Abstract
Microcirculation influences peripheral vascular resistance and therefore contributes to arterial blood pressure. The aim of this study was to investigate the correlation between serum markers of inflammation and microcirculatory parameters observed by nailfold videocapillaroscopy (NVC) in patients with resistant (RH, 58 [50–63] years, n = 25) or mild-to-moderate hypertension (MMH, 56 [47–64] years, n = 25) compared to normotensive patients (control group (CG), 33 [27–52] years, n = 25). C-reactive protein (CRP), endothelin, adiponectin, I-CAM and V-CAM levels were obtained by laboratory analysis. Functional capillary density (FCD; the number of capillaries with flowing red blood cells by unit tissue area), capillary diameters, maximum red blood cell velocity (RBCVmax) during the reactive hyperemia response/RBCVbaseline after 1 min of arterial occlusion at the finger base and time to reach RBCVmax were determined by NVC. A sub-analysis was also conducted on hypertensive patients not taking statins, with controlled/uncontrolled blood pressure. The RH group showed lower RBCV and RBCVmax values and longer TRBCVmax compared to MMH and CG patients, with worse values in those with uncontrolled blood pressure. FCD and diameters showed no significant differences among the three groups, with higher CRP values in the RH and MMH groups. An increase in endothelin was observed only in patients not taking statins in both hypertensive groups. Patients with severe hypertension and uncontrolled blood pressure levels presented more pronounced microvascular dysfunction, as well as higher serum values for CRP and endothelin (without statin treatment), suggesting that the use of statins decreases endothelin release.
Similar content being viewed by others
Introduction
Worldwide, cardiovascular diseases (CVD) are associated with high cardiovascular mortality and morbidity rates [1, 2] in addition to numerous hospital admissions with high medical, social, and economic costs. Owing to its prevalence, hypertension is one of the main drivers of CVD and damage to target organs, such as the kidneys (renal sclerosis), brain (brain stroke), blood vessels (peripheral vascular diseases), and heart (coronary artery disease) [3]. The global prevalence of hypertension is growing rapidly, and many patients present masked hypertension, including 18% of young sub-Saharan African adults [4].
Hypertension is an important public health problem in Brazil [5], with its prevalence exceeding 30% of the population (approximately 61.4 million persons), of whom only 20% have controlled blood pressure (BP) [3]. In the United States of America, data from the National Health and Nutrition Examination Survey [6] between 2005 and 2008 indicate that 29–31% of adults have hypertension, i.e., approximately 76.4 million US citizens >20 years [7, 8].
Inflammatory markers are emerging as risk factors for potential use in the clinical stratification of CVD to establish prognostic and diagnostic values for atherosclerotic disease [9]. In fact, many cross-sectional studies have shown an association between high levels of inflammation and hypertension [10]. Increasing evidence has also shown an association between hypertension and increased levels of C-reactive protein (CRP), one of the strongest and most reliable markers of vascular inflammation recognized for its participation in the pathophysiology of the disease, especially in resistant (RH) or malignant forms [11].
Microcirculatory alterations are also present in hypertension pathophysiology. In the presence of left ventricle hypertrophy, abnormalities, such as rarefaction, gradual loss of capillaries, and small arterioles, have already been described [12], leading to increased peripheral vascular resistance. This imbalance between angiogenesis and vascular remodeling is characteristic of more advanced forms of the disease [12,13,14,15]. Currently, there is no routine recommendation for the use of inflammatory markers or microcirculatory evaluation in the diagnostic, prognostic assessment, or primary prevention of hypertension. This is likely due to the lack of robust evidence on this matter, although it could be a promising field for future research.
This study aimed to evaluate the behavior of serum inflammatory markers and the microcirculation through nailfold videocapillaroscopy (NVC) in patients with RH, mild-to-moderate hypertension (MMH), and controls with normal BP (control group [CG]).
