Abstract
Objectives
To determine whether subconjunctival Mitomycin-C (MMC) injections are as safe and effective as sponge-soaked MMC in phaco-trabeculectomy.
Methods
This prospective, randomized, interventional study was conducted on consecutive patients with visually significant cataract and an uncontrolled primary open-angle glaucoma. One hundred thirty-nine patients were recruited but 15 were ineligible for analysis. The patients were randomized into a sponge/injection group. All participants received a twin-site phaco-trabeculectomy. They were followed up on days 1, 15, 30, 3 months and 6 months post-operatively. A p-value < 0.05 was considered significant.
Interventions
Participants in the sponge group received an augmentation of their phaco-trabeculectomy with sponges soaked in a mixture of 0.04% MMC and 2% preservative-free Lignocaine in a 1:1 ratio, placed in the subconjunctival space for four minutes. Participants in the injection group received the same mixture as a subconjunctival injection, after surgical draping.
Results
There were 62 patients in each group. The groups had no significant differences in their baseline characteristics. The mean IOP at 6 months was significantly lower in the injection group (14.8 ± 3.7 mm Hg) than in the sponge group (17.1 ± 6.4 mm Hg) (p = 0.02). There was no notable difference in the complications or the final post-operative visual outcome but a significantly greater number of patients in the sponge arm required removal of the releasable suture (p = 0.001) and additional anti-glaucoma medications (p = 0.04) at six months post-operatively.
Conclusions
Subconjunctival MMC achieves a lower IOP with fewer anti-glaucoma medications than sponge-soaked MMC at six months for twin-site phaco-trabeculectomy in primary open-angle glaucoma with no additional risks.
Similar content being viewed by others
Introduction
Cataract and glaucoma may co-exist and when both are significant, a simultaneous surgical management or a phaco-trabeculectomy is preferred in low-economy nations to avoid repeat surgeries and multiple hospital admissions. A phaco-trabeculectomy is augmented using anti-metabolites like Mitomycin-C (MMC) to improve outcomes.
Classically, sponges soaked in MMC are placed in the subconjunctival space for augmentation [1]. However, with this technique, the actual dose delivered varies in every case [2]. Moreover, there is a risk of intra-operative loss of sponges and formation of focal, encapsulated blebs (“ring of steel”) in the long-term leading to surgical failure [3, 4]. An alternate method of MMC augmentation is via a subconjunctival/ intra-tenon/ subtenon injection [5,6,7]. With a subconjunctival injection, an exact dose is delivered and less stress is put on the conjunctival wound edges leading to fewer tears and leaks [8]. It improves the ease of construction of a fornix-based flap and prevents the backflow of MMC into the anterior chamber through the fistulous tract [4, 8].
A large number of publications have compared sponge-soaked and injectable modes of MMC augmentation in patients undergoing trabeculectomy [7, 9,10,11,12]. However, one cannot directly extrapolate this data to phaco-trabeculectomy. A recent systematic review with meta-analysis, which has evaluated data from non-randomized sources, has concluded that phaco-trabeculectomy is associated with a similar IOP control and fewer complications than a standalone trabeculectomy [13]. But this can at best be considered a level 3a evidence [14]. On the contrary, many authors have reported poorer outcomes following phaco-trabeculectomy compared to an isolated trabeculectomy [15,16,17,18].
There is only a single publication which has compared sponges with injectable MMC augmentation in phaco-trabeculectomy but the data was collected retrospectively [10]. Here we report the results of a randomized control trial (RCT) comparing the safety and efficacy of these two modes of MMC augmentation for phaco-trabeculectomy.
Materials and methods
This was a prospective, randomized, interventional study conducted in the Glaucoma department of Aravind Eye Hospital and Post-Graduate Institute of Ophthalmology, Tirunelveli. It adhered to the tenets of the Declaration of Helsinki and complied with the requirements of the Health Insurance Portability and Accountability Act and local patient privacy protection policies. The study protocol was reviewed and approved by the Tirunelveli Medical College Institutional Research Ethics Committee before patient recruitment (Certificate Reference Number: 591/DNB/2014/02). The period of patient recruitment was from December 2014 to May 2015 and all participants were followed-up for a period of six months post-surgery.
