Abstract
Objective
To evaluate the efficacy and safety of a single-dose intravitreal umedaptanib pegol (anti-FGF2, investigational new drug) for the treatment of neovascular age-related macular degeneration (nAMD).
Methods
Nine participants who had a diagnosis of refractory nAMD were enrolled and received a single intravitreal injection of umedaptanib pegol at increasing doses of 0.2, 1.0 or 2.0 mg in the study eye.
Results
All three doses of umedaptanib pegol evaluated in the study were safe and well tolerated. No severe adverse event (AE) was observed in the study. There was an improvement in retinal fluid measured by central subfield thickness (CST) in most subjects. Remarkably, all three subjects who received 2.0 mg/eye showed improvement of more than 150 μm.
Conclusions
Intravitreal umedaptanib pegol was safe, well tolerated, and demonstrated an indication of bioactivity in participants that have persistent subretinal fluid refractory to the treatment with anti-VEGFs.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual loss in the elderly population [1]. The number of people with AMD was estimated to be 196 million in 2020 and will reach 288 million in 2040 globally [2]. The loss of central vision in neovascular age-related macular degeneration (nAMD) is caused by choroidal neovascularization (CNV), resulting in macular haemorrhage, effusion, and fibrosis [3] (Fig. 1).
Upon inflammation, FGF2 in the presence of TGFβ2 stimulates RPE cells to undergo EMT to fibroblasts, leading to scar formation. In parallel, FGF2 and VEGF act as vascular endothelial cell mitogens in the initiation and maturation of angiogenic vessels. Hence, FGF2 should be a new therapeutic target in nAMD by its dual action.
The current standard therapy for nAMD is to target vascular endothelial growth factor (VEGF) using ranibizumab (Lucentis®, Roche/Genentech), aflibercept (Eylea®, Regeneron Pharmaceuticals), bevacizumab (Avastin®, Roche/Genentech) and faricimab (Vabysmo®, bispecific anti-VEGF/anti-Ang2 drug, Roche/Genentech) [4, 5]. Frequent intravitreal injections of anti-VEGF drugs have been shown to be associated with major visual benefits in participants with AMD [6,7,8]. Despite their present efficacy, anti-VEGF agents also have several limitations. Participants may require a high injection frequency during years of treatment, leading to a high treatment burden. In addition, compared with participants in clinical trials, real-world participants showed worse visual outcomes, possibly due to poor compliance [9, 10]. Moreover, the risk of developing retinal scarring and geographic atrophy was increased after 2–5 years of treatment [11].
Recent studies have shed light on the role of fibroblast growth factor-2 (FGF2) in disease progression of nAMD. In mammals, FGFs have 22 known members that exert important functions in regulating cell proliferation, differentiation, and migration [12, 13]. Upon binding to tyrosine kinase FGF receptors, FGFR1-FGFR4, FGFs activate essential signalling pathways, such as the mitogen-activated protein kinase (MAPK)/ERK and JNK pathways, that are centrally involved in angiogenesis, tissue remodelling, and regeneration, including repair of neuronal damage, skin wound healing, joint protection, and control of hypertension. FGF2 is a major member of the FGF family along with FGF1 and has been implicated in the pathophysiology of both angiogenesis and fibrosis [14, 15]. It has been demonstrated that FGF2 stimulates the growth of vascular endothelial cells and tubular structure formation [16] in addition to promoting VEGF expression [17, 18]. The angiogenic activity of FGF2 was reportedly stronger than that of VEGF in a mouse corneal micropocket assay [19, 20]. Moreover, FGF2 in combination with TGFβ2 stimulates epithelial-mesenchymal transformation (EMT) in retinal pigment epithelial (RPE) cells [21], which might contribute to submacular fibrosis.
To address the targetability of FGF2 in nAMD treatment, the anti-FGF2 aptamer, umedaptanib pegol (formerly called RBM-007 [22]), was examined for the treatment of named in animal models. Umedaptanib pegol is composed of 37 nucleotides, whose ribose 2′ positions are modified to resist ribonucleases, in addition to being 5′-PEGylated and 3′-conjugated with an inverted dT to confer an advantageous pharmacokinetic profile [22]. Umedaptanib pegol binds strongly and specifically to FGF2 with the dissociation constant of 2 pM and blocks the interaction between human FGF2 and its receptors FGFR1-FGFR4 [21, 22]. In the in vivo studies conducted in mice and rats, umedaptanib pegol was able to inhibit FGF2-induced angiogenesis, laser-induced CNV, and CNV with fibrosis [21]. Pharmacokinetic studies of umedaptanib pegol in the rabbit vitreous revealed high and relatively long-lasting profiles [21]. Moreover, combined treatment with umedaptanib pegol and ranibizumab showed a synergistic effect in preventing CNV [21]. This therapeutic potential is further supported by the finding that FGF receptor double-conditional knockout (Fgfr1/2) mice showed a marked reduction in CNV accompanied by a decrease in the level of FGF2 upon laser injury [23]. Additionally, FGF2 was the only essential ligand in the in vivo models of CNV, in keeping with FGF2 regulation of pathogenic angiogenesis via the STAT3 pathway [24]. The anti-angiogenic and anti-scarring dual action of umedaptanib pegol holds promise as an additive or alternative therapy to anti-VEGF treatments for nAMD (see Fig. 1).
