Abstract
Background
To assess the association between optical coherence tomography angiography (OCTA) retinal measurements and Alzheimer’s disease (AD).
Methods
We searched MEDLINE and EMBASE from inception up to October 28th, 2020 for studies assessing the association between OCTA retinal measurements and AD. Estimates from eligible studies were meta-analysed and pooled standardized mean differences (SMDs) between AD patients and healthy participants with corresponding 95% confidence intervals (95% CI) were calculated, using the Hartung–Knapp/Sidik–Jonkman random-effects method. In addition, we quantified the minimum strength on the risk ratio scale (E value) required for an unmeasured confounder to nullify these associations.
Results
Ten eligible studies for our systematic review were identified through our search strategy. The pooled SMD between the retinal vessel density of AD patients and healthy participants in the whole superficial vascular plexus (SVP), parafoveal SVP and foveal avascular zone (FAZ) was −0.41 (95% CI: −0.69 to −0.13, p value = 0.01, I2 = 15%, seven studies), −0.51 (95% CI: −0.84 to −0.18, p value = 0.01, I2 = 40%, six studies), and 0.87 (95% CI: −0.03 to 1.76, p value = 0.05, I2 = 91%, seven studies), respectively. An unmeasured confounder would need to be associated with a 2.26-, 2.56- and 3.82-fold increase in the risk of AD and OCTA retinal measurements, in order for the pooled SMD estimate of vessel density in whole SVP, parafoveal SVP and FAZ, respectively, to be nullified.
Conclusions
In our study, whole and parafoveal SVP vessel density were inversely associated with AD. However, prospective longitudinal studies with larger sample sizes are needed to furtherly assess these associations.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the most common subtype of dementia [1] as well as the most common cause of dependence and disability [2, 3]. According to population-based estimates, one in three individuals born in developed countries will develop AD at some point during their life [4].
AD is characterized by a progressive, irreversible impairment of cognitive function due to apoptosis of nerve cells and brain atrophy. Cerebral neuronal loss and small-vessel cerebrovascular changes, which are most strongly associated with cognitive impairments, are difficult to detect with existing brain imaging technologies. In contrast, the retinal tissue and microvascular network can be imaged directly and may provide a unique window to study cerebral pathologic features [5], because the retinal and cerebral microvasculature share similar embryologic origins as well as anatomic and physiologic properties [6, 7]. The retina is considered to be part of the CNS, connected to the CNS through the optic nerve [8]. It is a developmental extension of the brain and is the only part of the central nervous system that can be imaged non-invasively at sub-cellular resolutions. As a result, retina and the optic nerve are affected by neurodegenerative changes in patients with AD [9]. Previous studies have shown that retinal measurements of optical coherence tomography (OCT) e.g. ganglion cell-inner plexiform layer, ganglion cell complex and retinal nerve fibre layer, are associated with AD, highlighting the potential usefulness of OCT measurements as biomarkers of AD [10].
Recent technological advances in OCT provide refined measurements of the microvascular pathology which by extension, may also provide us with sensitive biomarkers reflecting underlying cerebral processes. The recently developed functional extension of OCT-termed OCT angiography (OCTA) enables a detailed angiographic view of the retinal vascular network, by measuring the size, shape and blood flow of retinal capillaries in a living human eye without injecting any contrast agents or dye [11]. As AD is a disease that is also characterized by vascular changes, OCTA may reflect such vascular changes in the retina, making it of interest as a potential new and possibly early biomarker for AD [12]. In the last 2 years, a few studies have already illustrated that specific OCTA biomarkers are associated with AD [13,14,15].
Therefore, in this study, we performed a systematic review and meta-analysis of the literature up to date and summarized eligible studies on the differences of OCTA retinal measurements within the macula in AD patients and healthy individuals. In addition, we performed bias analysis to assess the robustness of the association estimates to unobserved confounding.
