, 49]. Several differences in clinical characteristics between focal and multifocal renal artery FMD have been demonstrated: hypertensive patients with unifocal FMD are more often male (31% in unifocal vs. 17% in multifocal FMD) and are generally younger at the onset of hypertension (26 vs. 40 years) and time of diagnosis (30 vs. 49 years) [15]. Given these clinical, angiographic, and histopathological differences, it appears plausible that multifocal and unifocal FMD are in fact two different diseases [15, 50] with different effects on the kidney.
a Digital subtraction angiography of a 58-year-old female showing multifocal FMD in the right renal artery (with a typical string-of-beads pattern). She has had hypertension since she was 28 years old and underwent analysis because of a recent myocardial infarction due to spontaneous coronary artery dissection. b Digital subtraction angiography of a 43-year-old female showing unifocal FMD in both renal arteries. She was admitted for hypertensive emergency with a blood pressure of up to 230/110 mmHg with grade III hypertensive retinopathy and renal failure (eGFR 29 ml/min/1.73 m2)
Clinical presentation
The most common presenting symptom of renal artery FMD is hypertension, which was present in 66.6% of the patients in the US FMD Registry [51]. Interestingly, among ‘coincidentally’ diagnosed renal artery FMD in normotensive potential kidney donors, 26–29% developed hypertension within 4–7.5 years after diagnosis [6, 8]. Abdominal pain (17.2%), bruits (10.8%) [51], and (occasionally) renal infarction [52,53,54,55] are other presenting symptoms. However, as FMD is a systemic vascular disease, the first symptoms are often not directly related to the renal artery but rather are caused by FMD localizations in other vascular beds, such as pulsatile tinnitus (33.4%), neck pain (27.2)%, or stroke/transient ischemic attack (17.5%) in the case of cervical FMD [51], chest pain due to FMD-related coronary artery abnormalities [32, 33], or abdominal complaints in the case of visceral involvement.
Renal artery FMD and its effects on the kidney
Pathophysiological changes in the kidneys of patients with FMD have hardly been studied. Animal models are not available yet. Therefore, our view of the effects of FMD on the kidney is predominantly based on experiments in patients with atherosclerotic renal artery stenosis [56] and animal models with renal artery clipping [57]. However, recent data from patients with renal artery FMD have provided more insights into the effects of renal artery FMD on the intrarenal microvasculature, intrarenal renin–angiotensin system, and pathophysiological mechanisms leading to hypertension in these patients.
Intrarenal microvasculature
The intrarenal microvasculature appears to be relatively preserved in kidneys with multifocal FMD. In patients with multifocal FMD of the renal artery, renal blood flow is significantly higher than in patients with atherosclerotic renal artery stenosis and comparable to that in matched patients with essential hypertension (without renovascular abnormalities) [58,59,60]. Moreover, in patients with unilateral FMD, renal blood flow is comparable between the affected and unaffected kidney [58, 59]. This suggest that the presence of a string-of-beads does not seriously affect local renal perfusion.
Overall, renal function is also relatively normal in kidneys with multifocal FMD. In a French cohort of 334 patients with renal artery FMD, the mean estimated glomerular filtration rate (eGFR using the Cockcroft–Gault formula) was 88 ml/min/1.73 m2, and only 30 patients (8.9%) had an eGFR below 60 ml/min/1.73 m2 [9]. In the US FMD Registry, only 2.8% out of 615 patients had renal insufficiency upon diagnosis [51]. Moreover, we demonstrated (by side-selective determination of creatinine clearance) that there are no differences in the glomerular filtration rate between the affected and unaffected kidney in patients with unilateral FMD [59], indicating that the presence of a string-of-beads does not substantially alter glomerular filtration.
Furthermore, the intrarenal renin-angiotensin system and the ability to respond to vasoactive stimuli appears to be preserved in kidneys with FMD. Intrarenal blockade of the vasoconstrictory Ang II/AT1R-axis (angiotensin II/angiotensin II type-1 receptor axis) by intrarenal infusion of AT1R-blocker eprosartan results in vasodilation, whereas intrarenal blockade of the vasodilatory (angiotensin-(1–7) / Mas receptor) axis by L-NMMA (blocking the final common pathway of this axis) [61] results in vasoconstriction [59]. This finding indicates that both axes play a role in the regulation of intrarenal hemodynamics in kidneys with FMD. Moreover, the stimulation of these axes with either angiotensin II or angiotensin-(1–7) resulted in a vasoconstrictory or vasodilatory effect, respectively, with a magnitude similar to that in patients without renal artery abnormalities [59]. This result indicates that neither of these axes acts on its full strength as the infusion of more angiotensin II or angiotensin-(1–7) still results in a hemodynamic effect. It also suggests that microvascular and endothelial function are relatively intact in kidneys with FMD as these functions are a prerequisite to exerting a hemodynamic response to vasoactive stimuli. These findings are in strong contrast to those in kidneys with atherosclerotic renal artery stenosis, where the intrarenal renin–angiotensin system and the ability of the microvasculature to respond to vasoactive stimuli are clearly disturbed given the almost absent effects of angiotensin-(1–7) and L-NMMA infusion [62, 63].