We report on our clinical trials first designed as phase 1 (SUSHI), open-label, safety and feasibility study of a single-dose umedaptanib pegol in subjects with refractory nAMD, and then followed by phase 2 trials in the accompanying manuscript.
Methods
Investigational new drug, umedaptanib pegol
The drug substance for umedaptanib pegol intravitreal injection is a sodium salt of a single-stranded oligonucleotide aptamer with thirty-seven structure-forming nucleotides in length that terminates at the 3’-end in an inverted 2’-deoxy-thymidine and at the 5’-end in an aminohexyl linker. The aminohexyl linker is covalently conjugated via an N-alkyl amide linkage with one branched 2 × 20-kDa mono-methoxy polyethylene glycol (PEG) unit. The chemical formula of umedaptanib pegol is as follows: RNA, amC6-5’-(Guom-Guom-Guom-Adom-Urom-Adom-Cydm-(2’-deoxy-2’-fluoro)Uro-Adom-Guom-Guom-Guo-Cydm-Adom-Urom-(2’-deoxy-2’-fluoro)Uro-Adom-Adom-Urom-Guom-(2’-deoxy-2’-fluoro)Uro-Urom-Adom-Cydm-Cydm-Adom-Guo-(2’-deoxy-2’-fluoro)Uro-Guo-(2’-deoxy-2’-fluoro)Uro-Adom-Guom-Urom-Cydm-Cydm-Cydm)-3’-3’-(2’-deoxy)Thy-5’; 5’-ester with 2,3-Bis [methoxy-poly(oxy-ethylene)]-1-carbamyl 7-aza-hexylene-oxy phosphate, sodium salt. Where Ado = adenylyl; Cyd = cytidylyl; Guo = guanylyl; Uro = uridylyl; Thy = thymidinyl; amC6 = aminohexyl; Nnnm = 2’-methoxy-nucleotide. Umedaptanib pegol -007 injectable solution is a single-use, preservative-free, sterile solution formulated in 3.6% or 4.9% mannitol vehicle for intravitreal administration.
Phase 1 (SUSHI) study design and participants
The SUSHI study (www.clinicaltrials.gov, identifier, NCT03633084) enrolled eligible subjects aged 55 years or older and had been diagnosed with nAMD in the eye under study, for which previous standard treatment with intravitreal anti-VEGF agents (aflibercept, bevacizumab or ranibizumab) has recently demonstrated incomplete resolution of exudation, as assessed by spectral domain optical coherence tomography (SD-OCT). Other inclusion criteria were BCVA of 65–10 letters (≤20/50 and ≥20/640 Snellen vision equivalent), presence of macular oedema or subretinal fluid on SD-OCT, choroidal neovascular lesions ≤9-disc areas (DA) and lesion composed of ≤50% subretinal haemorrhage.
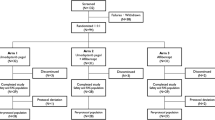
The study was conducted at four study sites in the United States between August 2018 and June 2019. Nine participants who had a diagnosis of refractory nAMD were enrolled. Following a screening evaluation, subjects received a single intravitreal injection of umedaptanib pegol at increasing doses of 0.2, 1.0 or 2.0 mg in the eye under study and no additional doses were administered thereafter (Fig. 2). The primary endpoint of the study was at 28 days post-injection of umedaptanib pegol, with safety evaluation through 56 days. The study was initiated with the lowest dose of 0.2 mg in the first cohort of three subjects, proceeding to a second cohort of three subjects at a dose of 1.0 mg, then a third cohort of three subjects at a dose of 2.0 mg. Decisions regarding proceeding to each sequential cohort were based on the recommendations of the Safety Review Team (SRT), consisting of external Retina Specialists and the Medical Monitor (Fig. 2). The first subject of each dose cohort was assessed by the SRT at 7 days after injection of umedaptanib pegol to determine if safety was acceptable. Umedaptanib pegol treatment was then given to the remaining two subjects in that dose cohort. Upon completion of the primary endpoint at 28 days post-umedaptanib pegol injection by all three subjects in the cohort, the SRT reviewed the safety of these subjects. The next dose cohort was then initiated, and the same schedule of interval SRT evaluations was repeated for that cohort.
