Abstract
Interspecific hybridization has varied consequences for offspring fitness, with implications for the maintenance of species integrity. Hybrid vigour, when it occurs, can peak in first-generation (F1) hybrids and then decline in advanced-generation (F2+) hybrids. This hybrid breakdown, together with the processes affecting patterns of hybridization and hybrid fitness, determine the evolutionary stability of hybrid zones. An extensive hybrid zone in North America involving the cattails Typha latifolia, T. angustifolia, and their invasive hybrid T. × glauca is characterized by hybrid vigour among F1s, but the fitness of advanced-generation hybrids has not been studied. We compared seed germination and plant growth of T. latifolia (parental L), F1 T. × glauca (F1), hybrid backcrosses to T. angustifolia (bcA) and T. latifolia (bcL), and advanced-generation (F2) hybrids. Consistent with expectations under hybrid breakdown, we found reduced plant growth for F2 hybrids in comparison with F1s (plant height and above-ground biomass) and parental Ls (above-ground biomass). Backcrossed hybrids had intermediate measures of plant growth and bcLs were characterized by reduced seed germination in comparison with parental Ls. Hybrid breakdown could make the formation of F1s in North America finite because (1) hybridization among cattails is asymmetric, with T. angustifolia but not T. latifolia subject to genetic swamping, and (2) T. angustifolia is less common and subject to competitive displacement by F1s. Hybrid breakdown is therefore expected to reduce hybrid frequencies over time, contributing to the long-term maintenance of T. latifolia – the only native cattail in the study region.
Similar content being viewed by others
Introduction
The fitness of hybrids has important consequences for the ecology and evolution of plant populations. Hybrid sterility or inviability can lead to steep geographic clines in species distributions (Barton and Hewitt 1985; Burton et al. 2013; Curry 2015; Bersweden et al. 2021) and the maintenance of species barriers (Orr 1995; Abbott 2017; Irwin 2020). Alternatively, fertile hybrids can obscure species boundaries via gene flow and introgression (Abbott et al. 2013). Hybridization can also be a source of adaptive (Barton 2008) or otherwise novel phenotypes, and can thereby facilitate speciation (Rieseberg 2006). Many hybridization events yield outcomes that fall somewhere between these extremes, and combinations of hybrid vigour with hybrid breakdown—the reduction in hybrid fitness among recombinant hybrids (e.g., advanced-generation hybrids including F2 hybrids; e.g., Johansen‐Morris and Latta 2006)—can lead to evolutionarily dynamic hybrid zones (Barton 2001; Buggs 2007; Runemark et al. 2019). Hybridization is widely recognized as an important evolutionary process (Stebbins 1959; Buggs 2007; Runemark et al. 2019), and recent genetic studies have underscored the range of outcomes that are possible in plant hybrid zones (Rendón-Anaya et al. 2021; Zalmat et al. 2021; Durán-Castillo et al. 2022; Mitchell et al. 2022).
In North America, and around the Laurentian Great Lakes (LGL) in particular, there is a geographically extensive hybrid zone involving the broadly distributed wetland plants—the cattails (Typha spp.). In this region, F1 hybrids (Typha × glauca Godr.) are formed asymmetrically following the pollination of narrow-leaf cattail (T. angustifolia L.) by the native broadleaf cattail (T. latifolia L.); T. angustifolia in most instances is the maternal parent (Pieper et al. 2017). All three taxa are clonal (rhizomatous), emergent aquatic perennials common to a variety of wetland habitats, including natural marshes and constructed wetlands such as road-side ditches. In the LGL F1 hybrids are considered invasive (Bansal et al. 2019), and characterized by hybrid vigour for vegetative traits (Bunbury-Blanchette et al. 2015; Zapfe and Freeland 2015) but also by partial sterility (Pieper et al. 2017). All three taxa (the two parental species and their hybrid) are highly abundant within the LGL, have overlapping ranges (Grace and Harrison 1986) and often co-occur within the same wetland without spatial segregation (McKenzie‐Gopsill et al. 2012; Pieper et al. 2018). The hybrid zone in the LGL region appears to have a relatively recent origin; molecular genetic evidence indicates that T. angustifolia was introduced to North America from Europe following European settlement (Ciotir et al. 2013). Population-genetic analyses indicate that the hybrid zone in the LGL region is overall dominated by F1 hybrids and T. latifolia, and that T. angustifolia, advanced-generation hybrids, and introgressed genotypes (i.e., back-crossed hybrids) occur at lower frequencies (Travis et al. 2011; Freeland et al. 2013; Pieper et al. 2020). Frequencies of F1, advanced-generation, and introgressed genotypes vary across regions of contact between T. angustifolia and T. latifolia in North America (Kirk et al. 2011; Freeland et al. 2013; Pieper et al. 2020; Tangen et al. 2021) despite high levels of gene flow among populations for both progenitor species (Pieper et al. 2020). Moreover, high levels of clonal diversity indicate frequent sexual recruitment and ongoing hybrid formation within the LGL hybrid zone (McKenzie‐Gopsill et al. 2012; Pieper et al. 2020). Recurrent hybrid formation, regional variation in hybrid frequencies, and strongly asymmetric hybridization that can lead to the genetic swamping of T. angustifolia but not T. latifolia (Pieper et al. 2017) collectively indicate that this hybrid zone is evolutionarily dynamic. Ultimately, these dynamics will be affected by the fitness of advanced generation and introgressed plants: selection against these plants might stabilize the hybrid zone (Barton 2008), but if these plants have similar or greater fitness than parental cattails, species boundaries may be eroded via introgression and (e.g., Behm et al. 2010). To date, the fitness of advanced generation and introgressed plants has not been assessed.