An informed consent was obtained from all subjects who were recruited. One eye of consecutive patients satisfying the inclusion and exclusion criteria were considered for the study. The inclusion criterion was a visually significant cataract along with a primary open-angle glaucoma demonstrating either a progression of visual field loss and/or an uncontrolled IOP with maximally tolerated medications in an individual aged more than 25 years. A visually significant cataract was defined as a lenticular opacity with a best corrected visual acuity of 0.18 logMAR or worse and an operable cataract as revealed on slit lamp evaluation.
Monocular individuals, eyes with no light perception, pregnant or nursing women, individuals with suspected scleral thinning on slit lamp examination or high axial myopia (≥−6.0 Dioptres sphere and an axial length ≥ 26 mm) and those with secondary or childhood glaucoma were excluded. Individuals with previous ocular surgeries, limbal stem cell deficiency, iris neovascularization, angle closure, chronic or recurrent uveitis and those unwilling/ unable to provide consent were also excluded.
There were two arms in the study - the sponge arm and the injection arm. Assuming a surgical success rate of 89% for injectable MMC and 70% for sponge-based augmentation, a sample size of 62 in each arm was calculated assuming a 5% level of significance, 80% power and 95% confidence interval [19, 20]. On the day of the surgery, recruited patients underwent a simple randomization to either group using a pre-determined random list of 150 numbers generated using the unweighted Bernoulli distribution protocol of the Analysis ToolPak™ add-in of Microsoft Excel© (Microsoft Corporation, Redmond, Washington, USA). Subjects, masked to the randomization, were assigned to a treatment group, based on the value at their rank (0 = Sponge group, 1 = Injection group). During follow-up, glaucoma specialists, masked to the patient’s group assignment, performed the post-operative examination and data collection.
All study participants underwent a twin-site MMC augmented phaco-trabeculectomy. High IOP was controlled with anti-glaucoma medications (AGMs) before surgery as needed. Miotics were stopped 48 h before surgery. If IOP was more than 30 mm Hg pre-operatively then the surgery was done 30 min after an intravenous infusion of 20% Mannitol (1 g/kg body weight).
In the sponge arm, two separate semi-circular surgical sponges prepared by bisecting a 7-mm Merocel® corneal light shield (Beaver-Visitech International, Waltham, MA, USA) soaked in a 1:1 mixture of 0.04% MMC and 2% preservative-free Lignocaine were used. They were placed underneath the conjunctival flap for 4 min and were removed. Thereafter, the surgical site was washed with 20 ml of balanced salt solution. In the injection arm, a mixture of 0.1 ml of 0.04% of MMC with 0.1 ml of 2% preservative-free Lignocaine was injected into the subconjunctival space with a 30-gauge needle 6–8 mm away from the limbus adjacent to the site of the future bleb after surgical draping. The surgeon waited for 4 min after the injection. During this interval the drug was massaged away from the limbus onto the future filtration site. The surgical site was then opened and the area was washed with 20 ml of balanced salt solution.
All surgeries were performed by two senior glaucoma surgeons (MAK and DM) with more than 7 years of experience in the subspeciality, in a similar manner. After a fornix-based superior conjunctival peritomy with MMC application (depending on the arm), a triangular partial thickness scleral flap with a side length of 4 mm and base at the limbus was fashioned. The phacoemulsification was performed using a 2.8 mm temporal clear corneal incision. A foldable posterior chamber Intraocular lens (IOL) was placed in the capsular bag. Thereafter, the trabeculectomy was completed using a Kelly’s punch and a peripheral iridectomy was made. The scleral flap was closed with an apical releasable 10–0 monofilament Nylon suture. Two additional fixed 10–0 monofilament Nylon sutures were placed on either side. The conjunctiva was closed with 8-0 polyglactin sutures. The anterior chamber was formed with balanced salt solution and Moxifloxacin (0.5%) was injected intracamerally at the end of surgery.