Clinical study sites
The SUSHI study was conducted in the following clinical sites: Retinal Consultants Medical Group, Sacramento, CA (PI: Joel Pearlman), Retinal Consultants Medical Group, Sacramento, CA (PI: Margaret Chang), Stanford University, Stanford, CA (PI: Diana Do), and Bay Area Retina Associates, Walnut Creek, CA (PI: Subhransu Ray).
Drug administration procedure
Umedaptanib pegol for intravitreal injection is formulated in a proprietary, clear, aqueous solution. Intravitreal injections were given according to standard of care (SoC) techniques used in modern retinal practice. Briefly, a sterile lid speculum was placed, and local/topical anaesthesia was administered. The conjunctiva and ocular adnexa were prepared with povidone-iodine. A 30-gauge needle was used for all injections, which were given 4.0 mm from the limbus. In this SUSHI study, intraocular pressure (IOP) was measured 30 (±10) min after the intravitreal injection, if ≥10 mmHg from pre-injection IOP, it would be re-evaluated in 60 (+10) min. If still ≥10 mmHg compared to pre-injection IOP, the subject was prescribed a topical IOP-lowering medication until the return for follow-up per the clinical investigator’s discretion, and the IOP increase of ≥10 mmHg was reported as an adverse event (AE).
Ethics statement
The SUSHI studies were conducted at four study sites in compliance with the Declaration of Helsinki, US Code 21 of Federal Regulations, and the Harmonized Tripartite Guidelines for Good Clinical Practice (1996); and were reviewed and approved by the appropriate Ethics Committees or institutional review boards. Informed consent was obtained from all study participants.
Results
Study design
The SUSHI study is a multicentre, open-label, dose-escalating study assessing the safety, tolerability, and bioactivity of a single intravitreal injection of umedaptanib pegol in nine adult patients between 71 and 92 years old (five females and four males, Caucasian) with nAMD refractory to treatment with anti-VEGF medications. Following a screening evaluation, subjects received a single intravitreal injection of umedaptanib pegol at a dose of 0.2 (cohort 1), or 1.0 (cohort 2) or 2.0 (cohort 3) mg in the eye tested (Fig. 2). There were three patients in each cohort.
Safety and tolerability
All three doses of umedaptanib pegol (0.2, 1.0, and 2.0 mg) evaluated in the study were safe and well tolerated. No severe AE was observed in the study. There was a single drug-related AE (mild iritis) that was resolved within one day by administration of topical corticosteroid drops. In addition, there were two cases of subconjunctival haemorrhage, which were related to the intravitreal injection procedure.
Efficacy
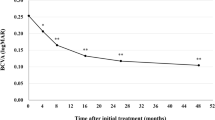
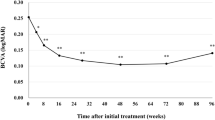
There was an improvement in retinal fluid quantities measured by central subfield thickness (CST) in 6/9 subjects at day 28 and in 7/9 subjects at day 56 (Fig. 3a). All three subjects in cohort 3 (2.0 mg/eye) showed improvement of more than 150 μm at day 28 and/or day 56 (Fig. 3a). Individual optic coherence tomography (OCT) images of cohort 3 are shown in Fig. 4. Improvement of best-corrected visual acuity (BCVA) over baseline at day 28 was observed in 5/9 subjects (Fig. 3b). Based on these results, there is an indication of bioactivity of umedaptanib pegol in patients that have persistent subretinal fluid refractory to the treatment with anti-VEGFs. Furthermore, based on the OCT data, umedaptanib pegol administration led to reabsorption of subretinal hyperreflective material (SHRM), which is a significant risk factor for scar formation, in patient 3C of cohort 3 (Fig. 4, right) [25]. Resolution of SHRM has been observed during anti-VEGF monotherapy or combination therapy in treatment-naïve nAMD patients [26, 27].
Nine refractory nAMD patients received a single intravitreal injection of umedaptanib pegol at doses of 0.2 (cohort 1), 1.0 (cohort 2) or 2.0 mg (cohort 3) in the eye under study. a Change of central subfield thickness (CST) monitored by OCT. b Change of best-corrected visual acuity (BCVA) monitored by EDTRS letters. Error bars represent standard error.