Characterizing hybrid zones facilitates comparison with other hybrid zones and can provide insights into their evolutionary stability (Curry 2015). However, the cattail hybrid zone is not easily classified and has features associated with different types of hybrid zones (Barton and Hewitt 1985; Curry 2015). The two parental species have broadly overlapping, circumpolar distributions and therefore the geographic clines in gene frequencies often associated with hybrid zones are not expected. Within the LGL region, F1 hybrids appear to benefit from heterosis (Bunbury-Blanchette et al. 2015), but in other parts of North America there may be selection against F1 hybrids (Tisshaw et al. 2020). This pattern could be consistent with some degree of bounded hybrid superiority (Abbott and Brennan 2014). The hybrid zone in the LGL can also be superficially be characterized as a mosaic. Hybrids are common in the LGL region, gene frequencies vary across wetlands, and hybrids are rare-to-absent in other regions of sympatry (Zhou et al. 2016; Ciotir et al. 2017). However, mosaic zones are typically characterized by environmentally-dependent selection against hybrids and F1 hybrids appear to be favoured in all environments across the entire LGL region (Bansal et al. 2019 and references therein). Moreover, in areas where introgression occurs, hybrid frequencies can be difficult to determine without the assistance of genetic markers. As a result, hybrid frequencies have been estimated in an insufficient number of sites to fully characterize the hybrid zone as a mosaic (Travis et al. 2010; McKenzie‐Gopsill et al. 2012; Pieper et al. 2020). Finally, there appears to be a paucity of F2s and introgressed hybrids (jointly referred to here as F2+; Travis et al. 2010; McKenzie‐Gopsill et al. 2012; Freeland et al. 2013; Pieper et al. 2020), which might indicate selection against advanced-generation hybrids. If so, dynamics typically associated with tension zones, within which gene frequencies are determined by a balance between selection and dispersal, might also apply (Barton and Hewitt 1985). Ultimately, the stability of the hybrid zone, and the persistence of invasive F1 genotypes, will depend on the occurrence of selection against advanced-generation hybrids.
Here, we investigated intrinsic selection against advanced-generation cattail hybrids by hand-crossing known parental and hybrid genotypes from the LGL region and growing the progeny under standardized conditions. We measured seed set and germination rates for each cross, and then transplanted seeds and compared patterns of seedling growth among cross types. We hypothesized that the apparently low frequency of F2 + hybrids is explained by selection against these plants. Accordingly, we predicted that F2 + hybrids have lower fitness than de novo F1 hybrids. Additionally, because we were also interested in processes that might contribute to the maintenance of native cattails, which remain abundant in the LGL region, we compared seed-set, germination, and plant growth between F2 + hybrids and T. latifolia.
Methods
Site selection
Sampling, hand crosses, and seed collection took place between June and October 2019. Cattails from multiple wetland types in and around Peterborough ON, Canada, were used as parents. Sites included roadside ditches, the edges of lakes and rivers, and municipal storm ponds (Supplementary Materials Table S1 and Fig. S1). Cattails at these sites grew in variable water depths, with some growing at depths > 1 m, however, for logistical reasons only plants growing in accessible areas in water depths < 60 cm were used as parents for our crosses.
Plant identification
Cattails were provisionally identified to taxon in the field using morphological characters (leaf width, plant height and the gap between the male and female florets; Smith 1967). However, morphological characteristics cannot differentiate F1, advanced-generation (AGH), and introgressed (back-crossed hybrids, BCH). We therefore collected leaf samples from all maternal plants and from all T. × glauca pollen donors for taxonomic verification of parent species and F1 hybrids via genotyping (Kirk et al. 2011). Parental T. angustifolia and T. latifolia can be identified by examining pollen characteristics: T. angustifolia has monad pollen, T. latifolia has tetrad pollen, and hybrids (F1s, AGH, and BCH) have variable pollen that is a mix of monads, dyads, triads, tetrads (Smith 1967). Pollen was examined in the field by removing part of the male segment of an inflorescence, immersing its pollen in water and examining it at 100× magnification using a Wild M11\111 compound microscope (Wild, Heerbrugg, Switzerland). Leaf samples were collected by removing a 12–15 cm segment from the youngest leaf of each plant, and placing it into a coin envelope. Leaf samples were desiccated by storing them in re-sealable bags containing Sorbead orange silica beads (eCompressedair, Oklahoma).
Hand crosses
Cattails are protogynous and monoecious, with distinct female (proximal) and male (distal) inflorescence segments, sometimes with a gap between the female and male segments (as for T. angustifolia and some hybrid genotypes). Cattails within sites were monitored daily to determine when female florets had started to mature but male florets remained immature. Maternal plants were selected prior to pollen dehiscence but after the male segment of the inflorescence had completely emerged from the sheath (a gap between female and male inflorescence segments can assist with taxonomic identification; Smith 1967). Once maternal plants were selected, the male inflorescence segment was removed, and the remaining female segment was covered with a Canvasback® (Seedburo, Des Plaines, IL) pollination bag to prevent pollen contamination.
To avoid crossing plants from the same clone, intra-taxon crosses involved pairs of plants from different sites (some inter-taxon crosses involved plants from the same site; Supplementary Materials Table S2). There was one exception to this rule involving site 11, and for this cross parents were located more than 3 m apart. To reduce the probability that different seed families shared the same maternal parent (i.e., to avoid using replicate shoots from the same clone in our crosses), maternal parents within each site were spaced a minimum of 2 m from other plants of the same taxon. We used a maximum of 10 maternal plants for each inter-taxon cross-type from a single site.