Methods
This was a cross-sectional study with a convenience sample consisting of patients from the hypertension clinics treated at Policlínica Piquet Carneiro, State University of Rio de Janeiro (UERJ).
All subjects signed the written Informed Consent Form enclosed in the protocol approved by the Hospital Ethics Committee (Policlínica Piquet Carneiro; no. CAAE 40803114.9.0000.5259) from the State University of Rio de Janeiro according to Helsinki Declaration.
The study population comprised 75 patients of both sexes aged >18 years and was stratified into three groups: RH (n = 25), MMH (n = 25), and normotensive controls (n = 25). We included RH patients (uncontrolled hypertension ≥140/90 mm Hg; using three or more antihypertensive drugs of different classes, including diuretics; or controlled hypertension with four or more drugs), MMH patients (hypertensive stages 1 and 2 of the VI Brazilian Guidelines of hypertension, with BP levels ranging from 140/90 mm Hg to 179/109 mm Hg, up to two antihypertensive drugs of different classes, and BP levels controlled in the past 2 months) and normotensive patients (BP < 140/90 mm Hg and no comorbidities).
The following exclusion criteria were adopted as they may cause changes in microcirculation and inflammatory markers: diabetes mellitus type 1 or 2, heart failure, myocardial infarction or stroke within the past 3 months, chronic kidney disease, use of hormonal or non-hormonal anti-inflammatory drugs, recent trauma (in the past 3 months), autoimmune disease, current infection, current neoplasia, aspirin use (anti-inflammatory dose), obesity grade III (body mass index (BMI) > 40 kg/m2), and chronic inflammatory disease.
A full clinical examination was performed, including measurements of at-rest BP, weight and height to calculate BMI, waist and hip measurements, bio-electrical impedance to measure fat and muscle mass percentages, blood draw for laboratory analyses, and NVC examination.
BP was measured in both arms with the patient seated, and the highest value obtained was recorded. High BP (levels ≥140/90 mm Hg) was defined as elevated BP in at least three different appointments during 2-month intervals. All patients underwent ambulatory BP monitoring and were investigated to exclude secondary causes of hypertension.
NVC variables and serum biomarker results were analyzed and compared among groups using two sub-analyses: (1) taking or not taking statins (all three groups); and (2) hypertensive (RH and MMH groups) with controlled/uncontrolled BP.
The following biomarker tests were performed: CRP using the turbidimetry/BioSystems/latex and vascular cell adhesion molecule (VCAM), intercellular cell adhesion molecule (ICAM), adiponectin, and endothelin using a Milliplex Kit (Human Cardiovascular Disease Panel 2 Kit 96-Well Plate Assay, Millipore Company, USA). Laboratory tests were conducted in the same place on a single morning using venous blood draws after an 8-h fast.
Microcirculation assessment by NVC
Patients were accommodated in an acclimatized room with a controlled temperature of 24 ± 1 °C for 20 min before procedures began.
Participants were examined after 8 h of fasting following these rules: (1) not removing cuticles of fingers and not painting nails within 14 days prior to the examination; (2) not smoking within 48 h prior to the examination; (3) not ingesting alcohol or liquids containing caffeine (tea, coffee, mate, guarana, chocolate, soft drinks) on the day of the examination; and (4) not handling paints, detergents, dyes, or solvents at least 7 days before the test.
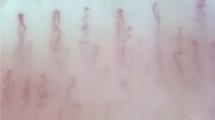
The patient sat comfortably in a chair and placed, at hearing level, the fourth finger of the left hand on the acrylic platform of a Leica MZFLIII stereoscopic microscope (Wetzlar, Germany) equipped with epi-illumination by a 45-degree optical fiber bundle (Leica GLS 100 set), polarized light, and a 100 W mercury vapor lamp coupled with a JVC TK-S250 video camera (Kyoto, Japan), Philips VR 999/78 VCR (São Paulo, Brazil) and Kodo KBM1700E monitor (Seoul, South Korea) for microcirculatory image recordings (Fig. 1a).