All patients were started on 2 hourly Dexamethasone (0.1%) with Chloramphenicol (0.5%) eye drops and 12 hourly Homatropine (2%) eye drops from the first post-operative day. The topical steroids were tapered depending upon the clinician’s discretion. The patients were reviewed on days 1, 15, 30 and at 3 and 6 months after surgery. The need for ocular massage, release of releasable sutures, LASER suturolysis, bleb needling and 5-Fluorouracil injections were decided by the reviewing glaucoma specialist at every visit.
At the time of recruitment and in all post-operative visits, the visual acuity was measured using a Snellen’s chart and was converted to logMAR for statistical evaluation. The following logMAR values were used for visual acuities which could not be mathematically converted to logMAR: finger counting close to face = 1.7 logMAR, hand movement = 2.0 logMAR and light perception = 2.3 logMAR. [21]. Intraocular pressure was measured both pre- and post-operatively using a Goldmann applanation tonometer (AT 900; Haag Streit International, Koeniz, Switzerland). A slit lamp evaluation was done at all the visits. Post-operatively, if a subject failed to turn up within one week from the given review date in any visit after discharge, they were considered lost to follow-up and were removed. The primary outcome measure was surgical success at 6 months post-operatively. “Complete” success was defined by a post-operative IOP of ≤18 mm Hg but >6 mm Hg and a ≥20% reduction in IOP from the pre-operative reading without addition of AGMs or other interventions with retention of light perception. Eyes that needed additional AGMs or minor glaucoma interventions (release of releasable suture, LASER suturolysis, ocular massage, bleb needling with MMC or 5FU injection) to meet the criteria of complete success were termed as “qualified” successes. The surgery was deemed a “failure” if IOP was >18 mm Hg or there was a <20% reduction in IOP from the pre-operative reading in spite of AGMs or minor interventions, or if there was persistent hypotony or a loss of light perception.
The statistical analysis was performed using the RcmdrPlugin.EZR package v 1.55 of the statistical software R v 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria) [22]. Continuous variables were summarized as mean ± standard deviation and categorical variables as frequency (percentage). The Fisher’s exact test/χ2 test was applied to assess the differences between two categorical variables, based on satisfaction of Cochran’s rule [23]. The Shapiro-Wilk test was used to check whether continuous variables followed the Gaussian distribution curve. Normally distributed continuous variables were compared using the Student’s t-test. For non-normally distributed variables, stochastic dominance was evaluated using the Mann–Whitney U test or the Wilcoxon signed-rank test based on whether the recordings were independent or paired. Kaplan–Meier survival curves were constructed for surgical success for both the groups and a log-rank test was used to compare them. Eyes that met the criteria for failure were censored at each time point. A p-value < 0.05 was considered statistically significant.
Results
A total of 139 eyes of 139 patients met the inclusion criteria during the recruitment window. After randomization using the random number chart, 72 participants were assigned to the sponge group and 67 were assigned to the injection group. One patient in the injection group suffered a panic attack in the operation theatre after draping and his surgery was postponed. None of the remaining recruited patients suffered from an intra-operative complication. MAK performed 38 surgeries in the sponge group and 32 surgeries in the injection group while DM performed 34 surgeries in each group (p = 0.739). After discharge, 10 patients in the sponge group and 4 patients in the injection group did not come for all the prescribed visits at the hospital and were therefore removed from the study. Overall, the data of 124 patients, 62 in each group, was eligible for analysis (Fig. 1).