Daily monitoring of sites continued so that the maturity of male florets could be tracked. Pollen was considered ready to collect when florets appeared bright yellow and pollen was dehiscing. Pollen was collected by removing the male segment of an inflorescence and shaking pollen into a 9.5 × 11 glassine envelope. The envelope was then labeled, sealed with tape, packed individually into a re-sealable bag, and stored in a refrigerator for up to 14 days. Cattail pollen maintains approximately 90% viability when stored for up to 50 days in a refrigerator (Buitink et al. 1998).
Pollinations were conducted using clean, 1-inch angular slash paint brushes. Brushes were sterilized the day before pollination by washing them in undiluted bleach, rinsed in hot tap water, and left to dry overnight. Each hand-pollination was conducted on maternal plants that had been bagged for 7–8 days. Pollen was applied using a brush saturated with pollen from a single donor by brushing the length of the inflorescence, re-coating the brush with pollen and repeating the application until female florets were evenly covered with pollen. After pollination, the inflorescence was re-covered with the pollination bag, with the opening stapled shut to prevent the wind from dislodging the bag. Pollination bags were removed approximately 2 weeks after hand-pollination and after all Typha at the site had completed their male phase.
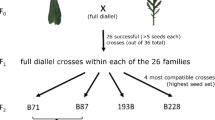
Pollinations yielded the following types of offspring: T. latifolia (L from Lm × Lp crosses, where m and p refer to the maternal and paternal parent, respectively), F1 T. × glauca (F1 from Am × Lp crosses), F2s (F2 from F1m × F1p crosses), backcrosses to T. latifolia (bcL from F1m × Lp crosses) and backcrosses to T. angustifolia (bcA from F1m × Ap crosses). Cross-types included within-taxon crosses (generating L and F2 offspring) and between-taxon crosses (generating F1, bcL, and bcA offspring). All BCH were generated using F1 T × glauca as the maternal parent, as a previous study demonstrated that F1s are receptive to pollen from both parental species but T. latifolia is not receptive to F1 pollen (Pieper et al. 2017). In total, we obtained 38–41 replicate crosses for each cross-type (Supplementary Materials Table S2).
Genotyping
Leaf samples were stored in Sorbead silica beads for a minimum of 72 h prior to genotyping. From each sample approximately 2 cm2 (~10–50 mg) of desiccated leaf tissue was ground into a semi-fine powder using a Retsch® MM300 mixer mill (Haan, Germany). DNA was extracted from the sample using EZNA extraction kits (Omega Bio-Tek, Inc., Georgia USA) following the manufacturer’s protocol for extracting from dried leaf samples, and eluted into 100 µL of buffer. Extracted DNA was then stored in the freezer (−18 °C).
DNA was amplified at four microsatellite loci (TA3, TA5, TA8, and TA20; Tsyusko‐Omeltchenko et al. 2003). The alleles at these loci are species-specific for Typha latifolia and T. angustifolia (Snow et al. 2010; Kirk et al. 2011). The forward primers were fluorescently labeled with hex (TA3, TA5) or fam (TA8, TA20). Loci TA3, TA8, and TA20 were amplified together in a multiplex reaction, while the TA5 locus was amplified separately; all reactions used Type It Microsatellite PCR Kits (Qiagen). Each multiplex reaction contained 5.5 uL of 1× Master Mix, either 0.2 µM (TA3 and TA8) or 0.1 uM (TA20) of primers, 2.95 µL of ddH2O, and 2 µL DNA, in a total volume of 11 µL. The TA5 amplifications included 1× Master Mix (5.5 µL), 0.2 µM of each primer, 3.28 µL ddH2O, and 2 µL DNA, in a total volume of 11 µL.
DNA was amplified using a Mastercycler thermocycler (Eppendorf). The multiplex reaction began with a 95 °C denaturation for 5 min, followed by 35 cycles of 30 s denaturation at 95 °C, 90 s annealing at 57 °C, and 30 s extension at 72 °C, with a final extension for 30 m at 60 °C. The TA5 amplifications followed the same cycling parameters, but with an annealing temperature of 51 °C. A subset of PCR amplifications was visualized using gel electrophoresis (1.5% agarose gel and 1× TBE buffer), with a 100 BP DNA Ladder (ThermoFisher Scientific) as a size standard. Multiplexed amplifications were then diluted with nuclease-free deionised water using a 1:20 ratio, while single amplifications were diluted using a 1:25 ratio. Each diluted PCR sample was added to a 9 µL HiDi/ROX-500 (Applied Biosystems) solution, made by adding 4 µL of ROX to 1 mL of HiDi® (myPOLS). Dilutions mixed with the HiDi/Rox solutions were submitted to the Natural Resources DNA Profiling and Forensic Centre in Trent University for genotyping on a 3730 DNA Analyzer (Applied Biosystems). DNA fragments were then sized using GeneMarker® v. 1.91 (Soft-Genetics).
Plants with only T. latifolia or T. angustifolia alleles were classified as T. latifolia or T. angustifolia respectively. Plants heterozygous for T. latifolia and T. angustifolia alleles at all four loci were classified as F1 T. × glauca. Plants that were heterozygous for T. latifolia and T. angustifolia alleles at some but not all loci were classified as F2 + (AGH or BCH). Crosses involving F2 + plants were omitted from the experiment, but with only four loci there is a possibility that some F2 + were incorrectly identified as F1s, T. latifolia, or T. angustifolia.