a A photo of the nailfold videocapillaroscopy technique and a description of the microcirculatory parameters studied. b Maximum red blood cell velocity (RBCVmax) in nailfold capillaries of control (CG) and hypertensive patients (MMH and RH) with and without statins. One can note the lowest values of maximum RBCV in patients with resistant hypertension. Representative images from the microcirculation (middle of the figure) demonstrate how RBCVmax was calculated. The contour in white shows one capillary analyzed. Plasma gaps are tracked (white circle) over time using software. Numbers 1, 2, and 3 indicate different positions of plasma gaps over time. *p < 0.05 between CG and RH. NA not applicable, FCD functional capillary density, AFD, APD and EFD afferent, apical, and efferent capillary diameters, respectively, TRBCVmax time to reach RBCVmax in seconds
A drop of mineral oil was placed on the nailfold bed to improve image quality. A pressure cuff connected to a mercury manometer was placed on the evaluated finger for functional testing of the microcirculation (reactive hyperemia response). Capillary morphology and functional density (number of capillaries in the microscopic field with flowing red blood cells (RBCs)) were evaluated with 250× magnification. At a magnification of 680×, we obtained the diameters of capillaries (DAFc—afferent, DAPc—apical, and DEFc—efferent; in micrometers), basal RBC velocity (RBCV; in mm/s), maximum RBCV after 1 min of arterial occlusion release in the evaluated finger (RBCVmax; in mm/s) during the reactive hyperemia response, and the time to reach RBCV in seconds (TRBCVmax) (Fig. 1a, b).
Mean values of the variables were obtained in the central, medial, and lateral fields of the nailfold bed to decrease variability. Only RBCVmax and TRBCVmax were obtained from single measurements due to ischemia.
Statistical analysis
Statistical analysis was performed with SAS® System, version 6.11 (SAS Institute, Inc., Cary, NC, USA) to compare numerical clinical, laboratory, and NVC data among the three groups (RH, MMH, and CG) using one-way analysis of variance (ANOVA)/Kruskal–Wallis [16] tests (nonparametric ANOVA), multiple comparison Tukey or Dunn [17] tests (nonparametric), and chi-square or Fisher’s exact test for categorical clinical data. NVC variables were adjusted for age and BMI through analysis of covariance.
Owing to the lack of normal distribution, the data are expressed as the medians and interquartile ranges in the tables, and a 5% level was adopted for significance.
Results
Overall, 75 patients were included. The mean ages were 55.5 ± 10.2 years for RH, 55.0 ± 9.8 years for MMH, and 39.4 ± 15.8 years for CG, and the mean arterial pressures were 147 ± 26/85 ± 15 mm Hg for RH, 131 ± 16/75 ± 12 mm Hg for MMH, and 110 ± 13/67 ± 9 mm Hg for CG.
CG participants had a lower mean age, BMI, waist circumference, waist/hip ratio, and fat mass compared to hypertensive patients (Table 1). No significant differences in these variables were found between the hypertensive groups (RH and MMH).5
All patients used at least an angiotensin II converting enzyme inhibitor, which could have changed their inflammation profiles. However, we could not stop the drug treatment. Other classes of antihypertensive medicaments have been used, such as thiazide diuretics (hydrochlorothiazide or chlorthalidone), calcium channel blockers (nifedipine or amlodipine), beta-blockers (atenolol or metoprolol), hydralazine, and clonidine, but there are no descriptions in the literature of their influence over endothelial function or inflammation. The vast majority of the participants were non-smokers (RH, 88.0%; MMH, 80.0%; and CG, 100%).
CRP showed higher levels in RH and MMH patients, without a significant difference between the two hypertensive groups (RH and MMH). Indeed, there were no other intergroup differences in inflammatory variables and adhesion molecules (Table 2).