Table 1 shows that the two groups had comparable baseline demographics and Glaucoma Medication Intensity Index (GMII) scores (Table 1). GMII, a validated predictor for the risk of filtration surgery failure due to pre-operative AGM use, was calculated using the formula by Wong et al [24]. The final logMAR visual acuity at 6 months post-operatively in the sponge and the injection arms were 0.081 ± 0.17 and 0.070 ± 0.12 respectively (p = 0.86). This was a statistically significant improvement compared to the pre-operative logMAR value (p < 0.001 in both the arms). However, there was no significant difference in visual acuity in between the two groups on any post-operative visit (Supplementary Table 1).
Table 2 summarizes the mean IOP and AGM use in the two groups in all the visits. At 6 months post-operatively, the injection group had a significantly lower mean IOP and a lesser requirement for AGMs (p = 0.02 and 0.04 respectively). There was no significant difference in the incidence of different complications in the two groups (Supplementary Table 2). None of the patients developed major complications associated with cataract extraction on the first post-operative day or during the course of follow-up (hyphaema, fibrinous uveitis, hypopyon or endophthalmitis). Analysis of the need for different minor interventions in the two groups revealed that a greater proportion of participants in the sponge group had to undergo removal of the apical releasable suture (p = 0.001) (Table 3).
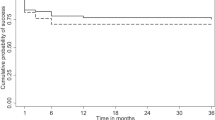
At the end of 6 months, complete success was achieved in 17.7% (n = 11) participants in the sponge group and 40.3% (n = 25) of those in the injection group (p = 0.01). The overall success rate (complete + qualified success) for the sponge and the injection groups were 37.10% (n = 23) and 56.45% (n = 35), respectively (p = 0.048). The Kaplan–Meier survival curve showed a statistically significant higher rate of success with/ without AGMs and minor interventions in the injection group compared to the sponge group (p = 0.003) (Fig. 2). Right-censoring in the survival analysis curve (Fig. 2) exclusively indicates the occurrence of failure.
Discussion
In a phaco-trabeculectomy, phacoemulsification generates additional inflammatory mediators which pass through the newly constructed fistula [25, 26]. This stimulates an exuberant healing reaction resulting in surgical-site scarring, an increased resistance to fluid egress and finally a surgical failure [18]. Wound-healing modulation using anti-metabolites, especially MMC, is a popular choice to circumvent this problem [27]. The efficacy of MMC varies depending on the method of application, the area of contact and essentially non-modifiable host factors [1]. Among the different modes of MMC augmentation in guarded filtration surgeries, subconjunctival application with pre-soaked sponges and tissue injections are the most common. Although studies in standalone trabeculectomy surgeries have shown that tissue injections are as safe and efficacious as pre-soaked sponges, there are only a few publications on the effect of tissue injections in phaco-trabeculectomy [10, 28]. This RCT prospectively looks at IOP lowering, complication profiles, the need for intervention(s)/ AGMs and overall success rates after these two modes of MMC augmentation in phaco-trabeculectomy over a 6 month follow-up period.
The final mean IOP, at 6 months, was significantly lower in our injection group (p = 0.021). However, Chiew et al. in his retrospective review has noted no statistically significant difference in the IOP between the two modes of augmentation at 6 months post phaco-trabeculectomy (p = 0.221) [10]. This is probably because they used 0.04% MMC for sponge-based augmentation and 0.02% MMC for injection-based augmentation instead of the same concentration in both the arms as in our study. Farooq et al. found a lower IOP at 6 months post-operatively in the injection group (12.30 ± 3.94 mm Hg) but their study population was a heterogeneous mix of primary and secondary glaucoma patients [28]. The decline in IOP noted in the sponge group in this study was similar to that found in other contemporary studies using sponge-based augmentation [12, 29].