Seed set and germination
In early autumn sites were monitored regularly to track fruit maturity. Mature fruits were placed into 13 × 7.9 × 27 cm paper bags and dried at room temperature for a minimum of 7 days. Achenes were then separated from the inflorescence stalk, placed into re-sealable plastic bags, and stored in a fridge at 4 °C. Seeds were separated from their achenes over a period of 10 days in February 2020 following protocols outlined in Ahee et al. (2015). Briefly, approximately 0.5 g of fruit from each of 194 maternal plants was weighed to the nearest mg using an XP105 DeltaRange weighing scale (Mettler Toledo, Ohio, USA) and placed into a blender filled with a soap and water solution. The solution was blended for 1 min to separate the seeds from other materials, including empty and otherwise inviable seeds. Seeds were then rinsed with tap water and transferred to a single, labelled petri dish filled with deionized (DI) water. Between 16 and 20 petri dishes were prepared per day, and the order in which different cross-types were processed was randomized each day.
The viability of seeds produced from each cross was evaluated using the index of seed-set developed by Ahee et al. (2015), in which seed-set is estimated as the number of seeds produced per gram of fruit tissue. Seed-set was counted using a macro script run using ImageJ software (Rasband 2014; for details see Ahee 2013). To estimate germination rate, processed seeds were kept in covered petri dishes in a greenhouse for 7 days at 15–20 °C and ambient light. Germination rate was scored as the proportion of germinated seeds in each petri dish after 8 days.
Growing seedlings
Immediately after counting germinated seeds, 1–20 seedlings from each cross (mean number of seedlings transplanted per offspring type F1: 16.86 ± 6.39 SD; F2: 17.98 ± 5.91 SD; L: 19.95 ± 0.33 SD; bcL: 17.33 ± 5.64 SD; bcA: 13.78 ± 8.06 SD) were transplanted into 200-cell plug trays filled with pre-moistened Sunshine Professional Soil Mix #3 (Sun Gro Horticulture, Brantford, Canada). Each tray contained seedlings from between 8 to 10 different crosses. The trays were kept under ambient greenhouse conditions (temperatures ranging from 15–28 °C), watered with reverse-osmosis (RO) water, and covered with a clear plastic dome to maintain higher-than ambient levels of humidity until the plants were tall enough to touch the dome (approximately 3 weeks), at which time the domes were removed. When seedlings were approximately 60 days old (in April 2020), up to 5 seedlings of each cross were transplanted into 4 round pots filled with Sunshine Mix #1. There were 34 F1, 30 L, 31 F2, 30 bcL, and 23 bcA crosses per offspring type yielding a total of 559 plants in the experiment. Up to 10 pots were randomly placed into propagation trays previously filled with RO water. Following transplantation, plants were fertilized using approximately 500 mL of 0.5 g/L 20:20:20 N:P:K fertilizer (Plant-Prod, Leamington, Ontario) per tray. The height of the tallest leaf of each plant was measured when plants were between 80–90 days old (in May 2020), all aboveground biomass from each plant was harvested After a total of 82 days in the greenhouse, and placed it into individual 8 ¼ × 5 5/16 × 16 1/8 paper bags. Biomass samples were dried at 65 °C for 24 h immediately after harvesting in a Binder BD 720 oven (Binder GmbH, Tuttlingen, Germany). The dry weight was recorded immediately after removing plants from the oven.
Statistical analyses
Variation in seed set among cross types was analysed using a linear mixed-effects model with the number of seeds per gram of fruit tissue as the dependent variable, cross type as the independent variable (five levels: L × L, A × L, F1 × F1, F1 × A, F1 × L), and maternal site of origin as a random effect. For this analysis, the dependent variable was square-root-transformed to meet model assumptions. Variation in the germination and growth of the progeny arising from the different cross types were also evaluated using linear mixed-effects models. For germination rate we first considered a generalized linear mixed model (GLMM) approach to logistic regression. Here, the number of germinated/ungerminated seeds was the dependent variable, progeny type (five levels: L, F1, F2, bcA, bcL) was the independent variable, and maternal site of origin was included as a random effect. However, even after correcting for overdispersion various aspects of model fit were poor. A linear mixed-effect model using the proportion of germinated seeds as the dependent variable fit model assumptions and was used for analysis of these data. Variation in plant height (dependent variable: height of the tallest shoot in cm) and above-ground biomass (dependent variable: dry biomass in g) were also evaluated using linear mixed effects models. For all of these models, progeny type was the fixed effect, and maternal site of origin was included as a random effect. Because we measured the height and biomass from replicate progeny from each cross (seed family), we included seed family as a nested random effect (nested within maternal site of origin) for analyses of those dependent variables. Biomass data were square-root-transformed to meet model assumptions. All model parameters, including for the analysis of seed set, were estimated using the ‘lmer’ function from the ‘lme4’ package (v. 1.1–27.1; Bates et al. 2016). Significance of fixed effects (cross type for seed set, progeny type for the other models) was assessed using a type-II Wald chi-square test, calculated using the ‘Anova’ function from the ‘car’ package (v. 3.0–12; Fox and Weisberg 2019) using R (v. 4.1.0; R Core Team 2021). Differences among cross- and progeny types were assessed by comparison of least-square means using the Tukey adjustment for pairwise tests, calculated using the ‘emmeans’ function from the ‘emmeans’ package (v. 1.7.1-1; Lenth et al. 2021).
Results
Seed set and germination
Seed-set was highly variable among cross types (Fig. 1A). All crosses involving F1 T. × glauca as the maternal parent had significantly lower seed-set (mean number of seeds per gram F1 × F1: 743.7 ± 891 SD; F1 × A: 489.4 ± 791.1 SD; F1 × L: 1349.6 ± 1359.4 SD) than the parental cross (mean number of seeds per gram L × L: 3125.0 ± 1843.1 SD), consistent with partial sterility of F1 T. × glauca (Fig. 1A; Supplementary Materials Table S3). Seed set was also highly variable for the A × L cross type (mean number of seeds per gram: 3124.5 ± 2814.3 SD) with a coefficient of variation (CV = 1.61) that exceeded those for all crosses combined (CV = 1.16).