The RH group showed lower RBCV and RBCVmax values and prolonged time to reach RBCVmax compared to the CG and MMH groups. There was no significant difference between MMH and CG for these variables. There were no significant differences in functional capillary density (FCD) or capillary diameters (afferent, apical, and efferent) among the groups. The statistical significance persisted after correcting for confounding variables, such as age and BMI (Fig. 2).
A subgroup analysis performed on individuals who were not taking statins (CG, n = 25; MMH, n = 11; and RH, n = 8) revealed an increase of endothelin, which reached higher serum values in the MMH and RH groups compared to the CG group (MMH ≠ CG, p = 0.0001; RH ≠ CG, p = 0.003). CRP was higher in the RH group than in the MMH and CG groups (p = 0.04), and RBCV presented lower values in the RH group than in the CG group through NVC (p = 0.01) (Table 3).
Intragroup analysis regarding the use or non-use of statins in only hypertensive individuals (RH and MMH) showed that endothelin reached higher values, with significant differences between hypertensive individuals who did and did not use statins. In the RH group, the values of endothelin, in pg/dl, were 24.3 [23.3–27.0] without statin (n = 8) and 16.5 [12.3–19.2] with statin, while in the MMH group, the endothelin vales were 26.7 [25.9–38.8] without statin (n = 11) and 13.1 [10.2–17.6] with statin (p < 0.01).
Statin did not interfere with BP in the hypertensive groups. Without statin, the results, in mm Hg, were 130 [118–149] for systolic and 75 [63–88] for diastolic, and the mean BP was 95 [81–107]. With statins, the results were 139 [123–161] for systolic and 78 [74–97] for diastolic, and the mean BP was 102 [89–117] mm Hg (p > 0.05). No other statistically significant differences could be found.
An analysis of BP in each hypertensive group revealed no significant differences. However, when the two groups were evaluated together, higher values for RBCV and RBCVmax and diminished time needed to reach RBCVmax (TRBCVmax) were found in patients with controlled BP (Table 4).
No significant differences were found in serum biomarker levels related to BP control, taken together or in isolation.
Discussion
Inflammation and hypertension cause microcirculatory impairment that worsens with the development of RH [18,19,20,21].
Vascular endothelial function includes inflammation modulation, maintenance of muscular tone, and coagulation control [18,19,20,21,22]. When hypertension damages the endothelium, all vascular-protective properties are lost or altered, and the endothelium becomes pro-constrictive, pro-thrombotic, and anti-fibrinolytic, leading to platelet aggregation, cellular migration, and the proliferation of vascular smooth muscle cells, low-density lipoprotein oxidation, monocyte and platelet adhesion, and inflammatory cytokine synthesis [22]. Vasoconstrictors, such as angiotensin lI, endothelin-1, thromboxane A2, and reactive oxygen species, overlap the effects of vasodilators, such as nitric oxide (NO), endothelium-derived hyperpolarizing factor, kinins, and prostacyclins [18, 23, 24]. Endothelial function is also influenced by genetics. Zhao et al. showed that variation in the methylation of the glucocorticoid receptor gene is associated with endothelial dysfunction, a marker of early atherosclerosis [25]. On the other hand, Tarnoki et al. quantified the contribution of genetics and the environment to BP related to obesity and arterial stiffness. Correlations between BMI and BP components were explained by genetic factors in 65–77% of all cases [26].
Many inflammatory markers, such as CRP, endothelin, cytokines, and adhesion molecules, have been found at high levels in hypertensive patients and seem to be associated with target organ damage and increased risks of future cardiovascular events. In normotensive patients, these markers have been associated with higher risk of future development of hypertension [20, 21, 27, 28]. Among inflammatory markers, CRP is the most widely studied and is generally used in clinical practice. Inflammation level as assessed by plasma CRP concentrations predicts the long-term risk of first myocardial infarction occurrence, ischemic stroke, or peripheral arterial disease [27,28,29,30,31,32] and contributes directly to increased vascular resistance by reducing NO synthesis by endothelial cells [33,34,35].