Although there was no statistically significant difference in the post-operative complication rates between the two groups, the frequency of different complications in our participants was different from contemporary publications. The common complications in both the groups of our study were a failed bleb and a tenons cyst. Shallow anterior chambers and hypotony are reported as the most common complications after a phaco-trabeculectomy especially after sponge-based MMC augmentation due to ciliary body toxicity and intraocular percolation of the drug [10]. But we found no statistically significant difference in the incidence of shallow anterior chambers [nsponge = 3 (4.8%); ninjection = 5 (8.1%); p = 0.454] or hypotony [nsponge = 5 (8.1%); ninjection = 4 (6.5%); p = 0.732] between participants in the two arms of our study. The incidence of hypotony following a guarded filtration surgery has been reported to vary markedly from 1.5% to 41%. [30]. The difference in the reported incidence of different complications in published literature may be related to the differences in the tightness and number of sutures, scleral flap configuration, IOP immediately after surgery and timing of suturolysis as well as a surgeon factor [31].
For tissue injections, unpreserved lignocaine is frequently mixed with reconstituted MMC as it is believed to provide an additive effect by preventing proliferation of fibroblasts [32]. However, mixing reconstituted MMC with unpreserved lignocaine has an antagonistic pharmacokinetic impact because lignocaine renders the solution more acidic (from 7.1 to 6.57) making MMC less stable [33]. Moreover, recent translational research has concluded that lignocaine has a supra-additive cytotoxic effect in vitro when combined with MMC.(ref. 34) In our study, a uniform concentration of reconstituted MMC with lignocaine has been used in all the study participants irrespective of the mode of augmentation. To our knowledge this is the first time that this mixture has been used for sponge-based MMC augmentation for phaco-trabeculectomy. The results of our study indirectly support the in vivo safety of this mixture for both modes of augmentation.
Analysis of the different minor post-operative interventions required in the study participants revealed a statistically significant greater need for removal of the apical releasable suture in the sponge arm (p = 0.001). The most likely reason would be an inherently poorer filtration rate resulting in a hypofunctional bleb in participants of the sponge group. This correlates with the significantly greater need for AGMs in the sponge arm at six months (p = 0.041). We believe that this is an example of retarded drug effect due to unfavourable pharmacokinetics. Contemporary studies have shown that maximum ocular tissue concentrations of MMC can be achieved with subconjunctival injections and not surface contact techniques [35]. Once injected, the drug can be massaged over a wide area translating to a more posterior and low-lying bleb. Such a bleb morphology has been shown to correlate well with good IOP control post-operatively [36]. Our findings correlate well with a similar RCT in trabeculectomy [12].
We compared the success rates of the interventions at 6 months. At 6 months, the frequency of complete success was low in both the groups but, it still remained higher in the injection group than in the sponge group (p = 0.01). The frequency of failure at this point of time was higher in the sponge group. Many studies have shown higher success rates in augmented filtration surgeries but ours had lower rates of success probably because a stringent cut-off of 18 mm Hg with ≥20% reduction in IOP from the pre-operative reading was used [37, 38]. Moreover, in a few studies, procedures performed under the slit lamp such as bleb needling and ocular massage were not considered as re-operations and were included in “complete success” while we have included them under “qualified success” [12].
There were a few limitations to our study. A simple randomization rather than a stratified randomization technique was used to assign the patient into either group as they were selected singly and not in bulk. Moreover, a simplified system of excluding all participants lost to follow-up was practised. An intention-to-treat strategy, another common technique of handling attrition, was avoided because complete exclusion of all drop-outs provides a more accurate risk estimation and labelling when the duration of a study is within a year [39]. Robust compensatory models like inverse probability of censoring weighted adjustment with causal diagrams were not implemented as all the confounders for dependant censoring could not be identified [40].
As a single eye in all these patients were included, systemic absorption of AGMs from the fellow eye might have been a confounding factor in the IOP lowering effect of the intervention. We did not collect any data regarding the number and type of AGMs in the fellow eye and cannot predict the extent to which this affected the type I error [41].
A literature review revealed that seepage of MMC into the anterior chamber is less common in injection-based augmentation [8]. Therefore, assessment of pre and post-intervention endothelial cell counts as well as evaluation of other confounders for endothelial loss like phaco power and phaco time would have helped assess the clinical significance of this advantage.