A Seed set (measured as the number of seeds per unit mass of fruit) for the parental cross, square-root transformed. B Germination rate, measured as the proportion of seeds that germinated. C Plant height measured as the tallest leaf per plant. D Total above-ground dry mass, square-root transformed. Boxes indicate the median (horizontal black line in each box), the first and third quartiles (the lower and upper edge of each box), and the minimum and maximum values that were no further than 1.5 the inter-quartile range (whiskers extending from each box) for each cross or offspring type. Data points show values for each replicate. Upper-case letters and codes below each box indicate taxon. In figure panel A, upper case letters refer to the maternal × paternal parent for each cross type, with A = Typha angustifolia; L = Typha latifolia; F1 = F1 hybrids. For panels B–D upper case letters refer to progeny classes, with L = the progeny of L × L crosses, F1 = the progeny of A × L crosses, F2 = the progeny of F1 × F1 crosses; bcA = the progeny of F1 × A crosses; and bcL = the progeny of F1 × L crosses. Lowercase letters above each box indicate the results of pairwise tests – crosses or offspring types with different letters are significantly different from each other.
Germination rates were also highly variable and bcL progeny had the lowest average germination rates (mean proportion of germinated seeds: 0.48 ± 0.33 SD), significantly lower than for L progeny (0.74 ± 0.21 SD; Fig. 1B; Supplementary Materials Table S4). The proportion of germinated seeds for the remaining progeny types were intermediate to L and bcL and not significantly different from them (F1: 0.61 ± 0.35 SD; F2: 0.64 ± 0.27 SD; bcA: 0.63 ± 0.26 SD; Fig. 1B; Supplementary Materials Table S4). Again, the cross yielding F1 progeny (cross type A × L) was associated with the most variation and germination rates for this progeny type spanned the entire range of values (from 0 to 0.99; Fig. 1B).
Plant growth
F2s grew poorly in comparison to the other types of progeny produced in our experiment. The height of F2 plants was, on average, the lowest among all types of cattails in the experiment (mean plant height F2: 86.2 cm ± 26.8 SD; Fig. 1C), and significantly lower than the height of F1s (F1: 100.1 cm ± 24.6 SD; Supplementary Materials Table S5). Backcrossed hybrids had intermediate plant heights (mean plant height bcA: 97.8 cm ± 18.9 SD; bcL: 95.9 cm ± 18.3 SD) that were, on average, not significantly different from those of parental L (L: 92.0 cm ± 23.4 SD) or F1s.
Patterns for above-ground biomass mirrored those for plant height: F2s produced the least above-ground biomass (mean dry biomass F2: 3.84 g ± 2.44 SD; Fig. 1D) and were significantly smaller than L (6.25 g ± 3.80 SD) and F1s (5.31 g ± 3.24 SD; Supplementary Materials Table S6). Backcrossed hybrids were again of intermediate size (mean dry biomass bcA: 4.35 g ± 2.05 SD; bcL: 5.29 g ± 2.86 SD) and, on average, not significantly different from parental L or F1 hybrids.
Discussion
Our study reveals that the heterotic effects of hybridization previously observed for F1 cattails do not extend to later generations: F2s, and to a more limited extent, introgressed hybrids (bcA and bcL) grew poorly in comparison with parental T. latifolia and de novo F1 hybrids. Our findings are consistent with expectations under hybrid breakdown and help explain the pattern identified from surveys of natural populations around the LGL that advanced-generation hybrids are less common than F1 hybrids (Travis et al. 2011; Freeland et al. 2013; Pieper et al. 2020). Our results also provide insights into the long-term stability of the cattail hybrid zone. Previous studies have inferred that F1s appear capable of competitively displacing both types of parental taxa and that asymmetric hybrid formation usurps the ovules of T. angustifolia and not T. latifolia (Freeland et al. 2013; Pieper et al. 2017, 2018). Moreover, parallel studies have provided evidence for regular sexual recruitment in Typha populations (Kirk et al. 2011; Travis et al. 2011; McKenzie‐Gopsill et al. 2012; Pieper et al. 2020). As we discuss in more detail below, the ongoing formation of advanced-generation hybrids together with asymmetric hybridization, competitive displacement, and—as shown here—hybrid breakdown, and should supress the recruitment of T. angustifolia in the hybrid zone, with downstream effects on the frequency of heterotic F1s in the LGL region.
Hybrid breakdown
F1 hybrid cattails are characterized by a mixture of partial sexual sterility and vegetative vigour, the balance of which favours F1s compared to T. latifolia when grown under the same conditions (Bunbury-Blanchette et al. 2015). This combination of reduced sexual fertility combined with enhanced vegetative growth has been observed in other plant hybrid zones (Marques et al. 2011). However, heterotic and partially sterile hybrids are not typical of plant hybrid zones—the fitness consequences of hybridization for F1 progeny are variable. In some cases F1s are neither sterile nor heterotic (Ross et al. 2012), and in others F1s are inferred to be fully fertile and heterotic (Johansen‐Morris and Latta 2006). For cattails, reduced sexual fertility of F1s should limit introgression and the rate of hybrid spread within hybrid zones (Pieper et al. 2017). However, these limitations have not prevented F1 hybrids from becoming the dominant cattail in the LGL region.