This study showed significantly higher CRP values (p < 0.05) in hypertensive patients (RH and MMH) compared to normotensive individuals (CG). Factors known to influence inflammation, such as older age, higher mean anthropometric variables (BMI, waist circumference, waist/hip ratio), higher bio-electrical impedance (body fat), and higher BP values, were observed mainly in the RH and MMH groups, reflecting the higher cardiovascular risk profile of such individuals and contributing to changes observed in CRP levels.
However, knowing that inflammation is generally present in hypertensive patients but to a greater extent in those with more severe forms of the disease, significantly higher CRP levels were expected in the RH compared to the MMH group, which was not observed. One possible explanation may be the aggressive treatment consisting of higher doses and greater numbers of antihypertensive drug classes in the RH group, which could attenuate or reduce inflammatory marker expression. All the hypertensive patients (RH and MMH) in this study used some form of a renin–angiotensin–aldosterone blocker, the class with greater potential to interfere with inflammation. It has already been demonstrated that administration of angiotensin II enhances the expression of CD68, a macrophage marker, in the rat hearts, suggesting increased infiltration of macrophages during inflammation in hypertension [36]. Other classes of antihypertensive drugs used by the patients in our study have not been shown to interfere with inflammation. All groups were balanced with regard to antihypertensive use.
No significant intergroup differences were noted in other analyzed inflammatory markers, including adiponectin, ICAM, VCAM, and endothelin, possibly related to drug use by hypertensive patients. In agreement with the findings of Ridker and co-workers [9, 32, 37, 38], this study showed that, despite its low specificity, CRP is an excellent high-sensitivity marker of inflammation in these patients.
Although there is a relationship between hypertension and inflammation, as demonstrated in this study by increased CRP in the RH group, other pathologies may present changes in inflammatory markers and in microcirculation without changing BP levels or atherosclerosis. For example, this may occur in autoimmune diseases, such as systemic lupus erythematosus and antiphospholipid syndrome [39, 40].
It should be noted that many commonly used drugs, such as statins and even antihypertensive drugs, may interfere with inflammatory marker expression [38, 41]. Accordingly, to test the possible effect of a drug known to have pleiotropic effects, we analyzed the data of individuals in the RH and MMH groups who did not use statins. Our findings showed higher endothelin values in this group compared to patients who used statins. The reasons for these findings are unclear but reinforce the notion that inflammatory markers are present in the context of hypertension and are possibly influenced by the drug used [35, 38, 41]. Ungvari and Koller suggested the participation of endothelium-derived endothelin and prostaglandin H2/Thromboxane A2 as factors that increase the Ca2+ sensitivity of arteriolar smooth muscle, thus improving myogenic constriction in hypertension [42, 43].
Microcirculatory changes have also been documented in hypertension, although the mechanisms are not fully understood. These changes may result in increased arteriolar sensitivity to vasoconstrictor substances, reduced endothelium-dependent dilatation, and increased oxidative stress in the vascular endothelium and contribute at least in part to increased systemic vascular resistance, closely linked to increased BP [44,45,46]. In this situation, arterioles contribute to peripheral resistance control by indirectly modulating the narrowing of capillary diameter and decreasing the number of capillaries. On the other hand, RBCV may fluctuate under the influence of peripheral vascular resistance changes [47].
Microcirculatory dysfunction appears to be both the cause and effect of increased arteriolar pressure [44]. Capillary rarefaction and arteriolar remodeling are characteristics of hypertension and have been disclosed by primary hypertension in clinical studies and animal models [44, 48]. We have shown lower RBCV, RBCVmax, and prolonged TRBCVmax values only in RH compared to the MMH and CG groups. On the other hand, MMH did not differ significantly from the CG group. These findings suggest that functional damage of the microcirculation occurs in late-stage disease; in other words, in RH, systemic small arteries and arterioles with myogenic capacity lose the ability to self-regulate and protect the capillary network [48, 49].
