Abstract
Background
To examine the association between optical coherence tomography angiography (OCTA) retinal measurements and Parkinson’s disease (PD).
Methods
We searched MEDLINE and EMBASE from inception up to November 5th, 2021 for studies examining the differences between OCTA retinal measurements in PD patients and healthy controls. We used the Hartung–Knapp–Sidik–Jonkman random-effects method to combine study-specific standardized mean differences (SMD) in pooled effect estimates and a meta-analytic extension of the E-value metric to quantify the confounding bias capable of nullifying the pooled estimates.
Results
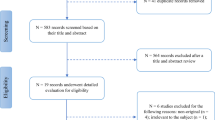
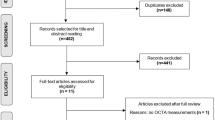
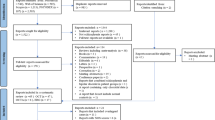
Nine eligible studies for our systematic review were identified through our search strategy. The pooled SMD between the retinal vessel density of PD patients and healthy participants in the whole superficial vascular plexus (SVP), foveal SVP, parafoveal SVP and foveal avascular zone (FAZ) was −0.68 (95% CI: −1.18 to −0.17, p value = 0.02, n = 7 studies), −0.14 (95% CI: −0.88 to 0.59, p value = 0.62, n = 5 studies), −0.59 (95% CI: −1.41 to 0.23, p value = 0.12, n = 5 studies) and −0.20 (95% CI: −0.79 to 0.38, p value = 0.39, n = 5 studies), respectively. An unmeasured confounder would need to be associated with a 3.01-fold, 1.54-fold, 2.81-fold and 1.70-fold increase in the risk of PD and OCTA retinal measurements, in order for the pooled SMD estimate of vessel density in whole SVP, parafoveal SVP and FAZ, respectively, to be nullified.
Conclusions
Our results provide evidence on an inverse association between whole SVP vessel density and PD.
摘要
背景: 探讨视网膜影像检查相干光断层扫描血流成像技术 (OCTA) 与帕金森病 (PD) 之间的相关性。
方法: 我们在MEDLINE和EMBASE上搜索了从开始时的相关文献到2021年11月5日之间的、研究PD患者和健康对照组的OCTA视网膜参数差异性的文献。我们使用Hartung-Knapp-Sidik-Jonkman随机效应法, 将研究特定的标准化平均差异 (SMD) 结合到集合效应估计中, 在合并效应估计和E值指标的元分析扩展中, 以量化能够合并估计无效的混杂偏差。
结果: 通过搜索, 我们确定了9项符合系统评价的研究。PD患者和健康参与者在整个浅层血管丛 (SVP) 、中心凹 (SVP) 、旁中心凹SVP和中心凹无血管区 (FAZ) 的视网膜血管密度集合的SMD为-0.68 (95% CI: −1.18 to −0.17, p = 0.02, n = 7)、−0.14 (95% CI: −0.88–0.59, p = 0.62, n = 5)、−0.59 (95% CI: −1.41–0.23, p = 0.12, n = 5) 和 -0.20 (95% CI: −0.79 至 0.38, p = 0.39, n = 5)。一个未测量的混杂因素需要与PD和OCTA视网膜测量的风险增加3.01倍、1.54倍、2.81倍和1.70倍相关, 以便使整个SVP、旁中心凹 SVP和FAZ的血管密度集合的SMD估计值失效。
结论: 我们的结果为整个SVP血管密度和PD之间呈负相关提供了证据。
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–80.
DeMaagd G, Philip A. Parkinson’s disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. J Formul Manag. 2015;40:504–32.
Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord. 2008;23:1428–34.
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27:27–42.
Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921–32.
Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology. 2019;126:497–510.
Chrysou A, Jansonius NM, van Laar T. Retinal layers in Parkinson’s disease: A meta-analysis of spectral-domain optical coherence tomography studies. Parkinsonism Relat Disord. 2019;64:40–9.
Ortuño-Lizarán I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Movem Disord. 2018;33:1315–24.
Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–31.
Rascuna C, Russo A, Terravecchia C, Castellino N, Avitabile T, Bonfiglio V, et al. Retinal thickness and microvascular pattern in early Parkinson’s disease. Front Neurol. 2020;11:533375.
Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, et al. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol. 2021;139:182–8.
Lauermann JL, Sochurek JAM, Plottner P, Alten F, Kasten M, Prasuhn J, et al. Applicability of optical coherence tomography angiography (OCTA) imaging in Parkinson’s disease. Sci Rep. 2021;11:5520.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. www.ohri.ca/programs/clinical_epidemiology/. Accessed 10 Nov 2019.