In summary, preserved renal blood flow, glomerular filtration, and the ability to respond to modulation of the renin–angiotensin system all indicate that intrarenal microvascular function is more or less intact in kidneys with multifocal FMD. This finding is in sharp contrast to that of kidneys with atherosclerotic renal artery stenosis (Fig. 2). Possibly, the lack of longstanding atherosclerotic burden (as in atherosclerotic renal artery stenosis [64, 65]) prevents tubulointerstitial atrophy and glomerulosclerosis [66,67,68]. However, we cannot rule out that the string-of-beads itself causes other hemodynamic changes (such as changes in local arterial pressure, wall stress, or pulse wave transmission) that have a protective effect on the intrarenal microvasculature.
Summary of the different effects on the kidney and blood pressure by fibromuscular dysplasia and atherosclerotic renal artery stenosis. Some caution is required about the efficacy of revascularization in fibromuscular dysplasia as it has only been studied in (non-randomized) observational studies, whereas the statement on the efficacy of revascularization in atherosclerotic renal artery stenosis is based on randomized controlled trials. References refer to the references in the main text
Mechanisms leading to hypertension
Until recently, it was assumed that renovascular FMD causes hypertension due to a decrease in renal blood flow, resulting in increased renin secretion, which in turn increases blood pressure (similar to experiments in animal models of renovascular hypertension and a subset of patients with renal artery stenosis caused by atherosclerosis) [2, 56]. However, several data argue against this prevailing concept of renovascular hypertension in patients with FMD.
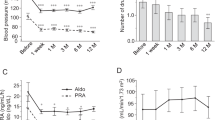
First and as discussed above, it was demonstrated that renal blood flow is not reduced in kidneys with multifocal FMD [58,59,60]. Second, renin secretion and renin–angiotensin system activity are relatively normal in kidneys with multifocal FMD. In a systematic analyses of 64 patients with multifocal FMD of the renal artery (off antihypertensive drugs and prior to balloon angioplasty), we found that systemic renin levels were within the normal range in all 64 patients and that renin secretion was comparable to that in patients with essential hypertension [58, 59]. Moreover, in patients with unilateral FMD, renin secretion in the affected kidney is comparable to that in the non-affected contralateral kidney. This finding is in contrast to that of patients with atherosclerotic renal artery stenosis in whom renin secretion is significantly higher and in whom lateralization in renin secretion is observed in a large patient subset [58, 69, 70]. These data suggest that the presence of a string-of-beads itself (generally) does not lead to an increase in renin secretion. In renovascular hypertension due to unifocal FMD, this observation is still being investigated, but preliminary data suggest that renal blood flow is reduced, resulting in more pronounced renin secretion than that found in multifocal FMD [71]. Third, several studies have demonstrated that revascularization can reduce blood pressure in patients with FMD without increased renin secretion, with no differences in renin secretion between patients who had a decrease in blood pressure and those who did not [59, 70, 72]. Fourth, whereas there is a direct relation (in cross-sectional data) between renin levels and blood pressure in patients with atherosclerotic renal artery stenosis, the association between these parameters appears to be inverse in patients with FMD: the higher the blood pressure, the lower the renin levels (Fig. 3) [58], or vice versa: the lower the blood pressure, the higher the renin levels, which is similar to the physiological response in healthy individuals [73].
Correlation between systolic blood pressure (24-h ambulatory blood pressure measurement) and renin level for patients with renal artery fibromuscular dysplasia (FMD, left panel) and patients with atherosclerotic renal artery stenosis (right panel) prior to revascularization and off antihypertensive drugs. Figure adapted from Van Twist et al. [54]
However, some caution is required as the abovementioned findings have not been replicated yet, and all these findings are at odds with the generally accepted view that multifocal FMD induces hypertension via decreased renal blood flow and increased renin secretion. In contrast to kidneys with atherosclerotic renal artery stenosis in whom renovascular resistance is so high that ischemic nephrons secrete excessive amounts of renin, it appears that central blood pressure can still be transmitted to the juxtaglomerular apparatus in kidneys with multifocal FMD, thus preventing an increase in renin secretion. Although increased renin secretion has been reported in some patients with multifocal FMD [74], this finding appears to be the exception rather than the rule.
The question remains why renin secretion was not increased in the abovementioned studies on multifocal FMD. An explanation could be that renin-secretion is only increased in the early phase of the disease: long-lasting renovascular hypertension would result in intrarenal parenchymal damage, leading to renal hypertension (secondary to parenchymal damage) with the normalization of renin secretion again in the long term. However, as intrarenal microvascular function is relatively preserved in kidneys with FMD and parenchymal damage would not be reversible by revascularization (which one would expect given the fairly good results of balloon angioplasty), this appears to be unlikely. Moreover, one would have expected at least some increase or lateralization in renin secretion in some patients (at least in patients in the early stage) but that is not the case either [58, 59]. Furthermore, as renovascular multifocal FMD is also observed in patients without hypertension [4], one could argue that FMD patients without increased renin secretion have in fact essential hypertension. In these patients, the string-of-beads would only be a bystander that is not contributing to hypertension. Unfortunately, it is not yet possible to differentiate between ‘true’ renovascular hypertension and essential hypertension with FMD as ‘an innocent bystander’. Measurement of the trans-stenotic pressure gradient has been suggested as a possible tool [2, 10], but data on its value in FMD are still restricted to some case reports [75, 76]. Therefore, it is regrettable that trans-stenotic pressure gradients were not routinely measured in the abovementioned mechanistic studies either [58, 59]. However, as the majority of patients with multifocal FMD in these studies had a blood pressure response to balloon angioplasty (48–51% one year after balloon angioplasty, according to pre-specified criteria) [58, 59], it is likely that the string-of-beads contributed to hypertension in many of these patients. Nevertheless, renin secretion was within normal range in all patients in that cohort. Naturally, such observational data on treatment efficacy could be biased by a variety of other factors (e.g., decreasing white-coat effect, regression to the mean, and improved adherence to drug treatment after revascularization), but it is unlikely that all these patients only had essential hypertension with the renovascular string-of-beads being nothing more than an innocent bystander.