It should be noted that, although hypertensive patients (RH and MMH) had a higher mean age, BMI, BP and body fat percentages than CG patients, there was no significant difference between the MMH and CG groups by NVC. When the data were corrected for age and BMI, no significant intergroup differences were observed. Another factor that can influence BP is vascular stiffness, which was not measured in our study, and its increase can lead to higher BP [26]. In future studies, vascular stiffness should be compared to microcirculatory impairment and different categories of high BP to determine how the vascular system in hypertensive patients modifies the microcirculatory environment.
BP control is a very important issue to consider. A meta-analysis of 11 randomized studies showed an absolute cardiovascular risk reduction with BP control and hypertension treatment [50]. Accordingly, adequate BP control could help to reduce microcirculatory damage and favorably influence NVC variables. Individuals with uncontrolled BP (≥140/90 mm Hg) showed lower RBCV values (RBCV and RBCVmax) and prolonged TRBCVmax compared to those with controlled pressure (<140/90 mm Hg) via antihypertensive treatment [14, 51]. Ungvari et al. proposed that the chronic presence of high pressure itself could elicit arterial oxidative stress, primarily by activating protein kinase C-dependent NAD(P)H oxidase pathway, leading to oxidative tissue damage. This finding reinforces the need for rigid BP control [52].
Microcirculatory and inflammatory changes are important components of microvascular dysfunction and contribute significantly to the pathophysiology of hypertension, potentially guiding antihypertensive treatment [44]. Another interesting point of view is modification of the gut microbiome in hypertensive patients. Yan et al. showed higher membrane transport, lipopolysaccharide biosynthesis, and steroid degradation by the gut microbiome of patients with high BP in contrast to healthy controls, where the metabolism of amino acids, cofactors, and vitamins was higher. Furthermore, Klebsiella spp. and Streptococcus spp. were frequently present in hypertensive gut microbiomes, while Roseburia spp. and Faecalibacterium prausnitzii were higher in controls [53]. Although causality between the gut microbiome and hypertension could not be proven, perhaps microbiome alterations could also be involved in inflammation and microcirculation, thus producing hypertension.
Thus the results of this study highlight the need for additional microcirculatory studies to investigate the intima-media thickness and calcium score in individuals with different pressure levels, as well as observations of the moment when dysfunction begins [51]. Perhaps microcirculation should be a new target for hypertension treatment [54].
Study limitations
The following limitations should be noted: a small sample size in the sub-analyses (patients without statin use), although statistical significance was very high (p < 0.001); the need to use and maintain various drugs prescribed by assistant physicians, such as antihypertensives and statins (because they are high cardiovascular risk patients); and the lowest mean patient age was for the CG group due to great difficulty in finding normotensive persons without comorbidities in the age group closest to the hypertensive groups. The difference in age did not affect the microvascular functional evaluation after multivariate analysis.
Conclusion
Patients with more severe forms of hypertension without BP control showed greater functional microvascular damage on NVC, as well as higher serum levels of CRP. Higher levels of endothelin were found in patients without statin use, which suggests that this drug might decrease endothelin release.
References
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71.
Marczak L, O’Rourke K, Shepard D, Leach-Kemon K, Institute for Health Metrics and Evaluation. Firearm deaths in the United States and globally, 1990-2015. JAMA. 2016;316:2347
Sociedade Brasileira de C, Sociedade Brasileira de H, Sociedade Brasileira, de N. [VI Brazilian Guidelines on Hypertension]. Arq Bras Cardiol. 2010;95(1 Suppl):1–51.
Thompson JE, Smith W, Ware LJ, Mels CMC, van Rooyen JM, Huisman HW, Malan L, Malan NT, Lammertyn L, Schutte AE. Masked hypertension and its associated cardiovascular risk in young individuals: the African-PREDICT study. Hypertens Res. 2016;39:158–65.