Another major limitation of this study was the absence of any objective method of bleb grading [42, 43]. With the current dataset, it is therefore impossible to predict the possible effect that the two modes of MMC administration had on bleb morphology. These differences in morphological characteristics may have been responsible for the difference in success rates. If available, this data could also have been used to calculate correlation coefficients between different bleb parameters and the amount of IOP reduction [44].
A long-term follow-up is essential in evaluating the outcomes of a filtration surgery. However, this is not possible in our patient pool where there is a high attrition rate, which exceeds 30% beyond 9 months after an intervention. With the current policy of removing all patients lost to follow-up from the analysis, only a miniscule subset of the initial participants would be available for final evaluation in case of a longer follow-up period. This would be a threat to the external validity of the study findings. The reader must therefore be cautious to neither extrapolate the findings beyond the reported timeline nor to extend it to patients with secondary forms of glaucoma.
Despite these limitations, this study provides some important insights into the effect of MMC augmentation on phaco-trabeculectomy procedures. To the best of our knowledge, this is the first RCT comparing the two modes of MMC augmentation for phaco-trabeculectomy. A subconjunctival MMC injection appears to be equally safe and more efficacious than sponge-based MMC in the short term in primary open-angle glaucoma patients with a significant cataract undergoing a twin-site phaco-trabeculectomy. But newer studies with longer follow-up periods need to be designed to compare the long-term success rates and complication profiles of these two methods of MMC application.
Summary
What was known before
-
Injectable MMC is as safe and efficacious as sponge-based MMC augmentation for trabeculectomy
What this study adds
-
Injectable MMC is as safe and more efficacious than sponge-based MMC augmentation for phaco-trabeculectomy in the short term
Data availability
The dataset analyzed during the current study has not been uploaded into a repository but will be available from the corresponding author on reasonable request approved by the Tirunelveli Medical College Institutional Research Ethics Committee and Aravind Eye Hospital & PG Institute of Ophthalmology, Tirunelveli.
References
Al Habash A, Aljasim LA, Owaidhah O, Edward DP. A review of the efficacy of mitomycin C in glaucoma filtration surgery. Clin Ophthalmol. 2015;9:1945–51.
Urbaneja D, Morilla-Grasa A, Jimenez E, Montemayor J, Marcobal N, Aragay C, et al. In vitro mitomycin C absorption and delivery with different sponge materials used in filtering surgery. Clin Ophthalmol. 2016;10:665–9.
Khamar M, Bhojwani D, Patel P, Vasavada A. Leftover mitomycin-c sponge causing blebitis. Indian J Ophthalmol. 2019;67:1753–5.
Bell K, de Padua Soares Bezerra B, Mofokeng M, Montesano G, Nongpiur ME, Marti MV, et al. Learning from the past: Mitomycin C use in trabeculectomy and its application in bleb-forming minimally invasive glaucoma surgery. Surv Ophthalmol. 2021;66:109–23.
Kandarakis SA, Papakonstantinou E, Petrou P, Diagourtas A, Ifantides C, Georgalas I, et al. One-year randomized comparison of safety and efficacy of trabeculectomy with mitomycin C sub-tenon injection versus mitomycin C-infused sponges. Ophthalmol Glaucoma. 2022;5:77–84.
Lee E, Doyle E, Jenkins C. Trabeculectomy surgery augmented with intra-Tenon injection of mitomycin C. Acta Ophthalmol. 2008;86:866–70.
Do JL, Xu BY, Wong B, Camp A, Ngai P, Long C, et al. A randomized controlled trial comparing subconjunctival injection to direct scleral application of mitomycin C in trabeculectomy. Am J Ophthalmol. 2020;220:45–52.
Hung PT. Mitomycin-C in glaucoma filtering surgery. Asia Pac Ophthalmol. 2000;2:21–4.
Maheshwari D, Kanduri S, Rengappa R, Kadar MA. Intraoperative injection versus sponge-applied mitomycin C during trabeculectomy: one-year study. Indian J Ophthalmol. 2020;68:615–9.
