Abstract
The field of gene therapy has made significant strides over the last several decades toward the treatment of previously untreatable genetic disease. Gene therapy techniques have been aimed at mitigating disease features of recessive and dominant disorders, as well as several cancers and other diseases. While there have been numerous disease targets of gene therapy trials, only four therapies have reached FDA and/or EMA approval for clinical use. Gene correction using CRISPR-Cas9 is an extension of gene therapy that has received considerable attention in recent years and boasts many possible uses beyond classical gene therapy approaches. While there is significant therapeutic potential using gene therapy and gene correction strategies, a number of hurdles remain to be overcome before they become more common in clinical use, particularly with regards to safety and efficacy. As research progresses in this exciting field, it is likely that these therapies will become first-line treatments and will have tremendous positive impacts on the lives of patients with genetic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Szybalska E, Szybalski W. Genetics of human cess line. IV. DNA-mediated heritable transformation of a biochemical trait. PNAS. 1962;48:2026–34.
Rogers S, Pfuderer P. Use of viruses as carriers added genetic information. Nature. 1968;219:749–51.
Rogers S, Lowenthal A, Terheggen H, Columbo J. Induction of arginase activity with the Shope papilloma virus in tissue culture cells from an argininemic patient. J Exp Med. 1973;137:1091–6.
Terheggen H. Unsuccessful trial of gene replacement in arginase deficiency. Z Kinderheilkd. 1975;119:1–3.
Blaese R, Culver K, Miller D, Carter C, Fleisher T, Clerici M. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science. 1995;270:475.
Wirth T, Parker N, Yla-Herttuala S. History of gene therapy. Gene. 2013;525:162–9.
Ribeil J, Hacein-Bey-Abina S, Payen E, Magnani A, Semerano M, Magrin E, et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;37:848–55.
Nathwani A, Reiss U, Tuddenham E, Rosales C, Chowary P, McIntosh J, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia b. N Engl J Med. 2014;371:1994–2004.
Griesenbach U, Pytel K, Alton E. Cystic fibrosis gene therapy in the UK and elsewhere. Hum Gene Ther. 2015;26:266–75.
Gaucer S, Lwin S, Titeux M, Abdul-Wahab A, Pironon N, Izmiryan A, et al. GENEGRAFT ex vivo phase I/II gene therapy trial for recessive dystrophic epidermolysis bullosa British. J Dermatol. 2019;182:788–818.
den Hollander A, Black A, Bennett J, Cremers F. Lighting a candle in the dark: adavances in genetics and gene therapy of recessive retinal dystrophies. JCI. 2011;120:3042–51.
Rodrigues G, Shalaev E, Karami T, Cunningham J, Slater N, Rivers H. Pharmaceutical development of AAV-based gene therapy products for the eye. Pharm Res. 2019;36:29.
Shibata S, Ranum P, Moteki H, Pan B, Goodwin A, Goodman S, et al. RNA interference prevents autosomal-dominant hearing loss. AJHG. 2016;98:1101–13.
Stoica L, Sena-Esteves M. Adeno associated viral vector delivered RNAi for gene therapy of SOD1 amyotrophic lateral sclerosis. Front Mol Neurosci. 2016;9:56.
Wallace L, Giesige C, Griffin D, Rodino-Klapaca L, Harper S. RNAi therapy for dominant limb girdle muscular dystrophy type 1A. Mol Ther. 2016;24:S248.
Maheshwari R, Tekade M, Sharma P, Tekade R. Nanocarriers assisted siRNA gene therapy for the management of cardiovascular disorders. Curr Pharm Des. 2015;21:4427–40.
Somasuntharam I, Boopathy A, Khan R, Martinez M, Brown M, Murthy N, et al. Delivery of Nox2-NADPH oxidase siRNA with polyketal nanoparticles for improving cardiac function following myocardial infarction. Biomaterials. 2013;34:7790–8.
Kwekkeboom R, Lei Z, Doevendans P, Musters R, Sluijter P. Targeted delivery of miRNA therapeutics for cardiovascular diseases: opportunities and challenges. Clin Sci. 2014;127:351–65.
Kurotaki N, Imaizumi K, Harada N, Masuno M, Kondoh T, Nagai T, et al. Haploinsufficiency of NSD1 causes Sotos syndrome. Nat Genet. 2002;30:365–6.
de Fusco M, Marconi R, Silvestri L, Atorino L, Rampoldi L, Morgante L, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33:192–6.
Shovlin C, Hughes J, Scott J, Seidman C, Seidman J. Characterization of endoglin and identification of novel mutations in hereditary hemorrhagic telangiectasia. Am J Hum Genet. 1997;61:68–79.