Ribeiro AL, Duncan BB, Brant LC, Lotufo PA, Mill JG, Barreto SM. Cardiovascular Health in Brazil: Trends and Perspectives. Circulation. 2016;133:422–33.
National Center for Health Statistics. NHANES 2007-2008 public data general release file documentation. 2015. http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/generaldoc_e.html. Accessed 04 Jul 2015.
Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–50.
Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001-2008. Natl Health Stat Report. 2011;1–22, 24.
Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM Jr., Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65.
Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M. Hypertension as an autoimmune and inflammatory disease. Hypertens Res. 2016;39:567–73.
Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–40.
Battegay EJ, de Miguel LS, Petrimpol M, Humar R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr Opin Pharmacol. 2007;7:151–7.
Hoefer IE, den Adel B, Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res. 2013;99:276–83.
Penna GL, Garbero Rde F, Neves MF, Oigman W, Bottino DA, Bouskela E. Treatment of essential hypertension does not normalize capillary rarefaction. Clin (Sao Paulo). 2008;63:613–8.
Triantafyllou A, Anyfanti P, Pyrpasopoulou A, Triantafyllou G, Aslanidis S, Douma S. Capillary rarefaction as an index for the microvascular assessment of hypertensive patients. Curr Hypertens Rep. 2015;17:33.
Hollander M, Wolfe DA, Chicken, E. Nonparametric statistical methods. 2nd ed. New York: John Wiley & Sons; 1999.
Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6:12.
Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138(5 Pt 2):S419–20.
Vicaut E. Hypertension and the microcirculation. Arch Mal Coeur Vaiss. 2003;96:893–903.
Tsounis D, Bouras G, Giannopoulos G, Papadimitriou C, Alexopoulos D, Deftereos S. Inflammation markers in essential hypertension. Med Chem. 2014;10:672–81.
Stefanadi E, Tousoulis D, Androulakis ES, Papageorgiou N, Charakida M, Siasos G, Tsioufis C, Stefanadis C. Inflammatory markers in essential hypertension: potential clinical implications. Curr Vasc Pharmacol. 2010;8:509–16.
Kalinowski L, Malinski T. Endothelial NADH/NADPH-dependent enzymatic sources of superoxide production: relationship to endothelial dysfunction. Acta Biochim Pol. 2004;51:459–69.
Widlansky ME, Gokce N, Keaney JF Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60.
Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–9.
Zhao J, An Q, Goldberg J, Quyyumi AA, Vaccarino V. Promoter methylation of glucocorticoid receptor gene is associated with subclinical atherosclerosis: a monozygotic twin study. Atherosclerosis. 2015;242:71–6.
Tarnoki AD, Tarnoki DL, Bogl LH, Medda E, Fagnani C, Nistico L, Stazi MA, Brescianini S, Lucatelli P, Boatta E, Zini C, Fanelli F, Baracchini C, Meneghetti G, Osztovits J, Jermendy G, Kiss RG, Preda I, Karlinger K, Lannert A, Molnar AA, Littvay L, Garami Z, Berczi V, Pucci G, Baffy G, Schillaci G, Pietilainen KH. Association of body mass index with arterial stiffness and blood pressure components: a twin study. Atherosclerosis. 2013;229:388–95.
Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97.
Davis SK, Gebreab SY, Xu R, Riestra P, Khan RJ, Sumner AE, Hickson D, Bidulescu A. Association of adiponectin with type 2 diabetes and hypertension in African American men and women: the Jackson Heart Study. BMC Cardiovasc Disord. 2015;15:13.
Gonzalez J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: New insights. World J Cardiol. 2014;6:353–66.
Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40.
Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11.
Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL, Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42.
Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–51.
Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–41.
Barbaro NR, Fontana V, Modolo R, De Faria AP, Sabbatini AR, Fonseca FH, Anhe GF, Moreno H. Increased arterial stiffness in resistant hypertension is associated with inflammatory biomarkers. Blood Press. 2015;24:7–13.