Chiew W, Guo X, Ang BCH, Lim APH, Yip LWL. Comparison of surgical outcomes of sponge application versus subconjunctival injection of mitomycin-C during combined phacoemulsification and trabeculectomy surgery in asian eyes. J Curr Ophthalmol. 2021;33:253–9.
Khouri AS, Huang GY, Huang L. Intraoperative Injection vs Sponge-applied Mitomycin C during Trabeculectomy: one-year study. J Curr Glaucoma Pract. 2017;11:101–6.
Esfandiari H, Pakravan M, Yazdani S, Doozandeh A, Yaseri M, Conner IP. Treatment outcomes of mitomycin C-augmented trabeculectomy, sub-tenon injection versus soaked sponges, after 3 years of follow-up: a randomized clinical trial. Ophthalmol Glaucoma. 2018;1:66–74.
Ahmadzadeh A, Kessel L, Subhi Y, Bach-Holm D. Comparative efficacy of phacotrabeculectomy versus trabeculectomy with or without later phacoemulsification: a systematic review with meta-analyses. J Ophthalmol. 2021;2021:6682534.
Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–10.
Ogata-Iwao M, Inatani M, Takihara Y, Inoue T, Iwao K, Tanihara H. A prospective comparison between trabeculectomy with mitomycin C and phacotrabeculectomy with mitomycin C. Acta Ophthalmol. 2013;91:e500–1.
Kleinmann G, Katz H, Pollack A, Schechtman E, Rachmiel R, Zalish M. Comparison of trabeculectomy with mitomycin C with or without phacoemulsification and lens implantation. Ophthalmic Surg Lasers. 2002;33:102–8.
Lochhead J, Casson RJ, Salmon JF. Long term effect on intraocular pressure of phacotrabeculectomy compared to trabeculectomy. Br J Ophthalmol. 2003;87:850–2.
Sacchi M, Monsellato G, Villani E, Lizzio RAU, Cremonesi E, Luccarelli S, et al. Intraocular pressure control after combined phacotrabeculectomy versus trabeculectomy alone. Eur J Ophthalmol. 2022;32:327–35.
Mostafaei A. Augmenting trabeculectomy in glaucoma with subconjunctival mitomycin C versus subconjunctival 5-fluorouracil: a randomized clinical trial. Clin Ophthalmol. 2011;5:491–4.
Singh J, O’Brien C, Chawla HB. Success rate and complications of intraoperative 0.2 mg/ml mitomycin C in trabeculectomy surgery. Eye. 1995;9:460–6.
Lee JWY, Lai JSM, Yick DWF, Tse RKK. Retrospective case series on the long-term visual and intraocular pressure outcomes of phacomorphic glaucoma. Eye. 2010;24:1675–80.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–51.
Wong JKW, Leung TK, Lai JS, Chan JC. Evaluation of adverse effects of topical glaucoma medications on trabeculectomy outcomes using the glaucoma medications intensity index. Ophthalmol Ther. 2022;11:387–401.
Husain R, Liang S, Foster PJ, Gazzard G, Bunce C, Chew PTK, et al. Cataract surgery after trabeculectomy: the effect on trabeculectomy function. Arch Ophthalmol. 2012;130:165–70.
Siriwardena D, Kotecha A, Minassian D, Dart J, Khaw P. Anterior chamber flare after trabeculectomy and after phacoemulsification. Br J Ophthalmol. 2000;84:1056–7.
Cabourne E, Clarke JCK, Schlottmann PG, Evans JR. Mitomycin C versus 5-Fluorouracil for wound healing in glaucoma surgery. Cochrane Database Syst Rev. 2015;2015:CD006259.
Farooq S, Farooq H, Faisal M. Outcome of intraoperative Mitomycin C injection in trabeculectomy and phacotrabeculectomy in patients with glaucoma. Pak J Ophthalmol. 2021;37:213–7.