Therefore, it appears that mechanisms other than increased renin secretion have to be involved in renovascular hypertension due to multifocal FMD. Without further research, we can only hypothesize about such alternative mechanisms. Other hemodynamic changes aside from reduced blood flow (such as local arterial pressure, turbulence of blood flow, wall stress, and pulse wave transmission) could be involved, but apparently, these changes do not result in increased renin secretion. However, such hemodynamic changes could lead to the activation of other pathways, such as the release of other paracrine stimuli, reactive oxygen species, or activation of the sympathetic nervous system. Future (prospective) studies should focus on changes before and after balloon angioplasty, particularly on other alterations in intrarenal hemodynamics (aside from perfusion alone), and on changes in other signaling pathways involved in hypertension, such as the sympathetic nervous system, reactive oxygen species, and various auto- and paracrine signaling systems, besides the renin–angiotensin system.
Clinical perspective
Diagnostic studies
Currently, the diagnosis of renal artery FMD is made based on imaging studies showing a non-atherosclerotic stenosis in the absence of syndromal or inflammatory disease [1, 2]. The radiographic presentation of multifocal FMD is quite typical with its string-of-beads appearance but is sometimes confused with vasospasms, resulting in typical standing waves that are transient and more regular and symmetrical than FMD [77]. Diagnosing unifocal FMD is often more challenging, as other diseases have similar radiographic presentations, especially atherosclerosis. Hence, the diagnosis can often only be made in younger patients (<40 years) [1] in the absence of multiple risk factors for atherosclerosis and vascular wall calcifications.
The gold standard for diagnosing renovascular FMD is still catheter-based digital subtraction angiography (DSA) [1, 2], optionally accompanied by intravascular ultrasound or optical coherence tomography to obtain a more detailed view in case of doubt regarding the severity of the stenosis or to evaluate the results of balloon angioplasty [78, 79] as the degree of stenosis in multifocal FMD cannot be obtained from the angiographical image alone. Selective catheterization of both renal arteries is required, as FMD lesions are often only found in the distal two-thirds of the renal artery and could easily be missed on aortic angiography.
Duplex ultrasound has been proposed as a non-invasive alternative for DSA, but this technique is highly operator-dependent and its negative predictive value is probably low, especially for more distal lesions [1, 10]. CTA and MRA could be better alternatives [1], but their diagnostic values are reduced as the spatial resolution of these imaging modalities is less than that of DSA. This difference was clearly demonstrated by two prospective studies (i.e., all patients underwent DSA, irrespective of the results of CTA or MRA): in an older study, sensitivity was only 28% for CTA and 22% for MRA [80], whereas a more recent study showed that MRA missed all cases of FMD that were detected with DSA [81]. Two retrospective studies suggested that the sensitivity of CTA and MRA is 100% compared to DSA [82, 83], but this finding could be biased as patients with negative CTA or MRA were not referred for DSA. Perhaps sensitivity has been improved with new CTA or MRA scanners with higher spatial resolution or increased use of reformatted images (affecting the assessment in up to 56% of the patients) [84] but that finding has to be evaluated in future trials. As we have several examples of patients who were diagnosed by DSA despite previous (false) negative CTA or MRA (especially multifocal FMD located distally in the renal artery), we recommend caution with ruling out FMD by CTA or MRA in cases of high clinical suspicion.
Systemic renin levels should not be used to screen for renovascular FMD as these levels are normal in the vast majority of patients with multifocal FMD [59]. Although elevated renin levels occasionally lead toward the diagnosis of FMD [74], this situation is the exception rather than the rule.
Management of renal artery FMD
In contrast to atherosclerotic renal artery stenosis [85,86,87], revascularization appears to be effective at lowering blood pressure in many patients with renal artery FMD. Some caution is required as randomized controlled trials on revascularization in FMD are lacking and one cannot exclude that observational studies are biased by other factors, such as decreasing white-coat effect, regression to the mean, or improved adherence to drug treatment after revascularization. Until a double blind, sham-controlled trial is available, we are restricted to observational studies. A large meta-analysis of such observational studies showed that hypertension was cured by balloon angioplasty in 40–52% of the cases and by surgery in 53–62% of the cases [12]. Moreover, in patients in whom cure cannot be achieved, improvements in blood pressure [88,89,90], renal function (6–8 ml/min/1.73 m2 on average) [91, 92], and the required number of antihypertensive drugs [93, 94] have been reported. The difference in response to revascularization between FMD and atherosclerotic renal artery stenosis could be explained by the fact that microvascular function is relatively preserved in kidneys with FMD: the qualitatively good kidney tissue distal to the string-of-beads would be able to function relatively well after revascularization.
Currently, revascularization is often considered the treatment of choice, especially in younger patients and patients with more severe or recent onset hypertension. Given its less invasive character and lower risk of major complications (6% vs. 15%) [12], balloon angioplasty is preferred over surgical revascularization. However, in case of complex lesions, ex vivo bench repair could be considered [95]. Stenting is generally not recommended (with the exception of treating renal artery dissection) as several cases of stent fractures or in-stent restenosis have been described [96, 97]. As restenosis occurs frequently (10–38%, depending upon duration of follow-up) [12, 13, 94, 98, 99], a second balloon angioplasty should be considered in patients in whom blood pressure rises after an initial response to balloon angioplasty.
