Abstract
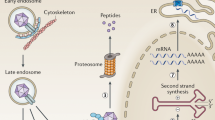
In the last two decades, recombinant adeno-associated virus has emerged as the most popular gene therapy vector. Recently AAV gene therapy has been approved by the FDA for the treatment of two rare genetic disorders, namely the early childhood blindness disease Leber congenital amaurosis and spinal muscular atrophy (SMA). As is the case for the treatment of SMA, if the AAV vector must be administered systemically, very high vector doses are often required for therapeutic efficacy. But higher vector doses inevitably increase the risk of adverse events. The tragic death of three children in a clinical trial to treat X-linked myotubular myopathy with an AAV vector has thrown this limitation into sharp relief. Regardless of the precise cause(s) that led to the death of the two children, it is critical that we develop better AAV vectors to achieve therapeutic levels of expression with lower vector doses. To transduce successfully a target cell, AAV has to overcome both systemic as well as cellular roadblocks. In this review, we discuss some of the most prominent cellular roadblocks that AAV must get past to deliver successfully its therapeutic payload. We also highlight recent advancements in our knowledge of AAV biology that can potentially be harnessed to improve AAV vector performance and thereby make AAV gene therapy safer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
26 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41434-021-00252-y
References
Kumaran N, Michaelides M, Smith AJ, Ali RR, Bainbridge JWB. Retinal gene therapy. Br Med Bull. 2018;126:13–25.
Hoy SM. Onasemnogene abeparvovec: first global approval. Drugs. 2019;79:1255–62.
Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. 2015;33:831–46.
Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–6.
Weitzman MD, Linden RM. Adeno-associated virus biology. In: Snyder RO, Moullier P (eds). Adeno-associated virus: methods and protocols. Humana Press: Totowa, NJ, 2011, pp 1-23.
Kotin RM, Linden RM, Berns KI. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–8.
Linden RM, Ward P, Giraud C, Winocour E, Berns KI. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1996;93:11288–94.
Linden RM, Winocour E, Berns KI. The recombination signals for adeno-associated virus site-specific integration. Proc Natl Acad Sci USA. 1996;93:7966–72.
McCarty DM, Young SM Jr, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–45.
Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci USA. 2010;107:10220–5.
Earley LF, Powers JM, Adachi K, Baumgart JT, Meyer NL, Xie Q et al. Adeno-associated virus (AAV) assembly-activating protein is not an essential requirement for capsid assembly of AAV serotypes 4, 5, and 11. J Virol. 2017;91:e02213–17. https://doi.org/10.1128/jvi.02213-17.
Sonntag F, Kother K, Schmidt K, Weghofer M, Raupp C, Nieto K, et al. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J Virol. 2011;85:12686–97.
Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–12.
Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci USA. 1984;81:6466–70.
Vandamme C, Adjali O, Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum Gene Ther. 2017;28:1061–74.
Wilson JM, Flotte TR. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum Gene Ther. 2020;31:695–6.
Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–22.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–65.
Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–22.
Samulski RJ, Berns KI, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci USA. 1982;79:2077–81.
Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol. 2016;21:75–80.
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–80.
Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010;3:81–9.
Ellis BL, Hirsch ML, Barker JC, Connelly JP, Steininger RJ 3rd, Porteus MH. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol J. 2013;10:74.
Grimm D, Kern A, Pawlita M, Ferrari F, Samulski R, Kleinschmidt J. Titration of AAV-2 particles via a novel capsid ELISA: packaging of genomes can limit production of recombinant AAV-2. Gene Ther. 1999;6:1322–30.
Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82:5887–911.
Zeltner N, Kohlbrenner E, Clement N, Weber T, Linden RM. Near-perfect infectivity of wild-type AAV as benchmark for infectivity of recombinant AAV vectors. Gene Ther. 2010;17:872–9.
Kronenberg S, Bottcher B, von der Lieth CW, Bleker S, Kleinschmidt JA. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J Virol. 2005;79:5296–303.
Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol. 2006;80:11040–54.
Nicolson SC, Samulski RJ. Recombinant adeno-associated virus utilizes host cell nuclear import machinery to enter the nucleus. J Virol. 2014;88:4132–44.
Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet. 2003;34:297–302.
Nguyen GN, Everett JK, Kafle S, Roche AM, Raymond HE, Leiby J, et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat Biotechnol. 2020;39:47–55.
Nowrouzi A, Penaud-Budloo M, Kaeppel C, Appelt U, Le Guiner C, Moullier P, et al. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol Ther. 2012;20:1177–86.
Nault JC, Datta S, Imbeaud S, Franconi A, Mallet M, Couchy G, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet. 2015;47:1187–93.
Buning H, Schmidt M. Adeno-associated vector toxicity-to be or not to be? Mol Ther. 2015;23:1673–5.
Berns KI, Byrne BJ, Flotte TR, Gao G, Hauswirth WW, Herzog RW, et al. Adeno-Associated Virus Type 2 and Hepatocellular Carcinoma? Hum Gene Ther. 2015;26:779–81.
Louis Jeune V, Joergensen JA, Hajjar RJ, Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods. 2013;24:59–67.
Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–45.
Kalia M, Jameel S. Virus entry paradigms. Amino Acids. 2011;41:1147–57.
Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–7.
Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82.
Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74:9184–96.
Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J Virol. 2006;80:8961–9.
Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, et al. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79:609–14.
Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–6.
Levy HC, Bowman VD, Govindasamy L, McKenna R, Nash K, Warrington K, et al. Heparin binding induces conformational changes in Adeno-associated virus serotype 2. J Struct Biol. 2009;165:146–56.
O’Donnell J, Taylor KA, Chapman MS. Adeno-associated virus-2 and its primary cellular receptor-Cryo-EM structure of a heparin complex. Virology. 2009;385:434–43.
Wallen AJ, Barker GA, Fein DE, Jing H, Diamond SL. Enhancers of adeno-associated virus AAV2 transduction via high throughput siRNA screening. Mol Ther. 2011;19:1152–60.
Qiu J, Brown KE. Integrin alphaVbeta5 is not involved in adeno-associated virus type 2 (AAV2) infection. Virology. 1999;264:436–40.
Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, et al. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–12.
Chen CL, Jensen RL, Schnepp BC, Connell MJ, Shell R, Sferra TJ, et al. Molecular characterization of adeno-associated viruses infecting children. J Virol. 2005;79:14781–92.
Cabanes-Creus M, Hallwirth CV, Westhaus A, Ng BH, Liao SHY, Zhu E et al. Restoring the natural tropism of AAV2 vectors for human liver. Sci Transl Med. 2020;12:eaba3312.
Kern A, Schmidt K, Leder C, Muller OJ, Wobus CE, Bettinger K, et al. Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J Virol. 2003;77:11072–81.
Afione S, DiMattia MA, Halder S, Di Pasquale G, Agbandje-McKenna M, Chiorini JA. Identification and mutagenesis of the adeno-associated virus 5 sialic acid binding region. J Virol. 2015;89:1660–72.
Dudek AM, Pillay S, Puschnik AS, Nagamine CM, Cheng F, Qiu J, et al. An alternate route for adeno-associated virus (AAV) entry independent of AAV receptor. J Virol. 2018;92:e02213–17.
Mizukami H, Young NS, Brown KE. Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology. 1996;217:124–30.
Zengel J, Carette JE. Structural and cellular biology of adeno-associated virus attachment and entry. Adv Virus Res. 2020;106:39–84.
Nonnenmacher M, Weber T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe. 2011;10:563–76.
Duan D, Li Q, Kao AW, Yue Y, Pessin JE, Engelhardt JF. Dynamin is required for recombinant adeno-associated virus type 2 infection. J Virol. 1999;73:10371–6.
Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74:2777–85.
Bess CD. Analysis of cellular factors involved in adeno- associated virus type 2 entry. Rockefeller University, 2009.