Guimarães ME, de Pádua Soares Bezerra B, de Miranda Cordeiro F, Carvalho CH, Danif DN, Prata TS, et al. Glaucoma surgery with soaked sponges with Mitomycin C vs sub-tenon injection: short-term outcomes. J Curr Glaucoma Pr. 2019;13:50–4.
Murthy SK, Damji KF, Pan Y, Hodge WG. Trabeculectomy and phacotrabeculectomy, with mitomycin-C, show similar two-year target IOP outcomes. Can J Ophthalmol. 2006;41:51–9.
Bindlish R, Condon GP, Schlosser JD, D’Antonio J, Lauer KB, Lehrer R. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002;109:1336–41.
Janani MK, Jaichandran V, Madhavan HNR, Vijaya L, George RJ, Ambastha PS, et al. Effect of lignocaine concentration on human fibroblasts growth in eyes undergoing trabeculectomy: an in vitro study. Biomed Hub. 2018;3:1–10.
Klifto MR, Fleischman D. pH of Mitomycin-C formulations for filtration surgery. J Glaucoma. 2019;28:647–8.
Park A, Hardin JS, Bora NS, Morshedi RG. Effects of lidocaine on mitomycin C cytotoxicity. Ophthalmol Glaucoma. 2021;4:330–5.
Mietz H, Diestelhorst M, Rump AF, Theisohn M, Klaus W, Krieglstein GK. Ocular concentrations of mitomycin C using different delivery devices. Ophthalmologica. 1998;212:37–42.
Sacu S, Rainer G, Findl O, Georgopoulos M, Vass C. Correlation between the early morphological appearance of filtering blebs and outcome of trabeculectomy with mitomycin C. J Glaucoma. 2003;12:430–5.
Lim MC, Hom B, Watnik MR, Brandt JD, Altman AR, Paul T, et al. A comparison of trabeculectomy surgery outcomes with Mitomycin-C applied by intra-tenon injection versus sponge. Am J Ophthalmol. 2020;216:243–56.
Mokbel TH, Ghanem AA, Moawad AI, Nafie EM, Nematallah EH. Comparative study of phacoemulsification-subscleral trabeculectomy versus phacoemulsification-deep sclerectomy. Saudi J Ophthalmol. 2009;23:189–96.
Reps JM, Rijnbeek P, Cuthbert A, Ryan PB, Pratt N, Schuemie M. An empirical analysis of dealing with patients who are lost to follow-up when developing prognostic models using a cohort design. BMC Med Inf Decis Mak. 2021;21:43.
Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ. Selection bias due to loss to follow up in cohort studies. Epidemiology. 2016;27:91–7.
Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52:19–26.
Wells AP, Crowston JG, Marks J, Kirwan JF, Smith G, Clarke JCK, et al. A pilot study of a system for grading of drainage blebs after glaucoma surgery. J Glaucoma. 2004;13:454–60.
Cantor LB, Mantravadi A, WuDunn D, Swamynathan K, Cortes A. Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana Bleb Appearance Grading Scale. J Glaucoma. 2003;12:266–71.
Smith M, Chipman ML, Trope GE, Buys YM. Correlation between the indiana bleb appearance grading scale and intraocular pressure after phacotrabeculectomy. J Glaucoma. 2009;18:217–9.
Author information
Authors and Affiliations
Contributions
SC was responsible for drafting the protocol, reviewing literature, extracting and analyzing data, interpreting the results, writing the manuscript and generating the tables and the graphs. MAK and DM were responsible for designing the study protocol, interpreting the results, and providing feedback on the manuscript. MRP, SkC and RR helped in interpreting the results and provided feedback on the manuscript. All authors approved the submitted version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chakrabarty, S., Kader, M.A., Maheshwari, D. et al. Short-term outcomes of Mitomycin-C augmented phaco-trabeculectomy using subconjunctival injections versus soaked sponges: a randomized controlled trial. Eye 38, 1196–1201 (2024). https://doi.org/10.1038/s41433-023-02869-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02869-2