Conservative management with antihypertensive drugs is often quite effective in patients with FMD [9, 100], which is probably also due to preserved microvascular function [59]. This finding might explain why many patients with FMD remain undetected: as hypertension responds to antihypertensive drug treatment fairly well, no diagnostic studies to detect secondary causes of hypertension (such as FMD) will be initiated. Since the efficacy of balloon angioplasty decreases with age, the presence of kidney damage and long-standing hypertension (presumably due to irreversible damage and remodeling of the vascular system) [12, 98], conservative management could be considered in elderly patients without severe hypertension that respond well to antihypertensive drugs [9]. To reduce thrombus formation on the intravascular webs, antiplatelet agents are recommended by several experts [2, 101]. However, studies on the effect of this intervention in patients with FMD are lacking. Screening for extrarenal FMD lesions, such as intracranial aneurysms (present in 4.6–12.9% of the patients) [28,29,30,31], should be considered if this screening would have therapeutic implications [10].
Conclusions and future directions
The effect of renal artery FMD on the kidney and its mechanisms leading to hypertension are complex and incompletely understood. Recent data demonstrate that renal blood flow, glomerular filtration, and the response to vasoactive stimuli are more or less intact in kidneys with multifocal FMD (summarized in Fig. 2), suggesting that intrarenal microvascular function is relatively preserved in these kidneys. Moreover, the assumption that hypertension in patients with renal artery FMD is caused by increased renin secretion due to reduced renal perfusion needs revision: renal blood flow, renin secretion, and the association between renin levels and blood pressure are relatively normal in patients with FMD. Further research is needed to answer questions about which alternative pathophysiological mechanisms are responsible for the development of hypertension in these patients. Furthermore, as renal artery FMD is underdiagnosed, improvement of screening strategies are needed to detect more patients with this potentially curable cause of hypertension.
References
Persu A, Giavarini A, Touze E, Januszewicz A, Sapoval M, Azizi M, Barral X, Jeunemaitre X, Morganti A, Plouin PF, de Leeuw P, ESH Working Group Hypertension and the Kidney. European consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens. 2014;32:1367–78.
Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, Gray WA, Gupta R, Hamburg NM, Katzen BT, Lookstein RA, Lumsden AB, Newburger JW, Rundek T, Sperati CJ, Stanley JC. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the american heart association. Circulation. 2014;129:1048–78.
Hendricks NJ, Matsumoto AH, Angle JF, Baheti A, Sabri SS, Park AW, Stone JR, Patrie JT, Dworkin L, Cooper CJ, Murphy TP, Cutlip DE. Is fibromuscular dysplasia underdiagnosed? A comparison of the prevalence of fmd seen in coral trial participants versus a single institution population of renal donor candidates. Vasc Med. 2014;19:363–7.
McKenzie GA, Oderich GS, Kawashima A, Misra S. Renal artery fibromuscular dysplasia in 2,640 renal donor subjects: a CT angiography analysis. J Vasc Interv Radiol. 2013;24:1477–80.
Blondin D, Lanzman R, Schellhammer F, Oels M, Grotemeyer D, Baldus SE, Rump LC, Sandmann W, Voiculescu A. Fibromuscular dysplasia in living renal donors: still a challenge to computed tomographic angiography. Eur J Radiol. 2010;75:67–71.
Andreoni KA, Weeks SM, Gerber DA, Fair JH, Mauro MA, McCoy L, Scott L, Johnson MW. Incidence of donor renal fibromuscular dysplasia: does it justify routine angiography? Transplantation. 2002;73:1112–6.
Neymark E, LaBerge JM, Hirose R, Melzer JS, Kerlan RK Jr., Wilson MW, Gordon RL. Arteriographic detection of renovascular disease in potential renal donors: incidence and effect on donor surgery. Radiology. 2000;214:755–60.
Cragg AH, Smith TP, Thompson BH, Maroney TP, Stanson AW, Shaw GT, Hunter DW, Cochran ST. Incidental fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology. 1989;172:145–7.
Giavarini A, Savard S, Sapoval M, Plouin PF, Steichen O. Clinical management of renal artery fibromuscular dysplasia: temporal trends and outcomes. J Hypertens. 2014;32:2433–8. discussion 2438
Persu A, Van der Niepen P, Touze E, Gevaert S, Berra E, Mace P, Plouin PF, Jeunemaitre X. Revisiting fibromuscular dysplasia: rationale of the european fibromuscular dysplasia initiative. Hypertension. 2016;68:832–9.
Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH, Jaff MR, Kim ES, Mace P, Matsumoto AH, McBane RD, Kline-Rogers E, White CJ, Gornik HL. The United States registry for fibromuscular dysplasia: results in the first 447 patients. Circulation. 2012;125:3182–90.
Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension. 2010;56:525–32.
de Fraissinette B, Garcier JM, Dieu V, Mofid R, Ravel A, Boire JY, Boyer L. Percutaneous transluminal angioplasty of dysplastic stenoses of the renal artery: results on 70 adults. Cardiovasc Interv Radiol. 2003;26:46–51.