Our findings indicate that any beneficial effects of hybridization for F1 cattails are transient and by the F2 generation hybrid cattails are subject to hybrid breakdown. In this study, F2s were smaller than F1s in terms of their height and biomass, and smaller than parental T. latifolia in terms of biomass. This is consistent with earlier studies showing that F2 + plants did not grow as tall as F1s (Zapfe and Freeland 2015), and that hybrids with unbalanced alleles from the two parent species (presumed F2 + plants) were smaller (in terms of biomass) than presumed F1s under controlled growth conditions (Bunbury-Blanchette et al. 2015). Transient effects of heterosis and subsequent hybrid breakdown have also been demonstrated for Avena barbata and A. fatua hybrids, although F2s remained heterotic and evidence for hybrid breakdown occurred among later-generation hybrids (F6 hybrids; Johansen‐Morris and Latta 2006). The loss of heterosis and the expression of hybrid breakdown after a single generation could be explained by epistatic interactions between loci (e.g., parental AAbb and aaBB genotypes yielding heterozygous AaBb F1s and recombinant AABB and aabb F2s, i.e., Bateson-Dobzhansky-Muller incompatibilities, or BDMIs; Orr 1995). Indeed, epistatic effects, including cyto-nuclear incompatibilities, have been proposed as contributing to the asymmetry of F1 hybrid formation and/or to the partial sterility of F1 hybrids in the gametophyte (haploid) phase of the life cycle (Pieper et al. 2017). Although asymmetric hybridization, which characterizes this hybrid zone, is consistent with cyto-nuclear incompatibilities, patterns of F2 + hybrid breakdown are not. Cyto-nuclear incompatibilities should have resulted differences in seed set between backcrosses and the two parental taxa that were opposite to what was found. Average seed set was lower for bcA than for bcL, however, because F1 hybrids have T. angustifolia cytoplasms (Kuehn et al. 1999; Freeland et al. 2017; Pieper et al. 2017) we would expect backcrossing to T. angustifolia to have ameliorated rather than exacerbated any negative epistasis between nuclear and cytoplasmic genes. Indeed, the effects of hybrid breakdown were weaker among introgressed hybrids than for F2s, a pattern that points to the possible importance of epistatic effects between nuclear loci in the regulation of hybrid breakdown.
Several mechanisms underlying hybrid incompatibility and breakdown have been characterized, each with their own mode of operation and diagnostic phenotypes. These include cyto-nuclear incompatabilities, chromosomal rearrangements, and nuclear BDMIs (reviewed in Fishman and Sweigart 2018). None of these mechanisms of hybrid incompatibility can be the sole explanation for the combination of asymmetric hybridization, partial hybrid sterility, and – as shown in this study—hybrid breakdown that characterizes cattails in the LGL region. As argued in the previous paragraph, cyto-nuclear incompatibilities are inconsistent with patterns of seed set among backcrosses. Nuclear BDMIs and chromosomal rearrangements are not expected to yield strongly asymmetric patterns of hybridization (Tiffin et al. 2001). Moreover, other phenotypes commonly found in other incompatible plant hybrids, including chlorotic or necrotic hybrids, and unfertilized or aborted seeds (Fishman and Sweigart 2018), were either not evident among our crosses or - in the case of seed phenotpyes - not observable because of how seeds were cleaned prior to germination. A profitable first step in identifying the mechanisms of hybrid incompatability would be to compare the phenotypes of seeds and offspring among F1 crosses using plants whose origins span regions of sympatry within which hybridization does and does not occur (e.g., LGL versus China; Zhou et al. 2016).
Hybrid zone stability
The evolutionary stability of hybrid zones is determined by (1) patterns of selection against hybrids and (2) the net movement of genes across the zone (Barton and Hewitt 1985; Curry 2015). In terms of selection, our results indicate that the persistence of hybrid cattails in the LGL region of North America requires the ongoing recruitment of F1 hybrids. Although F1s are heterotic (Travis et al. 2011; Larkin et al. 2012; Bunbury-Blanchette et al. 2015; Zapfe and Freeland 2015) and appear to be competitively dominant (Tuchman et al. 2009), their reduced sexual fertility (Pieper et al. 2017), combined with the reduced germination and vegetative vigour of their advanced-generation hybrid offspring found in this study indicate that the maintenance of hybrids in the LGL depends on the recurrent formation of F1s: any process that reduces the formation of F1s should also limit the stability of the hybrid zone. In terms of the movement of genes across the hybrid zone, high rates of gene dispersal (Pieper et al. 2020) together with asymmetric hybrid formation (Pieper et al. 2017) point to the net movement of genes from T. latifolia populations into T. angustifolia populations – a unidirectional form of genetic swamping (Todesco et al. 2016). The erosion of T. angustifolia populations via unidirectional gene flow from T. latifolia and selection against advanced-generation hybrids should ultimately combine to limit the formation of new hybrids. This process could be assisted by the targeted removal of non-native T. angustifolia from wetlands in the LGL region.
Data availability
The data and R scripts are available on the Dryad Digital Repository at https://doi.org/10.5061/dryad.q83bk3jm2.