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11:e0147601.
IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25.
Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016;7:55–79.
Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140–4.
Mathur MB, VanderWeele TJ. Sensitivity analysis for unmeasured confounding in meta-analyses. J Am Stat Assoc. 2020;115:163–72.
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane, 2019. www.training.cochrane.org/handbook.
R Core Team (2018). R: a language and environment for statistical computing. R Foundation for Statistical Computing V, Austria. https://www.R-project.org/.
Miri S, Shrier EM, Glazman S, Ding Y, Selesnick I, Kozlowski PB, et al. The avascular zone and neuronal remodeling of the fovea in Parkinson’s disease. Ann Clin Transl Neurol. 2015;2:196–201.
Bhullar PK, Pead E, Thompson AC, Yoon SP, Grewal DS, Polascik B, et al. Evaluation of potential biomarkers in multimodal retinal images for diagnosis of Parkinson’s disease: a pilot study. In Proc. Investigative Ophthalmology and Visual Science Conference. 2019;60.
Berkowitz S, Patel S. Pilot study for neurological and retinal imaging as biomarkers for Parkinson’s disease using optical coherence tomography-angiography. In: Proc. Investigative Ophthalmology and Visual Science Conference. 2020;61.
Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci. 2018;59:4115–22.
Murueta-Goyena A, Del Pino R, Galdós M, Arana B, Acera M, Carmona-Abellán M, et al. Retinal thickness predicts the risk of cognitive decline in Parkinson’s disease. Ann Neurol. 2021;89:165–76.
Shi C, Chen Y, Kwapong WR, Tong Q, Wu S, Zhou Y, et al. Characterization by fractal dimension analysis of the retinal capillary network in Parkinson’s disease. Retina. 2020;40:1483–91.
Zhang Y, Zhang D, Gao Y, Yang L, Tao Y, Xu H, et al. Retinal flow density changes in early-stage Parkinson’s disease investigated by swept-source optical coherence tomography angiography. Curr Eye Res. 2021;46:1886–91.
Zhou M, Wu L, Hu Q, Wang C, Ye J, Chen T, et al. Visual impairments are associated with retinal microvascular density in patients with Parkinson’s disease. Front Neurosci. 2021;15:718820.
Zou J, Liu K, Li F, Xu Y, Shen L, Xu H. Combination of optical coherence tomography (OCT) and OCT angiography increases diagnostic efficacy of Parkinson’s disease. Quant Imaging Med Surg. 2020;10:1930–9.
Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M. Optical coherence tomography angiography using the optovue device. Dev Ophthalmol. 2016;56:6–12.
HJ Schünemann, GE Vist, JPT Higgins, N Santesso, JJ Deeks, P Glasziou, et al. Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook.
Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O’Carroll SJ, Green CR, et al. Vascular degeneration in Parkinson’s disease. Brain Pathol. 2013;23:154–64.
van der Holst HM, van Uden IWM, Tuladhar AM, de Laat KF, van Norden AGW, Norris DG, et al. Cerebral small vessel disease and incident parkinsonism: the RUN DMC study. Neurology. 2015;85:1569–77.
Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, et al. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012;32:9359–68.
Yang P, Pavlovic D, Waldvogel H, Dragunow M, Synek B, Turner C, et al. String Vessel Formation is Increased in the Brain of Parkinson Disease. J Parkinsons Dis. 2015;5:821–36.
Christou EE, Asproudis I, Asproudis C, Giannakis A, Stefaniotou M, Konitsiotis S. Macular microcirculation characteristics in Parkinson’s disease evaluated by OCT-Angiography: a literature review. Semin Ophthalmol. 2022;37:399–407.
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–50.
Rees RN, Acharya AP, Schrag A, Noyce AJ. An early diagnosis is not the same as a timely diagnosis of Parkinson’s disease. F1000Research. 2018;7:F1000 Faculty Rev-106.
Pappelis K, Jansonius NM. Quantification and repeatability of vessel density and flux as assessed by optical coherence tomography angiography. Transl Vis Sci Technol. 2019;8:3.
Author information
Authors and Affiliations
Contributions
AK conceived and designed the presented study and performed the analysis. All authors wrote and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41433_2023_2438_MOESM2_ESM.docx
Supplementary Table 2: MEDLINE and EMBASE were searched using the Ovid interface on 05/11/2021 from inception until 05/11/2021
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katsimpris, A., Papadopoulos, I., Voulgari, N. et al. Optical coherence tomography angiography in Parkinson’s disease: a systematic review and meta-analysis. Eye 37, 2847–2854 (2023). https://doi.org/10.1038/s41433-023-02438-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02438-7