Sanlioglu AD, Karacay B, Benson PK, Engelhardt JF, Sanlioglu S. Novel approaches to augment adeno-associated virus type-2 endocytosis and transduction. Virus Res. 2004;104:51–9.
Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–9.
Malecz N, McCabe PC, Spaargaren C, Qiu R, Chuang Y, Symons M. Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr Biol. 2000;10:1383–6.
Chung SH, Frese KK, Weiss RS, Prasad BV, Javier RT. A new crucial protein interaction element that targets the adenovirus E4-ORF1 oncoprotein to membrane vesicles. J Virol. 2007;81:4787–97.
Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–23.
Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–12.
Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG, et al. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol. 2008;18:1802–8.
Iwasaki M, Ngo N, de la Torre JC. Sodium hydrogen exchangers contribute to arenavirus cell entry. J Virol. 2014;88:643–54.
Wittrup A, Zhang SH, Svensson KJ, Kucharzewska P, Johansson MC, Morgelin M, et al. Magnetic nanoparticle-based isolation of endocytic vesicles reveals a role of the heat shock protein GRP75 in macromolecular delivery. Proc Natl Acad Sci USA. 2010;107:13342–7.
Bantel-Schaal U, Hub B, Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J Virol. 2002;76:2340–9.
Johnson JS, Gentzsch M, Zhang L, Ribeiro CM, Kantor B, Kafri T, et al. AAV exploits subcellular stress associated with inflammation, endoplasmic reticulum expansion, and misfolded proteins in models of cystic fibrosis. PLoS Pathog. 2011;7:e1002053.
Meisen WH, Nejad ZB, Hardy M, Zhao H, Oliverio O, Wang S, et al. Pooled screens identify GPR108 and TM9SF2 as host cell factors critical for AAV transduction. Mol Ther Methods Clin Dev. 2020;17:601–11.
Nonnenmacher ME, Cintrat JC, Gillet D, Weber T. Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction. J Virol. 2015;89:1673–87.
Pajusola K, Gruchala M, Joch H, Luscher TF, Yla-Herttuala S, Bueler H. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J Virol. 2002;76:11530–40.
Douar AM, Poulard K, Stockholm D, Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75:1824–33.
Hansen J, Qing K, Kwon HJ, Mah C, Srivastava A. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J Virol. 2000;74:992–6.
Ding W, Zhang LN, Yeaman C, Engelhardt JF. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol Ther. 2006;13:671–82.
Forrester A, Rathjen SJ, Daniela Garcia-Castillo M, Bachert C, Couhert A, Tepshi L, et al. Functional dissection of the retrograde Shiga toxin trafficking inhibitor Retro-2. Nat Chem Biol. 2020;16:327–36.
Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525.
Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. The EMBO journal. 1993;12:677–82.
Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–6.
Dudek AM, Zabaleta N, Zinn E, Pillay S, Zengel J, Porter C, et al. GPR108 is a highly conserved AAV entry factor. Mol Ther. 2020;28:367–81.
Dong D, Zhou H, Na SY, Niedra R, Peng Y, Wang H, et al. GPR108, an NF-kappaB activator suppressed by TIRAP, negatively regulates TLR-triggered immune responses. PLoS One. 2018;13:e0205303.
Tanaka A, Tumkosit U, Nakamura S, Motooka D, Kishishita N, Priengprom T, et al. Genome-wide screening uncovers the significance of N-sulfation of heparan sulfate as a host cell factor for Chikungunya virus infection. J Virol. 2017;91:e00432–17.
Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–93.
Tian S, Muneeruddin K, Choi MY, Tao L, Bhuiyan RH, Ohmi Y, et al. Genome-wide CRISPR screens for Shiga toxins and ricin reveal Golgi proteins critical for glycosylation. PLoS Biol. 2018;16:e2006951.
Akache B, Grimm D, Shen X, Fuess S, Yant SR, Glazer DS, et al. A two-hybrid screen identifies cathepsins B and L as uncoating factors for adeno-associated virus 2 and 8. Mol Ther. 2007;15:330–9.