Materials
Eligibility criteria
We conducted this systemic review and meta-analysis of the published literature on the differences of OCTA measurements in AD and controls, following the Meta-analysis of Observational Studies in Epidemiology guidelines (Supplementary Table 1) [16]. The literature search was created to answer the question: ‘Is there an association between OCTA retinal measurements within the macula and AD?’. Studies included in the meta-analysis fulfilled the following criteria: (1) cross-sectional, case-control or prospective design; (2) data of OCTA measurements within the macula were reported; (3) data from retinal measurements was reported as mean or mean difference and standard deviation between AD patients and healthy participants. Studies where the mean difference could be calculated indirectly from data provided in the study were considered eligible for inclusion in our meta-analysis; (4) the diagnosis of AD in participants was based on established diagnostic systems (e.g. International Classification of Diseases, Diagnostic and Statistical Manual of Mental Disorders, National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association); (5) AD patients were recruited in addition to controls; (6) sample size of the study was >10. We excluded conference abstracts, letters to the editor, case-report studies, systematic reviews, meta-analyses, non-human studies, non-English studies and low-quality studies using the Newcastle–Ottawa Scale (NOS) [17].
Literature search
Two authors (AK, JK) independently performed a literature search via the databases MEDLINE and EMBASE (OvidSP) from database inception up to October 28th, 2020, for studies assessing the association between OCTA measurements and AD. We tailored the search strategy to the research question and the relevant inclusion and exclusion criteria of our review. We used terms from the hierarchically organized terminology for indexing and cataloguing and synonyms of these keywords. We also used free-text words in order to retrieve ‘in process’ and ‘publisher-supplied citations’ as they are not indexed with structured terminology. Finally, in order not to miss relevant articles, we manually searched the reference lists of all the studies, which met the inclusion criteria, in order to retrieve additional articles relevant to our research question (‘snowball’ procedure). The exact combination of search terms that was put in the search query of the OvidSP databases is provided in the Supplementary Table 2. In addition, we searched other sources of grey literature, notably Google Scholar and suggested citations.
Study selection and study quality assessment
After removing duplicate articles, we identified the eligible studies using a two-step selection process. In the first phase, two authors (AK, JK) independently screened the titles and abstracts of the studies yielded from the computerized literature search, while in the second phase, they assessed the full texts of the remaining studies to identify potentially relevant articles. We excluded any study that did not meet the prespecified eligibility criteria and any discrepancies were resolved by consensus.
The methodologic quality of included studies was assessed by same two investigators through a modified version of NOS for cross-sectional studies, which has been described previously [18]. They independently reviewed and graded the eligible articles obtained from the literature search to assess their quality. The main domains assessed with the modified NOS are representativeness of the sample, whether the sample size is justified and satisfactory, description of respondents and non-respondents, characteristics and response rate, ascertainment of the exposure, comparability of the subjects in different outcome groups, assessment of the outcome and adequacy of statistical analysis. In the ‘selection’ section of the NOS, studies including individuals diagnosed with AD according to established diagnostic systems would be considered representative of the average exposed cohort in the target population and were allotted a star. Age was set as the most important factor for controlling confounding in the ‘comparability’ section, which can be awarded a maximum of two stars. Similarly, the ‘ascertainment of the exposure’ and ‘assessment of the outcome’ sections can be awarded a maximum of two starts each, while each of the remaining sections can be awarded a maximum of one star. The maximum score of 10 can be given to a study. Studies with scores of <6 were considered of low-quality and excluded from this meta-analysis, while studies with scores of 6 or more were considered of moderate-to-high quality.
Data extraction
Two authors (AK, JK) independently checked the data of the eligible studies and entered them in a customized extraction form. The extracted information from each of the eligible study included: first author’s name, publication year, country in which the study was conducted, sample size, number of male and female participants, mean age, mean score of the Mini-Mental State Examination (MMSE), OCTA machine type used, the OCTA parameters that each study assessed on each outcome group and control covariates. All reported data were extracted from published articles and we also contacted the authors for additional information.
