Abstract
Pathogenic variants of HECW2 have been reported in cases of neurodevelopmental disorder with hypotonia, seizures, and absent language (NDHSAL; OMIM #617268). A novel HECW2 variant (NM_001348768.2:c.4343 T > C,p.Leu1448Ser) was identified in an NDHSAL infant with severe cardiac comorbidities. The patient presented with fetal tachyarrhythmia and hydrops and was postnatally diagnosed with long QT syndrome. This study provides evidence that HECW2 pathogenic variants can cause long QT syndrome along with neurodevelopmental disorders.
Similar content being viewed by others
Congenital long QT syndrome (LQTS) is a genetic disorder characterized by propensity for life-threatening cardiac arrhythmias that affects 1 in 2000 infants1. It is caused by pathogenic variants most commonly involving potassium and sodium ion channels and can be inherited or caused by a de novo variant2. To date, pathogenic variants in 17 different genes have been reported in LQTS; however, over 90% of variant-positive LQTS cases can be accounted for by three major LQTS genes—KCNQ1, KCNH2, and SCN5A2. A causal variant is found in approximately 75% of patients with LQTS with a definite phenotype; however, the genetic background of the remaining 25% of patients is unknown3. Here, using whole exome sequencing and variant filtering analysis, we identified a de novo heterozygous missense variant in HECW2 in an infant with congenital long QT syndrome who had no pathogenic variant in the known LQTS-associated genes. HECW2 encodes an E3 ubiquitin-protein ligase that stabilizes and enhances the transcriptional activity of p73, a key regulator of cell proliferation and apoptosis4. The encoded protein is highly expressed in the brain, lung, and heart tissue4. Recently, HECW2 variants have been reported to cause neurodevelopmental disorder with hypotonia, seizures, and absent language (NDHSAL; OMIM #617268). Of the 37 cases of NDHSAL reported to date, only 4 had cardiac comorbidities (Supplementary Table 1)5,6. This study reports an additional case of HECW2-related disorder with cardiac involvement and provides further evidence that HECW2 pathogenic variants can cause LQTS along with neurodevelopmental disorders.
The patient, now aged 2 years, is the first child of healthy, non-consanguineous Japanese parents. The mother was referred at 26 weeks of gestation for fetal echocardiography as tachycardia was discovered during an incidental ultrasound examination. M-mode echocardiography revealed intermittent tachycardia with a heart rate of 190 beats per minute (Fig. 1). Supraventricular tachycardia was suspected, and the fetal heart rate was closely monitored. Ultrasound examination at 27 weeks of gestation revealed fetal hydrops, most likely resulting from tachycardia. After confirming that the maternal electrocardiogram (ECG) was normal, trans-maternal treatment was initiated with digoxin followed by sotalol. However, the fetus remained in intermittent tachycardia. A 1689 g female neonate was delivered by cesarean section at 33 weeks of gestation due to maternal drug intolerance. A postnatal ECG demonstrated prolongation of the corrected QT interval (QTc [Fridericia], 682 msec) and torsade de pointes (Fig. 2A), which led to a diagnosis of congenital LQTS.
The patient was started on a regimen of magnesium sulfate and landiolol, which eliminated the torsade de pointes. Thereafter, treatment with mexiletine and propranolol gradually shortened the QT interval and effectively prevented ventricular tachycardia (Fig. 2B). The patient is still on medical therapy at 2 years of age and remains free of cardiac events. Neurological examinations revealed muscle hypotonia. At 3 months of age, the patient developed epileptic spasms. The interictal electroencephalography (EEG) results indicated hypsarrhythmia. The epileptic seizures were refractory to anti-seizure medication. Psychomotor development was severely delayed with no head control, eye pursuit, or speech sounds. The patient was fed via a gastrostomy tube due to feeding problems. Brain magnetic resonance imaging scans performed at 5 months of age showed no abnormal findings (Supplementary Fig. 1). These clinical features including hypotonia, seizures, and profound developmental delay were compatible with those of NDHSAL.
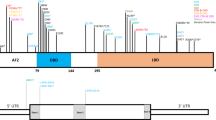
To reveal the underlying genetic etiology, the genomic DNA of the patient and the patient’s parents was extracted from the peripheral blood after obtaining written informed consent. This study was approved by the Asahikawa Medical University Research Ethics Committee (approval number 17112). Genetic testing for the known LQTS-associated genes identified no pathogenic variants in this patient7. Next, whole exome sequencing and variant filtering analysis were performed on the genes of the patient-parent trio, which identified a de novo heterozygous missense variant in HECW2, NM_001348768.2:c.4343 T > C,p.(Leu1448Ser). This missense variant was located in the C-terminal HECT domain of HECW2 and has been reported in another patient with NDHSAL in ClinVar (VCV001320205.1). A different missense change at the same codon (p.Leu1448Trp) has also been reported as likely pathogenic (ClinVar ID: VCV000801845.1)8. This missense variant was absent from the controls in public databases, such as the Exome Variant Server (http://evs.gs.washington.edu/EVS/), dbSNP (http://www.ncbi.nlm.nih.gov/snp/), and Genome Aggregation Database (gnomAD, https://gnomad.broadinstitute.org/). It was predicted to be damaged by in silico tools including SIFT, PolyPhen-2, and CADD (score 29.6). According to the American College of Medical Genetics and Genomics guidelines9, the variant was classified as pathogenic (PS2, PM1, PM2, PM5, PP3, and PP4).