Pingault V, Bondurand N, Kuhlbrodt K, Goerich D, Prehu M, Puliti A, et al. SOX10 mutations in patients with Waardenburg-Hirschprung disease. Nat Genet. 1998;18:171–3.
Kuehn H, Caminha I, Niemela J, Rao K, Davis J, Fleisher T, et al. FAS haploinsufficiency is a common disease mechanism in the human autoimmune lymphoproliferative syndrome. J Immunol. 2011;186:6035–43.
Kortum F, Das S, Flindt M, Morris-Rosendahl D, Stefanova I, Goldstein A, et al. The coreFOXG1syndrome phenotype consists ofpostnatal microcephaly, severe mental retardation,absent language, dyskinesia, and corpuscallosum hypogenesis. J Med Genet. 2011;48:396–406.
Huang V, Qin Y, Wang J, Wang X, Place R, Lin G, et al. RNAa is conserved in mammalian cells. PLoS ONE. 2010;5:e8848.
Zhang Z, Wang Z, Liu X, Wang J, Feng L, Li C, et al. Up-regulation of p21WAF1/CIP1 by small activating RNA inhibits the in vitro and in vivo growth of pancreatic cancer cells. Tumori. 2012;98:804–11.
Place R, Wang J, Noonan E, Meyers R, Manoharan M, Charisse K, et al. Forumulation of small activating RNA into lipidoid nanoparticles inhibits xenograft prostate tumor growth by inducing p21 expression. Molecular Therapy. Nucleic Acids. 2012;1:e15.
Wright D, Li T, Yang B, Spalding M. TALEN-mediated genoe editing: prospects and perspectives. Biochem J. 2014;462:15–24.
Le Provost F, Lillico S, Passet B, Young R, Whitelaw B, VIlotte J. Zinc finger nuclease technology heralds a new era in mammalian transgenesis. Trends Biotechnol. 2010;28:134–41.
Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–70.
Cong L, Ran F, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Ran F, Hsu P, Wright J, Agarwala V, Scott D, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308.
Srivastava M, Raghavan S. DNA double-strand break repair inhibitors as cancer therapeutics. Chem Biol. 2014;22:17–29.
Hoeijmakers J. Genomic maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74.
Vilenchik M, Knudson A. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. PNAS. 2003;100:12871–6.
Jacobson N, Andrews M, Shepard A, Nishimura D, Searby C, Fingert J, et al. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–25.
Joe M, Sohn S, Hur W, Moon Y, Choi Y, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;312:592–600.
Yam G, Gaplovska-Kysela K, Zuber C, Roth J. Aggregated myocilin induces Russell bodies and causes apoptosis: implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am J Pathol. 2007;170:100–9.
Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–79.
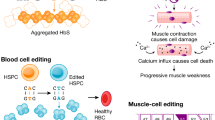
Jain A, Zode G, Kasetti R, Ran F, Yan W, Sharma T, et al. CRISPR-Cas9–based treatment of myocilin-associated glaucoma. PNAS. 2017;114:11199–204.
Jackow J, Guo Z, Hansen C, Abaci H, Doucet Y, Shin J, et al. CRISPR/Cas9-based targeted genome editing for correction of recessive dystrophic epidermolysis bullosa using iPS cells. PNAS. 2019;116:26846–52.
Burnight E, Gupta M, Wiley L, Anfinson K, Tran A, Triboulet R, et al. Using CRISPR-Cas9 to generate gene-corrected autologous iPSCs for the treatment of inherited retinal degeneration. Mol Ther. 2017;25:1999–2013.
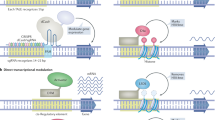
Chavez A, Scheiman J, Vora S, Pruitt B, Tuttle M, Iyer E, et al. Highly-efficient Cas9-mediated transcriptional programming. Nat Methods. 2015;12:326–8.
Saayman S, Lazar D, Scott T, Hart J, Takahashi M, Burnett J, et al. Potent and targeted activation of latent HIV-1 using the CRISPR/dCas9 activator complex. Mol Ther. 2016;24:488–98.
Hsu M, Liao H, Truong V, Huang K, Yu F, Chen H, et al. CRISPR-based activation of endogenous neurotrophic genes in adipose stem cell sheets to stimulate peripheral nerve regeneration. Theranostics. 2019;9:6099–111.
Vojta A, Dobrinic P, Tadic V, Bockor L, Korac P, Julg B, et al. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acid Res. 2016;44:5615–28.