Statistical analysis
We used means and standard deviations from each outcome group to calculate standardized mean differences (SMDs) of each OCTA measurement between different outcome groups, with corresponding 95% confidence intervals (95% CI). In cases where values of OCTA measurements outcomes were not directly available, we calculated them indirectly by combining means and standard deviations. We adopted the Hartung–Knapp/Sidik–Jonkman random-effects method to combine study-specific SMDs in pooled effect estimates with respective 95% confidence intervals and calculate the between-study variance (τ2). The advantages of the Hartung–Knapp/Sidik–Jonkman method have been described previously [19, 20] and they are evident especially when there is high heterogeneity among the studies and the number of studies in the meta-analysis is small. We calculated the percentage of total variation due to heterogeneity (I2) and used the Cochran Q to test for between-study heterogeneity. We performed meta-analysis on the association between OCTA retinal measurements and AD, when five or more studies were eligible for a specific OCTA parameter.
Moreover, we conducted sensitivity analyses to assess unmeasured confounding, since random-effect meta-analyses can produce biased estimates when the included studies are subject to unmeasured confounding [21]. Specifically, we calculated the minimum magnitude of unmeasured confounding on the risk ratio scale that is needed to nullify the SDM between the outcome groups. This approach is a meta-analytic extension of the E value metric [22] that measures the confounding bias capable of bringing the effect estimate, of single studies, to a specific threshold. Due to the relatively low number of eligible studies, we did not conduct tests for assessment of publication bias nor meta-regression to identify sources of heterogeneity [23]. P values < 0.05 were considered as statistically significant, and all statistical tests were two-sided. The statistical software R (version 3.5.1, Foundation for Statistical Computing, Vienna, Austria; package) [24] was used for all the analyses.
Results
Systematic review
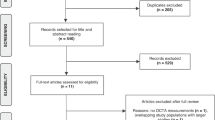
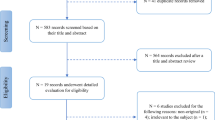
Figure 1 summarizes the screening process and the identification of eligible studies. We identified 600 articles in total through our literature search. After removal of duplicates, 452 articles were selected for title and abstract screening and 441 of them were excluded. The remaining 11 articles were eligible for full-text review. We excluded one article since it did not include any OCTA retinal measurements [25]. Thus, ten articles [13,14,15, 26,27,28,29,30,31,32] were included in our systematic review and were eligible for meta-analysis. All eligible studies were of moderate or high quality and the NOS score ranged from 7/10 to 9/10 with a median score of 9/10 (Supplementary Table 3).
Tables 1 and 2 summarize the main characteristics of the eligible studies. Among the ten included articles, four were conducted in Europe [26,27,28,29], four in Asia [13, 30,31,32] and two in the U.S.A. [14, 26]. All studies were cross-sectional and the summarized number of participants was 610 (248 AD patients and 362 healthy participants), ranging from 33 to 172 participants in individual studies. Regarding the mean age of participants, it ranged from 63.38 to 74.23 years and from 60.6 to 74.26 years for AD patients and healthy participants, respectively. The mean MMSE score of AD patients was reported from all eligible studies and ranged from 16.92 to 24.9, while seven studies [13,14,15, 28,29,30,31] reported the mean MMSE score of healthy participants, ranging from 26.81 to 29.84. The AngioVue software of Optovue spectral domain OCT [33] was utilized in four studies [13, 26, 28, 31] to assess the retinal microvasculature, while the remaining six [14, 15, 27, 29, 30, 32] used the AngioPlex software of Carl Zeiss spectral domain OCT [34].