In the present case, LQTS was diagnosed based on a prolonged QT interval and history of torsade de pointes. The patient harbored no pathogenic variants in the known LQTS-associated genes, but a pathogenic variant in HECW2. Cardiac comorbidities were reported in four cases of HECW2-related disorders (Supplementary Table 1). The first reported case of HECW2-related disorder with LQTS had prenatal-onset cardiomyopathy and harbored the heterozygous missense variant in EIF2B2, p.(Gly200Val), in addition to HECW2 variant, NM_001348768.2:c.4334 A > G,p.(Glu1445Gly)5. Recessive variants in EIF2B2 cause early onset vanishing white matter disease; however, heterozygous carriers of EIF2B2 variants are asymptomatic10. Thus, the cardiac phenotype of the patient was considered to be due to HECW2 variant. This is the second case that considers LQTS as a possible phenotype of a HECW2 variant. Ubiquitylation has been shown to be implicated in the regulation of cardiac ion channels11,12. HECW2 is a member of the neuronal precursor cell-expressed developmentally down-regulated protein 4 (Nedd4) family of E3 ubiquitin ligases, which comprises nine members, including Nedd4-2. In particular, Nedd4-2 has been shown to regulate cell surface expression of cardiac voltage-gated sodium channel Nav1.5 through post-translational modification11. Nedd4-2 deficiency increases the cell surface expression of Nav1.5, resulting in cardiac proarrhythmic changes such as a prolonged QT interval12. LQTS patients with gain-of-function mutations of SCN5A, the gene encoding Nav1.5, have been reported to benefit from sodium channel blockers13. In the present case with HECW2 variant, the use of mexiletine, a voltage-gated sodium channel blocker, in combination with a beta-blocker was effective in shortening the QT interval and suppressing cardiac events. Both HECW2 and Nedd4-2 are highly expressed in the heart tissue. Thus, future studies are required to elucidate the cardiac targets of HECW2. In conclusion, this study reports an additional case of a HECW2-related disorder with cardiac involvement and provides further evidence that the clinical manifestations of this disorder are not confined to the central nervous system.
HGV Database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at https://doi.org/10.6084/m9.figshare.hgv.3305.
References
Schwartz, P. J. et al. Prevalence of the congenital long-QT syndrome. Circulation 120, 1761–1767 (2009).
Mizusawa, Y., Horie, M. & Wilde, A. A. Genetic and clinical advances in congenital long QT syndrome. Circ. J. 78, 2827–2833 (2014).
Tester, D. J., Will, M. L., Haglund, C. M. & Ackerman, M. J. Effect of clinical phenotype on yield of long QT syndrome genetic testing. J. Am. Coll. Cardiol. 47, 764–768 (2006).
Miyazaki, K. et al. A novel HECT-type E3 ubiquitin ligase, NEDL2, stabilizes p73 and enhances its transcriptional activity. Biochem Biophys. Res. Commun. 308, 106–113 (2003).
Berko, E. R. et al. De novo missense variants in HECW2 are associated with neurodevelopmental delay and hypotonia. J. Med. Genet. 54, 84–86 (2017).
Acharya, A. et al. Delineating the genotypic and phenotypic spectrum of HECW2-related neurodevelopmental disorders. J. Med. Genet. 59, 669–677 (2022).
Ohno, S., Ozawa, J., Fukuyama, M., Makiyama, T. & Horie, M. An NGS-based genotyping in LQTS; minor genes are no longer minor. J. Hum. Genet. 65, 1083–1091 (2020).
Yanagishita, T. et al. HECW2-related disorder in four Japanese patients. Am. J. Med. Genet. A 185, 2895–2902 (2021).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Leegwater, P. A. et al. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat. Genet. 29, 383–388 (2001).
van Bemmelen, M. X. et al. Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4-2 mediated ubiquitination. Circ. Res. 95, 284–291 (2004).
Minegishi, S. et al. An isoform of Nedd4-2 plays a pivotal role in electrophysiological cardiac abnormalities. Int J. Mol. Sci. 18, 1268 (2017).
Li, G. & Zhang, L. The role of mexiletine in the management of long QT syndrome. J. Electrocardiol. 51, 1061–1065 (2018).
Acknowledgements
This study was supported in part by a grant from the Initiative on Rare and Undiagnosed Diseases (IRUD) project of the Japan Agency for Medical Research and Development (AMED). We thank the patient and the patient’s parents for their cooperation in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imanishi, R., Nakau, K., Shimada, S. et al. A novel HECW2 variant in an infant with congenital long QT syndrome. Hum Genome Var 10, 17 (2023). https://doi.org/10.1038/s41439-023-00245-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41439-023-00245-w