Chira S, Jackson C, Oprea I, Ozturk F, Pepper M, Diaconu I, et al. Progresses towards safe and efficient gene therapy vectors. Oncotarget. 2015;6:30675–703.
Dong J, Fan P, Frizzell R. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–12.
Lundstrom K. Viral vectors in gene therapy. Diseases. 2018;6:42.
Sakamoto M, Yuasa K, Yoshimura M, Yokota T, Ikemoto T, Suzuki M, et al. Micro-dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem Biophys Res Commun. 2002;293:1265–72.
Simonelli F, Maguire A, Testa F, Pierce E, Mingozzi F, Bennicelli J, et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–50.
Cideciyan A, Hauswirth W, Aleman T, Kaushal S, Schwartz S, Boye S, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20:999–1004.
McTaggart S, Al-Rubeai M. Retroviral vectors for human gene delivery. Biotechnol Adv. 2002;20:1–31.
Zhang W, Li L, Li D, Liu J, Li X, Li W, et al. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum Gene Ther. 2018;29:160–79.
Milone M, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–41.
Yin H, Kanasty R, Eltoukhy A, Vegas A, Dorkin J, Anderson D. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–55.
Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–52.
Jayakumar R, Chennazhi K, Muzzarelli R, Tamura H, Nair S, Selvamurugan N. Chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr Polym. 2010;79:1–8.
de la Fuente M, Seijo B, Alonso M. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. IOVS. 2008;49:2016–24.
Zheng F, Shi X, Yang G, Gong L, Yuan H, Cui Y, et al. Chitosan nanoparticle as gene therapy vector via gastrointestinal mucosa administration: Results of an in vitro and in vivo study. Life Sci. 2007;80:388–96.
Cheong S, Lee C, Kim S, Jeong H, Kim E, Park E, et al. Superparamagnetic iron oxide nanoparticles-loaded chitosan-linoleic acid nanoparticles as an effective hepatocyte-targeted gene delivery system. Int J Pharm. 2009;372:169–76.
Kim T, Jin H, Kim H, Cho M, Cho C. Mannosylated chitosan nanoparticle-based cytokinegene therapy suppressed cancer growth in BALB/c mice bearing CT-26 carcinoma cells. Mol Cancer Ther. 2006;5:1723–32.
Xue Y, Wang N, Zeng Z, Huang J, Xiang Z, Guan Y. Neuroprotective effect of chitosan nanoparticle gene delivery system grafted with acteoside (ACT) in Parkinson’s disease models. J Mater Sci Technol. 2020;43:197–207.
Tan A, Rajadas J, Seifalaian A. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65:357–67.
Toh W, Lai R, Hui J, Lim S. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64.
Akao Y, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y, Naoe T. Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol Ther. 2011;19:395–9.
Xitong D, Xiaorong Z. Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene. 2016;575:377–84.
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543.
Gyorgy B, Sage C, AA I, Scheffer D, Brisson A, Tan S, et al. Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Mol Ther. 2017;25:379–91.
Stolberg S. The biotech death of Jesse Gelsinger. N Y Times Mag. 1999;136-140:149–50.
Braun C, Boztug K, Paruynski A, Witzel M, Schwartzer A, Rothe M, et al. Gene therapy for Wiskott-Aldrich syndrome-long-term efficacy and genotoxicity. Sci Transl Med. 2014;6:227ra33.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack M, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9.
Xiong W, Wu D, Xue U, Wang S, Chung M, Ji X, et al. AAV cis-regulatory sequences are correlated with ocular toxicity. PNAS. 2019;116:5785–94.
Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell J, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–60.
Wilson J, Flotte T. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum Gene Ther. 2020;31:695–6.
Hinderer C, Katz N, Buza E, Dyer C, Goode T, Bell P, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus expressing human SMN. Hum Gene Ther. 2018;29:285–98.
Therapeutics A. 2020. https://myotubulartrust.org/wp-content/uploads/23JUNE2020-Letter-to-Patient-Community_Sent.pdf.
Yang Y, Chi Y, Tang X, Ertl H, Zhou D. Rapid, efficient, and modular generation of Adenoviral vectors via isothermal assembly. Curr Protoc Mol Biol. 2016;16:S113.
Sumida S, Truitt D, Lemckert A, Vogels R, Custers J, Addo M, et al. Neutralizing antibodies to Adenovirus serotype 5 vaccine vectors are directed primarily against the Adenovirus hexon protein. J Immunol. 2005;174:7179–85.