Gottsater A, Lindblad B. Optimal management of renal artery fibromuscular dysplasia. Ther Clin Risk Manag. 2014;10:583–95.
Savard S, Steichen O, Azarine A, Azizi M, Jeunemaitre X, Plouin PF. Association between 2 angiographic subtypes of renal artery fibromuscular dysplasia and clinical characteristics. Circulation. 2012;126:3062–9.
Cutts S, Grewal RS, Downing R. Bilateral brachial artery fibromuscular dysplasia. Eur J Vasc Endovasc Surg. 2000;19:667–8.
De Waele M, Lauwers P, Hendriks J, Van Schil P. Fibromuscular dysplasia of the brachial artery associated with unilateral clubbing. Interact Cardiovasc Thorac Surg. 2012;15:1080–1.
Higashimori A, Yokoi Y. The interventional therapy for axillary stenosis with fibromuscular dysplasia of renal artery. Cardiovasc Interv Ther. 2013;28:184–7.
Steinmetz EF, Berry P, Shames ML, Buckley C, Goeddel LA, Thompson RW. “Grape cluster” aneurysm of the right subclavian artery: an unusual manifestation of fibromuscular dysplasia. Ann Vasc Surg. 2003;17:296–301.
Honjo O, Yamada Y, Kuroko Y, Kushida Y, Une D, Hioki K. Spontaneous dissection and rupture of common iliac artery in a patient with fibromuscular dysplasia: a case report and review of the literature on iliac artery dissections secondary to fibromuscular dysplasia. J Vasc Surg. 2004;40:1032–6.
Ketha SS, Bjarnason H, Oderich GS, Misra S. Clinical features and endovascular management of iliac artery fibromuscular dysplasia. J Vasc Interv Radiol. 2014;25:949–53.
Niizeki T, Ishino M, Kitahara T, Yamauchi S, Ikeno E, Kubota I. Endovascular therapy for fibromuscular dysplasia of the bilateral external iliac arteries visualized with optical coherence tomography. Am J Case Rep. 2015;16:187–90.
Erwin PA, Blas JV, Gandhi S, Romero ME, Gray BH. Images in vascular medicine. Visceral fibromuscular dysplasia in a patient with chronic abdominal pain. Vasc Med. 2016;21:170–1.
Patel NC, Palmer WC, Gill KR, Wallace MB. A case of mesenteric ischemia secondary to fibromuscular dysplasia (fmd) with a positive outcome after intervention. J Interv Gastroenterol. 2012;2:199–201.
Sekar N, Shankar R. Fibromuscular dysplasia with multiple visceral artery involvement. J Vasc Surg. 2013;57:1401.
Miller MB, Flores DR III. Fibromuscular dysplasia of the brachial artery. N Engl J Med. 2017;376:e2.
Plouin PF, Baguet JP, Thony F, Ormezzano O, Azarine A, Silhol F, Oppenheim C, Bouhanick B, Boyer L, Persu A, Hammer F, Gosse P, Mounier-Vehier C, Le Hello C, Jeunemaitre X, Azizi M, Amar L, Chatellier G, Mousseaux E, Touze E, Investigators A. High prevalence of multiple arterial bed lesions in patients with fibromuscular dysplasia: the arcadia registry (assessment of renal and cervical artery dysplasia). Hypertension. 2017;70:652–8.
Cloft HJ, Kallmes DF, Kallmes MH, Goldstein JH, Jensen ME, Dion JE. Prevalence of cerebral aneurysms in patients with fibromuscular dysplasia: a reassessment. J Neurosurg. 1998;88:436–40.
Touze E, Oppenheim C, Trystram D, Nokam G, Pasquini M, Alamowitch S, Herve D, Garnier P, Mousseaux E, Plouin PF. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke. 2010;5:296–305.
Kadian-Dodov D, Gornik HL, Gu X, Froehlich J, Bacharach JM, Chi YW, Gray BH, Jaff MR, Kim ES, Mace P, Sharma A, Kline-Rogers E, White C, Olin JW. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the U.S. Registry for fmd. J Am Coll Cardiol. 2016;68:176–85.
Lather HD, Gornik HL, Olin JW, Gu X, Heidt ST, Kim ESH, Kadian-Dodov D, Sharma A, Gray B, Jaff MR, Chi YW, Mace P, Kline-Rogers E, Froehlich JB. Prevalence of intracranial aneurysm in women with fibromuscular dysplasia: a report from the US registry for fibromuscular dysplasia. JAMA Neurol. 2017;74:1081–7.
Michelis KC, Olin JW, Kadian-Dodov D, d’Escamard V, Kovacic JC. Coronary artery manifestations of fibromuscular dysplasia. J Am Coll Cardiol. 2014;64:1033–46.
van Twist DJL, de Leeuw PW, Kroon AA. Coronary tortuosity: a clue to the diagnosis of fibromuscular dysplasia? Am J Hypertens. 2017 ;30:776–80.
Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–88.
Toggweiler S, Puck M, Thalhammer C, Manka R, Wyss M, Bilecen D, Corti R, Amann-Vesti BR, Luscher TF, Wyss CA. Associated vascular lesions in patients with spontaneous coronary artery dissection. Swiss Med Wkly. 2012;142:w13538.
Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013;6:44–52.
Prasad M, Tweet MS, Hayes SN, Leng S, Liang JJ, Eleid MF, Gulati R, Vrtiska TJ. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672–7.
Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, Vrtiska TJ, Prasad M, Rihal CS, Hayes SN, Gulati R. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv. 2014;7:656–62.