Salganik M, Venkatakrishnan B, Bennett A, Lins B, Yarbrough J, Muzyczka N, et al. Evidence for pH-dependent protease activity in the adeno-associated virus capsid. J Virol. 2012;86:11877–85.
Zadori Z, Szelei J, Lacoste MC, Li Y, Gariepy S, Raymond P, et al. A viral phospholipase A2 is required for parvovirus infectivity. Dev Cell. 2001;1:291–302.
Girod A, Wobus CE, Zadori Z, Ried M, Leike K, Tijssen P, et al. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J Gen Virol. 2002;83(Pt 5):973–8.
Stahnke S, Lux K, Uhrig S, Kreppel F, Hosel M, Coutelle O, et al. Intrinsic phospholipase A2 activity of adeno-associated virus is involved in endosomal escape of incoming particles. Virology. 2011;409:77–83.
Xiao W, Warrington KH Jr, Hearing P, Hughes J, Muzyczka N. Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2. J Virol. 2002;76:11505–17.
Seisenberger G, Ried MU, Endress T, Buning H, Hallek M, Brauchle C. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science. 2001;294:1929–32.
Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–22.
Johnson JS, Samulski RJ. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83:2632–44.
Lux K, Goerlitz N, Schlemminger S, Perabo L, Goldnau D, Endell J, et al. Green fluorescent protein-tagged adeno-associated virus particles allow the study of cytosolic and nuclear trafficking. J Virol. 2005;79:11776–87.
Kelich JM, Ma J, Dong B, Wang Q, Chin M, Magura CM, et al. Super-resolution imaging of nuclear import of adeno-associated virus in live cells. Mol Ther Methods Clin Dev. 2015;2:15047.
Grieger JC, Snowdy S, Samulski RJ. Separate basic region motifs within the adeno-associated virus capsid proteins are essential for infectivity and assembly. J Virol. 2006;80:5199–210.
Bevington JM, Needham PG, Verrill KC, Collaco RF, Basrur V, Trempe JP. Adeno-associated virus interactions with B23/Nucleophosmin: identification of sub-nucleolar virion regions. Virology. 2007;357:102–13.
Qiu J, Brown KE. A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid. Virology. 1999;257:373–82.
Bernaud J, Rossi A, Fis A, Gardette L, Aillot L, Buning H, et al. Characterization of AAV vector particle stability at the single-capsid level. J Biol Phys. 2018;44:181–94.
Cotmore SF, Tattersall P. Mutations at the base of the icosahedral five-fold cylinders of minute virus of mice induce 3’-to-5’ genome uncoating and critically impair entry functions. J Virol. 2012;86:69–80.
Ros C, Baltzer C, Mani B, Kempf C. Parvovirus uncoating in vitro reveals a mechanism of DNA release without capsid disassembly and striking differences in encapsidated DNA stability. Virology. 2006;345:137–47.
Rossi A, Dupaty L, Aillot L, Zhang L, Gallien C, Hallek M, et al. Vector uncoating limits adeno-associated viral vector-mediated transduction of human dendritic cells and vector immunogenicity. Sci Rep. 2019;9:3631.
Hsu HL, Brown A, Loveland AB, Lotun A, Xu M, Luo L, et al. Structural characterization of a novel human adeno-associated virus capsid with neurotropic properties. Nat Commun. 2020;11:3279.
Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–34.
Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–32.
Qing K, Wang XS, Kube DM, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci USA. 1997;94:10879–84.
Schwartz RA, Palacios JA, Cassell GD, Adam S, Giacca M, Weitzman MD. The Mre11/Rad50/Nbs1 complex limits adeno-associated virus transduction and replication. J Virol. 2007;81:12936–45.
Mano M, Ippodrino R, Zentilin L, Zacchigna S, Giacca M. Genome-wide RNAi screening identifies host restriction factors critical for in vivo AAV transduction. Proc Natl Acad Sci USA. 2015;112:11276–81.