Yu B, Wang Z, Dong J, Wang C, Gu L, Sun C, et al. A serological survey of human adenovirus serotype 2 and 5 circulating pediatric populations in Changchun, China, 2011. Virol J. 2012;9:287.
Halbert C, Miller A, McNamara S, Emerson J, Gibson R, Ramsey B, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17:440–7.
Manno C, Pierce G, Arruda V, Glader B, Ragni M, Rasko J, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–7.
Wang Z, Allen J, Riddell Sg P, Storb R, Tapscott S, Chamberlain J, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26.
Luo Y. Refining CRISPR-based genome and epigenome editing off-targets. Cell Biol Toxicol. 2019;35:281–3.
Kang S, Lee W, JAn, Lee J, Kim Y, Kim H. et al. Prediction-based highly sensitive CRISPR off-target validation using target-specific DNA enrichment. Nat Commun. 2020;11:3596.
Wang D, Mou H, Li S, Li Y, Hough S, Tran K, et al. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum Gene Ther. 2015;26:432–42.
Cutting G. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45–56.
Ashley-Koch A, Yang Q, Olney R. Sickle hemoglobin (HbS) allele and sickle cell disease: a HuGE review. Am J Epidemiol. 2000;151:839–45.
Russell S, Bennett J, Wellman J, Chung D, Yu Z, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hPRE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–60.
Sheffield V, Zhang Q, Heon E, Drack A, Stone E, Carmi R. The Bardet–Biedl syndrome. In: Erickson R, Wynshaw-Boris A, editors. Epstein’s Inborn Errors of Development, 3rd ed. New York, NY: Oxford University Press; 2016.
Reuter J, Spacek D, Snyder M. High-throughput sequencing technologies. Mol Cell. 2016;58:586–97.
Sertkaya A, Birkenbach A, Berlind A, Eyraud J. Examination of clinical trial costs and barriers for drug development. Asian J Pharmaceutical and Clinical Research. 2014;10:53–6.
Darrow J. Luxturna: FDA documents reveal the value of a costly gene therapy. Drug Discov Today. 2019;24:949–54.
Aartsma-Rus A, Krieg A. FDA approves eteplirsen for Duchenne muscular dystrophy: the next chapter in the eteplirsen saga. Nucleic Acid Ther. 2017;27:1–3.
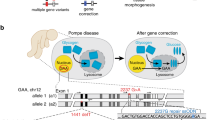
Seo S, Mullins R, Dumitrescu A, Bhattarai S, Gratie D, Wang K, et al. Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1. Investig Ophthalmol Vis Sci. 2013;11:6118–32.
Singh G, Dash D. Electrostatic mis-interactions cause overexpression toxicity of proteins in E. coli. PLoS ONE. 2013;8:e64893.
Ferrua F, Cicalese M, Galimberti S, Giannelli S, Dionisio F, Barzaghi F, et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019;6:e239–53.
Pendaries V, Gasc G, Titeux M, Tonasso L, Majia J, Hovnanian A. siRNA-mediated allele-specific inhibition of mutant type VII collagen in dominant dystrophic epidermolysis bullosa. J Investig Dermatol. 2012;132:1741–3.
Leachman S, Hickerson R, Schwartz S, Bullough E, Hutcherson S, Boucher K, et al. First-in-human mutation-targeting siRNA phase Ib trial of an inherited skin disorder. Mol Ther. 2010;18:442–6.
Funding
This work was funded by the National Institutes of Health, grant numbers R01 EY011298 (VCS), R01 EY017168 (VCS) and an institutional grant to the Department of Ophthalmology and Visual Sciences at the University of Iowa (P30EY025580), as well as the Roy J. Carver Charitable Trust (VCS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cring, M.R., Sheffield, V.C. Gene therapy and gene correction: targets, progress, and challenges for treating human diseases. Gene Ther 29, 3–12 (2022). https://doi.org/10.1038/s41434-020-00197-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-00197-8
This article is cited by
-
CRISPR/Cas9-mediated base editors and their prospects for mitochondrial genome engineering
Gene Therapy (2024)
-
Engineered compact pan-neuronal promoter from Alphaherpesvirus LAP2 enhances target gene expression in the mouse brain and reduces tropism in the liver
Gene Therapy (2023)
-
Low generational cystamine core PAMAM derivatives modified with nuclear localization signal derived from lactoferrin as a gene carrier
Korean Journal of Chemical Engineering (2023)
-
The roles of small extracellular vesicles in cancer and immune regulation and translational potential in cancer therapy
Journal of Experimental & Clinical Cancer Research (2022)
-
Recent advances of the biological and biomedical applications of CRISPR/Cas systems
Molecular Biology Reports (2022)