Boutouyrie P, Gimenez-Roqueplo AP, Fine E, Laloux B, Fiquet-Kempf B, Plouin PF, Jeunemaitre X, Laurent S. Evidence for carotid and radial artery wall subclinical lesions in renal fibromuscular dysplasia. J Hypertens. 2003;21:2287–95.
Kiando SR, Tucker NR, Castro-Vega LJ, Katz A, D’Escamard V, Treard C, Fraher D, Albuisson J, Kadian-Dodov D, Ye Z, Austin E, Yang ML, Hunker K, Barlassina C, Cusi D, Galan P, Empana JP, Jouven X, Gimenez-Roqueplo AP, Bruneval P, Hyun Kim ES, Olin JW, Gornik HL, Azizi M, Plouin PF, Ellinor PT, Kullo IJ, Milan DJ, Ganesh SK, Boutouyrie P, Kovacic JC, Jeunemaitre X, Bouatia-Naji N. Phactr1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genet. 2016;12:e1006367.
Guo DC, Duan XY, Regalado ES, Mellor-Crummey L, Kwartler CS, Kim D, Lieberman K, de Vries BB, Pfundt R, Schinzel A, Kotzot D, Shen X,Yang ML, University of Washington Center for Mendelian G, Bamshad MJ, Nickerson DA, Gornik HL, Ganesh SK, Braverman AC, Grange DK, Milewicz DM. Loss-of-function mutations in yy1ap1 lead to grange syndrome and a fibromuscular dysplasia-like vascular disease. Am J Hum Genet. 2017;100:21–30.
Ganesh SK, Morissette R, Xu Z, Schoenhoff F, Griswold BF, Yang J, Tong L, Yang ML, Hunker K, Sloper L, Kuo S, Raza R, Milewicz DM, Francomano CA, Dietz HC, Van Eyk J, McDonnell NB. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered tgf-beta expression and connective tissue features. FASEB J. 2014;28:3313–24.
Silhol F, Sarlon-Bartoli G, Daniel L, Bartoli JM, Cohen S, Lepidi H, Piquet P, Bartoli MA, Vaisse B. Intranuclear expression of progesterone receptors in smooth muscle cells of renovascular fibromuscular dysplasia: a pilot study. Ann Vasc Surg. 2015;29:830–5.
Savard S, Azarine A, Jeunemaitre X, Azizi M, Plouin PF, Steichen O. Association of smoking with phenotype at diagnosis and vascular interventions in patients with renal artery fibromuscular dysplasia. Hypertension. 2013;61:1227–32.
Miller DJ, Marin H, Aho T, Schultz L, Katramados A, Mitsias P. Fibromuscular dysplasia unraveled: the pulsation-induced microtrauma and reactive hyperplasia theory. Med Hypotheses. 2014;83:21–24.
Harrison EG Jr., Hunt JC, Bernatz PE. Morphology of fibromuscular dysplasia of the renal artery in renovascular hypertension. Am J Med. 1967;43:97–112.
Lummus S, Breeze R, Lucia MS, Kleinschmidt-DeMasters BK. Histopathologic features of intracranial vascular involvement in fibromuscular dysplasia, ehlers-danlos type iv, and neurofibromatosis I. J Neuropathol Exp Neurol. 2014;73:916–32.
Tanaka H, Zaima N, Sasaki T, Yamamoto N, Inuzuka K, Sano M, Konno H, Urano T, Setou M, Unno N. Characteristic distribution pattern of lysophosphatidylcholine in fibromuscular dysplasia-associated visceral artery aneurysms compared with atherosclerotic visceral artery aneurysms. J Atheroscler Thromb. 2016;23:673–80.
Varennes L, Tahon F, Kastler A, Grand S, Thony F, Baguet JP, Detante O, Touze E, Krainik A. Fibromuscular dysplasia: what the radiologist should know: a pictorial review. Insights Imaging. 2015;6:295–307.
Olin JW. Is fibromuscular dysplasia a single disease? Circulation. 2012;126:2925–7.
Kim ES, Olin JW, Froehlich JB, Gu X, Bacharach JM, Gray BH, Jaff MR, Katzen BT, Kline-Rogers E, Mace PD, Matsumoto AH, McBane RD, White CJ, Gornik HL. Clinical manifestations of fibromuscular dysplasia vary by patient sex: a report of the united states registry for fibromuscular dysplasia. J Am Coll Cardiol. 2013;62:2026–8.
Van den Driessche A, Van Hul E, Ichiche M, Verpooten GA, Bosmans JL. Fibromuscular dysplasia presenting as a renal infarction: a case report. J Med Case Rep. 2010;4:199.
Afshinnia F, Sundaram B, Rao P, Stanley J, Bitzer M. Evaluation of characteristics, associations and clinical course of isolated spontaneous renal artery dissection. Nephrol Dial Transplant. 2013;28:2089–98.
van Twist DJ, van de Laar RJ, Brans RJ, Refos JW, Kroon AA. A 34-year-old man with back pain. Neth J Med. 2017;75:45.
Faucon AL, Bobrie G, Jannot AS, Azarine A, Plouin PF, Azizi M, Amar L. Cause of renal infarction: a retrospective analysis of 186 consecutive cases. J Hypertens. 2017 36:634–40.
Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens. 2010;23:1159–69.
Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Ren Physiol. 2009;297:F1055–68.
van Twist DJ, Houben AJ, de Haan MW, de Leeuw PW, Kroon AA. Pathophysiological differences between multifocal fibromuscular dysplasia and atherosclerotic renal artery stenosis. J Hypertens. 2017 ;35:845–52.
van Twist DJ, Houben AJ, de Haan MW, de Leeuw PW, Kroon AA. Renal hemodynamics and renin-angiotensin system activity in humans with multifocal renal artery fibromuscular dysplasia. J Hypertens. 2016;34:1160–9.
Lerman LO, Taler SJ, Textor SC, Sheedy PF 2nd, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996;49:846–54.
van Twist DJ, Kroon AA, de Leeuw PW. Angiotensin-(1-7) as a strategy in the treatment of hypertension? Curr Opin Nephrol Hypertens. 2014;23:480–6.
Van Twist D, Houben A, De Haan M, Mostard G, De Leeuw P, Kroon A. Angiotensin-(1-7)-induced renal vasodilation is reduced in human kidneys with renal artery stenosis. J Hypertens. 2014;32:2428.
Wierema TK, Houben AJ, Kroon AA, Koster D, van der Zander K, van Engelshoven JM, de Leeuw PW. Nitric oxide dependence of renal blood flow in patients with renal artery stenosis. J Am Soc Nephrol. 2001;12:1836–43.
Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295–301.
Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis. 2009;52:196–203.
Zhang X, Eirin A, Li ZL, Crane JA, Krier JD, Ebrahimi B, Pawar AS, Zhu XY, Tang H, Jordan KL, Lerman A, Textor SC, Lerman LO. Angiotensin receptor blockade has protective effects on the poststenotic porcine kidney. Kidney Int. 2013;84:767–75.
Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrol Dial Transplant. 2010;25:3615–22.
Eirin A, Lerman LO. Mesenchymal stem cell treatment for chronic renal failure. Stem Cell Res Ther. 2014;5:83.
Luscher TF, Greminger P, Kuhlmann U, Siegenthaler W, Largiader F, Vetter W. Renal venous renin determinations in renovascular hypertension. Diagnostic and prognostic value in unilateral renal artery stenosis treated by surgery or percutaneous transluminal angioplasty. Nephron. 1986;44(Suppl 1):17–24.
Luscher TF, Vetter H, Studer A, Pouliadis G, Kuhlmann U, Glanzer K, Largiader F, Hauri D, Greminger P, Siegenthaler W, Vetter W. Renal venous renin activity in various forms of curable renal hypertension. Clin Nephrol. 1981;15:314–20.
De Heer PWM,Van Twist DJL, Ho uben AJHM, De Leeuw PW, Kroon AA. [op.3a.05] differences in renal hemodynamics and renin secretion between patients with unifocal and multifocal fibromuscular dysplasia. J Hypertens. 2017;35:e26.
Vetter W, Vetter H, Tenschert W, Kuhlmann U, Studer A, Glanzer K, Pouliadis G, Largiader F, Furrer J, Siegenthaler W. [renovascular hypertension. Prognostic value of renal venous renin determinations (author’s transl)]. Klin Wochenschr. 1979;57:863–73.
De Leeuw P, Birkenhäger WH. Renal mechanisms of blood pressure regulation: clinical evidence. In: Zanchetti A, editor. Handbook of hypertension. Elsevier Science B.V.; Amsterdam, 1997.
Petruzzelli M, Taylor KP, Koo B, Brown MJ. Telling tails: very high plasma renin levels prompt the diagnosis of renal artery stenosis, despite initial negative imaging. Hypertension. 2016;68:11–16.
Mahmud E, Brocato M, Palakodeti V, Tsimikas S. Fibromuscular dysplasia of renal arteries: percutaneous revascularization based on hemodynamic assessment with a pressure measurement guidewire. Catheter Cardiovasc Interv. 2006;67:434–7.
Prasad A, Zafar N, Mahmud E. Assessment of renal artery fibromuscular dysplasia: angiography, intravascular ultrasound (with virtual histology), and pressure wire measurements. Catheter Cardiovasc Interv. 2009;74:260–4.
Sharma AM, Gornik HL. Standing arterial waves is not fibromuscular dysplasia. Circ Cardiovasc Interv. 2012;5:e9–e11.
Tanaka A, Suzuki K, Inoue N, Meguro T. Optical coherence tomography images of iliac artery fibromuscular dysplasia. Eur Heart J. 2014;35:2872.
Gowda MS, Loeb AL, Crouse LJ, Kramer PH. Complementary roles of color-flow duplex imaging and intravascular ultrasound in the diagnosis of renal artery fibromuscular dysplasia: should renal arteriography serve as the “gold standard”? J Am Coll Cardiol. 2003;41:1305–11.
Vasbinder GB, Nelemans PJ, Kessels AG, Kroon AA, Maki JH, Leiner T, Beek FJ, Korst MB, Flobbe K, de Haan MW, van Zwam WH, Postma CT, Hunink MG, de Leeuw PW, van Engelshoven JM, Renal Artery Diagnostic Imaging Study in Hypertension (RADISH) Study Group. Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med. 2004;141:674–82.discussion 682.
Neville C, House AA, Nguan CY, Beasley KA, Peck D, Thain LM, Rankin R, McAlister VC, Spouge AR, Luke PP. Prospective comparison of magnetic resonance angiography with selective renal angiography for living kidney donor assessment. Urology. 2008;71:385–9.