References
Abbott R, Albach D, Ansell S, Arntzen JW, Baird SJE, Bierne N et al. (2013) Hybridization and speciation. J Evol Biol 26:229–246
Abbott RJ, Brennan AC (2014) Altitudinal gradients, plant hybrid zones and evolutionary novelty. Philos Trans R Soc B 369:20130346
Abbott RJ (2017) Plant speciation across environmental gradients and the occurrence and nature of hybrid zones. J Syst Evol 55:238–258
Ahee J (2013) The spatial dynamics of wind pollination in broadleaf cattail (Typha latifolia). MSc Thesis. Trent University, Canada
Ahee JE, Van Drunen WE, Dorken ME (2015) Analysis of pollination neighbourhood size using spatial analysis of pollen and seed production in broadleaf cattail (Typha latifolia). Botany 93:91–100
Bansal S, Lishawa SC, Newman S, Tangen BA, Wilcox D, Albert D et al. (2019) Typha (cattail) invasion in North American wetlands: Biology, regional problems, impacts, ecosystem services, and management. Wetlands 39:645–684
Barton NH (2001) The role of hybridization in evolution. Mol Ecol 10:551–568
Barton NH (2008) The effect of a barrier to gene flow on patterns of geographic variation. Genet Res 90:139–149
Barton NH, Hewitt GM (1985) Analysis of hybrid zones. Ann Rev Ecol Syst 16:113–148
Bates D, Maechler M, Bolker B (2016) lme4: Linear mixed-effects models using Eigen and S4. R package version o1.1–13, http://CRAN.R-project.org/package-lme4
Behm JE, Ives AR, Boughman JW (2010) Breakdown in postmating isolation and the collapse of a species pair through hybridization. Am Nat 175:11–26
Bersweden L, Viruel J, Schatz B, Harland J, Gargiulo R, Cowan RS et al. (2021) Microsatellites and petal morphology reveal new patterns of admixture in Orchis hybrid zones. Am J Bot 108:1388–1404
Buggs RJA (2007) Empirical study of hybrid zone movement. Heredity 99:301–312
Bunbury-Blanchette AL, Freeland JR, Dorken ME (2015) Hybrid Typha× glauca outperforms native T. latifolia under contrasting water depths in a common garden. Basic Appl Ecol 16:394–402
Burton RS, Pereira RJ, Barreto FS (2013) Cytonuclear genomic interactions and hybrid breakdown. Ann Rev Ecol Evol Syst 44:281–302
Buitink J, Claessens MM, Hemminga MA, Hoekstra FA (1998) Influence of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant Phys 118:531–541
Ciotir C, Kirk H, Row JR, Freeland JR (2013) Intercontinental dispersal of T. angustifolia and T. latifolia between Europe and North America has implications for the Typha invasions. Biol Invasions 15:1377–1390
Ciotir C, Szabo J, Freeland J (2017) Genetic characterization of cattail species and hybrids (Typha spp.) in Europe. Aquat Bot 141:51–59
Curry CM (2015) An integrated framework for hybrid zone models. Evol Biol 42:359–365
Durán-Castillo M, Hudson A, Wilson Y, Field DL, Twyford AD (2022) A phylogeny of Antirrhinum reveals parallel evolution of alpine morphology. N. Phytol 233:1426–1439
Fishman L, Sweigart AL (2018) When two rights make a wrong: The evolutionary genetics of plant hybrid incompatibilities. Ann Rev Plant Biol 69:707–731
Fox J, Weisberg S (2019) An R Companion to Applied Regression, 3rd edn. Sage, Thousand Oaks, CA
Freeland J, Ciotir C, Kirk H (2013) Regional differences in the abundance of native, introduced, and hybrid Typha spp. in northeastern North America influence wetland invasions. Biol Invasions 15:2651–2665
Freeland JR, Ciotir C, Wensink L, Dorken M (2017) Widespread cytonuclear discordance in narrow-leaved cattail (Typha angustifolia) does not explain the dominance of its invasive hybrid (Typha × glauca). Hydrobiologia 792:53–65
Grace JB, Harrison JS (1986) The biology of Canadian weeds: 73. Typha latifolia L., Typha angustifolia L. and Typha x glauca. Can J Plan Sci 361–379
Irwin DE (2020) Assortative mating in hybrid zones is remarkably ineffective in promoting speciation. Am Nat 195:E150–E167
Johansen‐Morris AD, Latta RG (2006) Fitness consequences of hybridization between ecotypes of Avena barbata: hybrid breakdown, hybrid vigor, and transgressive segregation. Evolution 60:1585–1595
Kirk H, Connolly C, Freeland JR (2011) Molecular genetic data reveal hybridization between Typha angustifolia and Typha latifolia across a broad spatial scale in eastern North America. Aquat Bot 95:189–193
Kuehn MM, Minor JE, White BN (1999) An examination of hybridization between the cattail species Typha latifolia and Typha angustifolia using random amplified polymorphic DNA and chloroplast DNA markers. Mol Ecol 8:1981–1990
Larkin DJ, Freyman MJ, Lishawa SC, Geddes P, Tuchman NC (2012) Mechanisms of dominance by the invasive hybrid cattail Typha× glauca. Biol Invasions 14:65–77
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2021) Emmeans: Estimated marginal means, aka least-squares means. R package version, 1.7.1–1
Marques I, Nieto Feliner G, Martins‐Loução MA, Fuertes Aguilar J (2011) Fitness in Narcissus hybrids: low fertility is overcome by early hybrid vigour, absence of exogenous selection and high bulb propagation. J Ecol 99:1508–1519
Mitchell N, Luu H, Owens GL, Rieseberg LH, Whitney KD (2022) Hybrid evolution repeats itself across environmental contexts in Texas sunflowers (Helianthus). Evolution https://doi.org/10.1111/evo.14536
McKenzie‐Gopsill A, Kirk H, Van Drunen W, Freeland JR, Dorken ME (2012) No evidence for niche segregation in a North American Cattail (Typha) species complex. Ecol Evol 2:952–961