Case examples showing reduction in retinal fluid in cohort 3 patients. SD-OCT images at baseline (top), day 28 (primary endpoint, middle) and day 56 (bottom) are shown. Each patient CST mean change from baseline is presented under the SD-OCT image. Patient 3A, 86-year-old female (Caucasian) who received 58 injections of anti-VEGFs for 9 years before this study; Patient 3B, 92-year-old female Caucasian) who received 19 injections of anti-VEGFs for 3 years before this study; Patient 3C, 86-year-old female (Caucasian) who received 5 injections of anti-VEGFs for 1 year before this study. SHRM is marked by arrows in subject 3.
Blood PK profile
There was small or negligible (below quantitation limit) exposure of umedaptanib pegol in plasma. No drug was detected in plasma in any subjects in cohort 1 at day 28. The highest concentration of drug detected at day 28 was 17.5 ng/mL in one subject in cohort 3. The highest concentration of drug detected in any cohort during the trial was 269 ng/mL in one subject at day 1 in cohort 3, demonstrating that the systemic exposure of umedaptanib pegol is quite low when administered via the intravitreal route.
Discussion
A significant unmet need exists with anti-VEGF monotherapy regardless of the benefit in anti-VEGF medications in patients with nAMD [8, 28,29,30,31]. Numerous clinical trials have been completed or are underway to fill the remaining unmet needs of the anti-VEGFs.
In the present investigation, escalating intravitreal doses of an anti-FGF2 pegylated-aptamer umedaptanib pegol were administered in refractory nAMD patients. This is the first in human clinical trial to target FGF2 via intravitreal administration. Accordingly, umedaptanib pegol was given at increasing doses at 0.2, 1.0, and 2.0 mg/eye. Significantly, no unexpected local or systemic adverse safety signals were observed after the administration of the investigational drug.
The participants in this study had long histories of intravitreal anti-VEGF therapy and were considered refractory to these treatments. It was remarkable that there appeared to be an improvement in retinal fluid levels as measured by central subfield thickness (CST) in most subjects. This was associated with vision improvement in a significant number of treated eyes. The improvement in visual acuity may have been limited by the long duration of CNV disease activity which may have resulted in structural damage to the fovea. These findings suggest that intravitreal umedaptanib pegol is safe and has potential in the treatment of eyes with refractory nAMD where the chorio-retina is no longer responsive to anti-VEGF drugs. This finding supports a distinct mode of action of umedaptanib pegol compared with anti-VEGFs.
Having established the safety of intravitreal umedaptanib pegol, and established a proof of concept, further studies are required to determine the efficacy in the treatment of nAMD. Phase 2 studies are underway.
Summary
What was known before
-
Intravitreal anti-VEGF drugs (e.g., bevacizumab, ranibizumab, and aflibercept) have become the standard treatment for nAMD.
-
As far as is known, VEGF is the only effective target molecule for nAMD monotherapy.
-
Participants may require a high injection frequency over years of treatment, leading to a high treatment burden or several complications.
-
The real-world studies showed worse visual outcomes, possibly due to poor compliance.
What this study adds
-
Intravitreal umedaptanib pegol was safe, well tolerated, and demonstrated bioactivity in refractory nAMD patients.
-
To the best of our knowledge, this is the first report to demonstrate efficacy in monotherapy of nAMD with targets other than VEGF.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16.
Tadayoni R. Choroidal neovascularization induces retinal edema and its treatment addresses this problem. J Ophthalmic Vis Res. 2014;9:405–6.
Drolet DW, Green LS, Gold L, Janjic N. Fit for the eye: aptamers in ocular disorders. Nucleic Acid Ther. 2016;26:127–46.
Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Trans Med. 2018;10:eaao4097.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. VIEW 1 and VIEW 2 study groups: Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98.
Ciulla TA, Huang F, Westby K, Williams DF, Zaveri S, Patel SC. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2018;2:645–53.
Mehta H, Tufail A, Daien V, Lee AY, Nguyen V, Ozturk M, et al. Real-world outcomes in participants with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–46.
Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ, et al. Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124:97–104.
Krejci P, Prochazkova J, Bryja V, Kozubik A, Wilcox WR. Molecular pathology of the fibroblast growth factor family. Hum Mutat. 2011;30:1245–55.
Marie PJ, Miraoui H, Severe N. FGF/FGFR signaling in bone formation: progress and perspectives. Growth Factors. 2012;30:117–23.
Schultz GS, Grant AB. Neovascular growth factors. Eye. 1991;1:70–80.