Sabharwal R, Vladica P, Coleman P. Multidetector spiral ct renal angiography in the diagnosis of renal artery fibromuscular dysplasia. Eur J Radiol. 2007;61:520–7.
Willoteaux S, Faivre-Pierret M, Moranne O, Lions C, Bruzzi J, Finot M, Gaxotte V, Mounier-Vehier C, Beregi JP. Fibromuscular dysplasia of the main renal arteries: comparison of contrast-enhanced mr angiography with digital subtraction angiography. Radiology. 2006;241:922–9.
Bolen MA, Brinza E, Renapurkar RD, Kim ES, Gornik HL. Screening ct angiography of the aorta, visceral branch vessels, and pelvic arteries in fibromuscular dysplasia. JACC Cardiovasc Imaging. 2016 ;10:554–61.
Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Sr., Dworkin LD, CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13–22.
Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150:840–8. W150-841
Investigators A, Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–62.
Alhadad A, Mattiasson I, Ivancev K, Gottsater A, Lindblad B. Revascularisation of renal artery stenosis caused by fibromuscular dysplasia: effects on blood pressure during 7-year follow-up are influenced by duration of hypertension and branch artery stenosis. J Hum Hypertens. 2005;19:761–7.
Mousa AY, Campbell JE, Stone PA, Broce M, Bates MC, AbuRahma AF. Short- and long-term outcomes of percutaneous transluminal angioplasty/stenting of renal fibromuscular dysplasia over a ten-year period. J Vasc Surg. 2012;55:421–7.
Yang YK, Zhang Y, Meng X, Yang KQ, Jiang XJ, Wu HY, Zhang HM, Song L, Wang LP, Gao LG, Zhou XL. Clinical characteristics and treatment of renal artery fibromuscular dysplasia with percutaneous transluminal angioplasty: a long-term follow-up study. Clin Res Cardiol. 2016;105:930–7.
Airoldi F, Palatresi S, Marana I, Bencini C, Benti R, Lovaria A, Alberti C, Nador B, Nicolini A, Longari V, Gerundini P, Morganti A. Angioplasty of atherosclerotic and fibromuscular renal artery stenosis: time course and predicting factors of the effects on renal function. Am J Hypertens. 2000;13:1210–7.
La Batide-Alanore A, Azizi M, Froissart M, Raynaud A, Plouin PF. Split renal function outcome after renal angioplasty in patients with unilateral renal artery stenosis. J Am Soc Nephrol. 2001;12:1235–41.
Smit JV, Wierema TK, Kroon AA, de Leeuw PW. Blood pressure and renal function before and after percutaneous transluminal renal angioplasty in fibromuscular dysplasia: a cohort study. J Hypertens. 2013;31:1183–8.
Iwashima Y, Fukuda T, Yoshihara F, Kusunoki H, Kishida M, Hayashi S, Nakamura S, Kamide K, Horio T, Kawano Y. Incidence and risk factors for restenosis, and its impact on blood pressure control after percutaneous transluminal renal angioplasty in hypertensive patients with renal artery stenosis. J Hypertens. 2016;34:1407–15.
Ham SW, Weaver FA. Ex vivo renal artery reconstruction for complex renal artery disease. J Vasc Surg. 2014;60:143–50.
Raju MG, Bajzer CT, Clair DG, Kim ES, Gornik HL. Renal artery stent fracture in patients with fibromuscular dysplasia: a cautionary tale. Circ Cardiovasc Interv. 2013;6:e30–31.
Barrier P, Julien A, Guillaume C, Philippe O, Herve R, Francis J. Technical and clinical results after percutaneous angioplasty in nonmedial fibromuscular dysplasia: outcome after endovascular management of unifocal renal artery stenoses in 30 patients. Cardiovasc Interv Radiol. 2010;33:270–7.
Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. The long-term outcomes of percutaneous therapy for renal artery fibromuscular dysplasia. J Vasc Surg. 2008;48:865–71.
Fujihara M, Fukata M, Higashimori A, Nakamura H, Odashiro K, Yokoi Y. Short- and mid-term results of balloon angioplasty for renal artery fibromuscular dysplasia. Cardiovasc Interv Ther. 2014;29:293–9.
Fyhrquist F, Gronhagen-Riska C, Tikkanen I, Junggren IL. Long-term monotherapy with lisinopril in renovascular hypertension. J Cardiovasc Pharmacol. 1987;9(Suppl 3):S61–65.
Persu A, Touze E, Mousseaux E, Barral X, Joffre F, Plouin PF. Diagnosis and management of fibromuscular dysplasia: an expert consensus. Eur J Clin Invest. 2012;42:338–47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
van Twist, D.J.L., de Leeuw, P.W. & Kroon, A.A. Renal artery fibromuscular dysplasia and its effect on the kidney. Hypertens Res 41, 639–648 (2018). https://doi.org/10.1038/s41440-018-0063-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0063-z
This article is cited by
-
Clinical characteristics and long-term outcomes of endovascular treatment of renal artery fibromuscular dysplasia with branch lesions
Pediatric Nephrology (2021)
-
Renovascular hypertension in pediatric patients: update on diagnosis and management
Pediatric Nephrology (2021)
-
Kidney enlargement effect of angioplasty for nonatherosclerotic renovascular disease: reversibility of ischemic kidney
Hypertension Research (2020)
-
Characterization of adenosine A2 receptors in peripheral blood mononuclear cells of patients with fibromuscular dysplasia
Hypertension Research (2020)