Orr HA (1995) The population genetics of speciation: the evolution of hybrid incompatibilities. Genetics 139:1805–1813
Pieper SJ, Nicholls AA, Freeland JR, Dorken ME (2017) Asymmetric hybridization in cattails (Typha spp.) and its implications for the evolutionary maintenance of native Typha latifolia. J Hered 108:479–487
Pieper SJ, Freeland JR, Dorken ME (2018) Coexistence of Typha latifolia, T. angustifolia (Typhaceae) and their invasive hybrid is not explained by niche partitioning across water depths. Aquat Bot 144:46–53
Pieper S, Dorken M, Freeland J (2020) Genetic structure in hybrids and progenitors provides insight into processes underlying an invasive cattail (Typha× glauca) hybrid zone. Heredity 124:714–725
R Core Development Team (2021) R: A language and environment for statistical computing v. 4.1.0. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
Rasband WS (2014) ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/,1997-2014
Rendón-Anaya M, Wilson J, Sveinsson S, Fedorkov A, Cottrell J, Bailey MES et al. (2021) Adaptive introgression facilitates adaptation to high latitudes in European Aspen (Populus tremula L.). Mol Biol Evol 38:5034–5050
Rieseberg LH (2006) Hybrid speciation in wild sunflowers. Ann Mo Bot 93:34–48
Ross RIC, Ågren JA, Pannell JR (2012) Exogenous selection shapes germination behaviour and seedling traits of populations at different altitudes in a Senecio hybrid zone. Ann Bot 110:1439–1447
Runemark A, Vallejo-Marin M, Meier JI (2019) Eukaryote hybrid genomes. PLoS Genet 15:e1008404
Smith SG (1967) Experimental and natural hybrids in North American Typha (Typhaceae). Am Midl Nat 78:257–287
Snow AA, Travis SE, Wildová R, Fér T, Sweeney PM, Marburger JE et al. (2010) Species‐ specific SSR alleles for studies of hybrid cattails (Typha latifolia × T. angustifolia; Typhaceae) in North America. Am J Bot 97:2061–2067
Stebbins GL (1959) The role of hybridization in evolution. Proc Am Philos Soc 103:231–251
Tangen B, Bansal S, Freeland J, Travis S, Wasko J, McGonigle et al. (2021) Distributions of native and invasive Typha (cattail) throughout the Prairie Pothole Region of North America. Wetl Ecol Manag 30:1–17
Tiffin P, Olsen S, Moyle LC (2001) Asymmetrical crossing barriers in angiosperms. Proc R Soc Lond B 268:861–867
Tisshaw K, Freeland J, Dorken M (2020) Salinity, not genetic incompatibilities, limits the establishment of the invasive hybrid cattail Typha × glauca in coastal wetlands. Ecol Evol 10:12091–12103
Todesco M, Pascual MA, Owens GL, Ostevik KL, Moyers BT, Hübner S et al. (2016) Hybridization and extinction. Evol Appl 9:892–908
Travis SE, Marburger JE, Windels S, Kubatova B (2010) Hybridization dynamics of invasive cattail (Typhaceae) stands in the Western Great Lakes Region of North America: a molecular analysis. J Ecol 98:7–16
Travis SE, Marburger JE, Windels SK, Kubátová B (2011) Clonal structure of invasive cattail (Typhaceae) stands in the Upper Midwest Region of the US. Wetlands 31:221–228
Tsyusko‐Omeltchenko OV, Schable NA, Smith MH, Glenn TC (2003) Microsatellite loci isolated from narrow‐leaved cattail Typha angustifolia. Mol Ecol Notes 3:535–538
Tuchman NC, Larkin DJ, GeddesP, Wildova R, Jankowski K, Goldberg DE (2009) Patterns of environmental change associated with Typha × glauca invasion in a Great Lakes coastal wetland. Wetlands 29:964–975
Zalmat AS, Sotola AV, Nice CC, Martin NH (2021) Genetic structure in Louisiana Iris species reveals patterns of recent and historical admixture. Am J Bot 108:2257–2268
Zapfe L, Freeland JR (2015) Heterosis in invasive F1 cattail hybrids (Typha× glauca). Aquat Bot 125:44–47
Zhou B, Yu D, Ding Z, Xu X (2016) Comparison of genetic diversity in four Typha species (Poales, Typhaceae) from China. Hydrobiologia 770:117–128
Acknowledgements
The authors thank Kathryn Tisshaw, Sara Pieper, and Verena Sesin for assistance in the field and in the lab, Avery Chambers and Braidy Chambers for assistance with Fig. S1, and three anonymous referees for constructive comments.
Funding
This work was funded by the Natural Sciences and Engineering Research Council via Discovery Grants to MED (RGPIN-2018-04866) and JRF (RGPIN-2017-04371).
Author information
Authors and Affiliations
Contributions
VVB contributed to the study design, data analysis and manuscript writing, and was responsible for conducting the hand crosses and for all data collection in the field and greenhouse. JRF was responsible for designing the study, supervising the work, and contributed to writing the manuscript. MED contributed to the study design and supervision, and was responsible for data analysis and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Matthew Hartfield
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhargav, V.V., Freeland, J.R. & Dorken, M.E. Evidence of hybrid breakdown among invasive hybrid cattails (Typha × glauca). Heredity 129, 195–201 (2022). https://doi.org/10.1038/s41437-022-00557-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41437-022-00557-7
This article is cited by
-
Self-fertilization does not lead to inbreeding depression in Typha parent species or hybrids
Evolutionary Ecology (2024)
-
Undetected but Widespread: the Cryptic Invasion of Non-Native Cattail (Typha) in a Pacific Northwest Estuary
Estuaries and Coasts (2023)