Schreiber CA, Sakuma T, Izumiya Y, Holditch SJ, Hickey RD, Bressin RK, et al. An siRNA screen identifies the U2 snRNP spliceosome as a host restriction factor for recombinant adeno-associated viruses. PLoS Pathog. 2015;11:e1005082.
Mitchell AM, Samulski RJ. Mechanistic insights into the enhancement of adeno-associated virus transduction by proteasome inhibitors. J Virol. 2013;87:13035–41.
Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76:2043–53.
Chaanine AH, Nonnenmacher M, Kohlbrenner E, Jin D, Kovacic JC, Akar FG, et al. Effect of bortezomib on the efficacy of AAV9.SERCA2a treatment to preserve cardiac function in a rat pressure-overload model of heart failure. Gene Ther. 2014;21:379–86.
Li B, Ma W, Ling C, Van Vliet K, Huang LY, Agbandje-McKenna M, et al. Site-directed mutagenesis of surface-exposed lysine residues leads to improved transduction by AAV2, but not AAV8, vectors in murine hepatocytes in vivo. Hum Gene Ther Methods. 2015;26:211–20.
Bogyo M, Wang EW. Proteasome inhibitors: complex tools for a complex enzyme. Curr Top Microbiol Immunol. 2002;268:185–208.
Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–16.
Chen Q, Njenga R, Leuchs B, Chiocca S, Kleinschmidt J, Muller M. Sumoylation targets adeno-associated virus capsids but mainly restricts transduction by cellular mechanisms. J Virol. 2020; 94. e00871–20.
Holscher C, Sonntag F, Henrich K, Chen Q, Beneke J, Matula P, et al. The sumoylation pathway restricts gene transduction by adeno-associated viruses. PLoS Pathog. 2015;11:e1005281.
Schreiner S, Wodrich H. Virion factors that target Daxx to overcome intrinsic immunity. J Virol. 2013;87:10412–22.
Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, Govindasamy L, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther. 2007;15:1323–30.
Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105:7827–32.
Zhong L, Qing K, Si Y, Chen L, Tan M, Srivastava A. Heat-shock treatment-mediated increase in transduction by recombinant adeno-associated virus 2 vectors is independent of the cellular heat-shock protein 90. J Biol Chem. 2004;279:12714–23.
Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202.
Aslanidi GV, Rivers AE, Ortiz L, Song L, Ling C, Govindasamy L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One. 2013;8:e59142.
Buning H, Srivastava A. Capsid modifications for targeting and improving the efficacy of AAV vectors. Mol Ther Methods Clin Dev. 2019;12:248–65.
Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol. 2014;6:a022616.
Gray JL, Delft F, Brennan PE. Targeting the small GTPase superfamily through their regulatory proteins. Angew Chem Int Ed. 2020;59:6342–66.
Acknowledgements
We would like to thank Dr. Kyle Chamberlain for the critical reading of our manuscript.
Funding
This work was supported by NHLBI grants HL131404 (T.W.) and HL117505 (T.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: After publication the authors noticed that a section of their paper was incorrect.
Rights and permissions
About this article
Cite this article
Riyad, J.M., Weber, T. Intracellular trafficking of adeno-associated virus (AAV) vectors: challenges and future directions. Gene Ther 28, 683–696 (2021). https://doi.org/10.1038/s41434-021-00243-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00243-z
This article is cited by
-
Engineering approaches for RNA-based and cell-based osteoarthritis therapies
Nature Reviews Rheumatology (2024)
-
Reactive oxygen species enhance rAAV transduction by promoting its escape from late endosomes
Virology Journal (2023)
-
AAV-based in vivo gene therapy for neurological disorders
Nature Reviews Drug Discovery (2023)
-
Changing trends in the development of AAV-based gene therapies: a meta-analysis of past and present therapies
Gene Therapy (2023)
-
Adeno-associated virus-based caveolin-1 delivery via different routes for the prevention of cholesterol gallstone formation
Lipids in Health and Disease (2022)