Osada-Oka M, Shiota M, Izumi Y, Nishiyama M, Tanaka M, Yamaguchi T, Sakurai E, Miura K, Iwao H. Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypertens Res. 2017;40:353–60.
Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, Group JS. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207.
Dancour MA, Vaz JL, Bottino DA, Bouskela E. Nailfold videocapillaroscopy in patients with systemic lupus erythematosus. Rheumatol Int. 2006;26:633–7.
Vaz JL, Dancour MA, Bottino DA, Bouskela E. Nailfold videocapillaroscopy in primary antiphospholipid syndrome (PAPS). Rheumatol (Oxf). 2004;43:1025–7.
Sahebkar A, Kotani K, Serban C, Ursoniu S, Mikhailidis DP, Jones SR, Ray KK, Blaha MJ, Rysz J, Toth PP, Muntner P, Lip GY, Banach M, Lipid, Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Statin therapy reduces plasma endothelin-1 concentrations: a meta-analysis of 15 randomized controlled trials. Atherosclerosis. 2015;241:433–42.
Huang A, Koller A. Endothelin and prostaglandin H2 enhance arteriolar myogenic tone in hypertension. Hypertension. 1997;30:1210–5.
Ungvari Z, Koller A. Endothelin and prostaglandin H(2)/thromboxane A(2) enhance myogenic constriction in hypertension by increasing Ca(2+) sensitivity of arteriolar smooth muscle. Hypertension. 2000;36:856–61.
Struijker-Boudier HA, Rosei AE, Bruneval P, Camici PG, Christ F, Henrion D, Levy BI, Pries A, Vanoverschelde JL. Evaluation of the microcirculation in hypertension and cardiovascular disease. Eur Heart J. 2007;28:2834–40.
Agabiti-Rosei E. [Structural and functional changes of the microcirculation in hypertension: influence of pharmacological therapy]. Drugs. 2003;63(Spec No 1):19–29.
Struijker Boudier HA, le Noble JL, Messing MW, Huijberts MS, le Noble FA, van Essen H. The microcirculation and hypertension. J Hypertens Suppl. 1992;10:S147–56.
Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33:998–1001.
Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens. 2001;19:921–30.
Roman RJ, Van Dokkum RP. Commentary on the special issue on the impact of myogenic tone in health and disease. Curr Vasc Pharmacol. 2014;12:779.
Blood Pressure Lowering Treatment Trialists C, Sundstrom J, Arima H, Woodward M, Jackson R, Karmali K, Lloyd-Jones D, Baigent C, Emberson J, Rahimi K, MacMahon S, Patel A, Perkovic V, Turnbull F, Neal B. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–8.
Martina B, Frach B, Surber C, Drewe J, Battegay E, Gasser P. Capillary blood cell velocity in finger nailfold: effect of enalapril and mibefradil in patients with mild to moderate hypertension. Microvasc Res. 1999;57:94–99.
Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol. 2004;165:219–26.
Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, Zhou J, Guan X, Ding Y, Wang S, Khan M, Xin Y, Li S, Ma Y. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017;24:1–9.
Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Junqueira, C.L.C., Magalhães, M.E.C., Brandão, A.A. et al. Microcirculation and biomarkers in patients with resistant or mild-to-moderate hypertension: a cross-sectional study. Hypertens Res 41, 515–523 (2018). https://doi.org/10.1038/s41440-018-0043-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0043-3
This article is cited by
-
Evaluation of endothelial dysfunction in hypertensive children and adolescents
Pediatric Nephrology (2024)
-
Association of serum 25-hydroxyvitamin D levels with primary hypertension: a study from south India
Hypertension Research (2020)
-
Analysis of Endothelin-1 Concentrations in Individuals with Periodontitis
Scientific Reports (2020)
-
Red blood cell abnormalities and hypertension
Hypertension Research (2020)
-
Comparisons of skin microvascular changes in patients with primary aldosteronism and essential hypertension
Hypertension Research (2020)