Vinding T. Occurrence of drusen, pigmentary changes and exudative changes in the macula with reference to age-related macular degeneration an epidemiological study of 1000 aged individuals. Acta Ophthalmol. 1990;68:410–4.
Tomanek RJ, Sandra A, Zheng W, Brock T, Bjercke RJ, Holifield JS. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res. 2001;88:1135–41.
Belgore F, Lip GYH, Blann AD. Basic fibrobrast growth factor induces the secretion of vascular endothelial growth factor by human aortic smooth muscle cells but not by endothelial cells. Eur J Clin Invest. 2003;33:833–9.
Malabanan KP, Kanellakis P, Bobik A, Khachigian LM. Activation transcription factor-4 induced by fibroblast growth factor-2 regulates vascular endothelial growth factor-A transcription in vascular smooth muscle cells and mediates intimal thickening in rat arteries following balloon injury. Circ Res. 2008;103:378–87.
Cao R, Bråkenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13.
Birsner AE, Benny O, D’Amato RJ. The corneal micropocket assay: a model of angiogenesis in the mouse eye. J Vis Exp. 2014;90:e51375.
Matsuda Y, Nonaka Y, Futakawa S, Imai H, Akita K, Nishihata T, et al. Anti-angiogenic and anti-scarring dual action of an anti-fibroblast growth factor 2 aptamer in animal models of retinal disease. Mol Ther Nucl Acids. 2019;17:819–28.
Jin L, Nonaka Y, Miyakawa S, Fujiwara M, Nakamura Y. Dual therapeutic action of a neutralizing anti-FGF2 aptamer in bone diseases and bone cancer pain. Mol Ther. 2016;24:1974–86.
Oladipupo SS, Smith C, Santeford A, Park C, Sene A, Wiley LA, et al. Endothelial cell FGF signaling is required for injury response but not for vascular homeostasis. Proc Natl Acad Sci USA. 2014;111:13379–84.
Dong Z, Santeford A, Ban N, Lee TJ, Smith C, Smith C, et al. FGF2-induced STAT3 activation regulates pathologic neovascularization. Exp Eye Res. 2019;187:107775.
Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:656–66.
Jaffe GJ, Ciulla TA, Ciardella AP, Devin F, Dugel PU, Eandi CM, et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: a phase IIb, multicenter, randomized controlled trial. Ophthalmology. 2017;124:224–24.
Leung KFC, Downes SM, Chong V. Retrospective analysis of the effect of subretinal hyper-reflective material and other morphological features of neovascular age-related macular degeneration on visual acuity outcomes in eyes. Treated with intravitreal aflibercept over one year. Vision. 2018;2:5.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201.
Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–56.
Holekamp NM, Liu Y, Yeh WS, Chia Y, Kiss S, Almony A, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Ophthalmol. 2014;157:825–33.
Rasmussen A, Bloch SB, Fuchs J, Hansen LH, Larsen M, LaCour M, et al. A 4-year longitudinal study of 555 patients treated with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120:2630–6.
Acknowledgements
We acknowledge the following principal investigators who participated in these studies: Joel Pearlman, Margaret Chang, Diana Do, and Subhransu Ray. We thank Quan Dong Nguyen, Linda Lam, Karl G. Csaky, Gary D. Novack, and Rajendra Apte for scientific discussion as consultants or as SRT members; Susan Srivatsa and Thomas Rupp for support in CMC; Satoshi Futakawa and Yosuke Nonaka for support in data analysis; Margaret Buckingham for critical reading of the manuscript and many valuable comments; and all members of RIBOMIC Inc. for technical supports and discussion.
Author information
Authors and Affiliations
Contributions
DSP worked as a physician/medical monitor of clinical trials. KA was responsible for CMC of investigational new drug. RBB designed and supervised the clinical studies. TN supervised the data analysis. YA designed and directed the SUSHI study. EN participated in the clinical study management. YN coordinated the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
DSP, YA and EN are employees of RIBOMIC USA Inc. KA, TN and YN are employees of RIBOMIC Inc. YA, KA, EN and YN are shareholders of RIBOMIC Inc. RBB serves on the scientific advisory board of RIBOMIC Inc. as a paid advisory board member.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, D.S., Akita, K., Bhisitkul, R.B. et al. Safety and tolerability of intravitreal umedaptanib pegol (anti-FGF2) for neovascular age-related macular degeneration (nAMD): a phase 1, open-label study. Eye 38, 1149–1154 (2024). https://doi.org/10.1038/s41433-023-02849-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02849-6