The way that the eyes of participants were selected varied among studies; in three studies [13, 27, 30, 32] one eye from each participant was randomly selected, in two [14, 29] studies values from both eyes of every participant were averaged, unless only one suitable image was present, in two studies [15, 31] both eyes of every participant were selected, unless only one eye was eligible for inclusion the analyses, in one [28] study, the eye with the highest signal quality score was selected for each participant and in one study [26], one eye of each participant was included in the analysis without reporting the reasoning behind the selection.
Meta-analysis
Since several OCTA parameters, which assess the vessel density of retinal microvasculature exist and these parameters may differ among different OCTA machines (Table 2), the SMD was used as a summary statistic in out meta-analyses. This method is particularly useful when studies assess the same outcome but measure it in various ways [35]. OCTA data on the vessel density of the whole superficial vascular plexus (SVP), parafoveal SVP and foveal avascular zone (FAZ), were obtained in more than five studies and thus, these were the OCTA metrics that were meta-analysed. Regarding the whole SVP, estimates were obtained from seven studies; in four studies [13, 15, 27, 28] the effect estimates were obtained directly from the data provided by each study, while in the remaining three studies, the estimates were indirectly calculated. More specifically, in these two studies [29,30,31], we calculated the estimates for the whole SVP by combining the means and standard deviations of parafoveal and perifoveal SVP of each outcome group [36]. The meta-analysed estimates of the parafoveal SVP vessel density and FAZ were directly obtained from six [13, 15, 26, 28,29,30] and seven studies [13,14,15, 28,29,30,31], respectively. One study was excluded from our meta-analyses because of inadequate information on OCTA retinal measurements in order to calculate SMD [32]. Last, although one study [14] provided eligible estimates of retinal vessel density, these estimates were not in the group of selected OCTA parameters to be meta-analysed, namely whole SVP, parafoveal SVP and FAZ, and, thus, we excluded it from our meta-analyses. When a study provided more than one metrics of retinal vessel density, we selected for the meta-analyses the one that was used by most of the remaining studies.
The pooled SMD between the retinal vessel density of AD patients and healthy participants in the whole SVP, parafoveal SVP and FAZ was −0.41 (95% CI: −0.69 to −0.13, p value = 0.01, I2 = 15%, seven studies), −0.51 (95% CI: −0.84 to −0.18, p value = 0.01, I2 = 40%, six studies) and 0.87 (95% CI: −0.03 to 1.76, p value = 0.05, I2 = 91%, seven studies), respectively (Fig. 2). High heterogeneity (I2 = 91%, Cochran’s Q p value < 0.01) was observed among the seven studies examining the association between FAZ and AD. In order for the pooled SMD estimate of vessel density in whole SVP, parafoveal SVP and FAZ to be nullified, an unmeasured confounder would have to be associated with a risk ratio of 2.26, 2.56 and 3.82, respectively, with the risk of AD and the corresponding OCTA metrics.
Discussion
To the best of our knowledge, this is the largest to-date systematic review and meta-analysis to quantify the associations between OCTA retinal measurements and AD. In our study, we found statistically significant inverse associations of whole and parafoveal SVP vessel density with AD, and a marginally nonsignificant positive association between FAZ and AD.
Extracellular deposition of amyloid-β plaques and intracellular neurofibrillary tangles are the characteristic neuropathological findings in AD, which lead to progressive, irreversible impairment of cognitive function due to apoptosis of nerve cells and brain atrophy [37]. In addition, vascular changes have been identified by numerous studies [38, 39]. These pathological changes can be found not only in brain but also in retina due to their similar embryological origin. The pathophysiological mechanism of reduced retinal vessel density in AD remains unknown. However, it has been postulated that the deposition of beta-amyloid plaques in retina apply compressive forces to retinal cells and blood vessels, reducing blood flow to retina, which is one of the most energy consuming tissues [40]. The vascular endothelia growth factors that are produced in response to retinal hypoxia cannot bind to their corresponding endothelial receptors and restore retinal blood supply to normal levels, since they are mechanically blocked by the diffuse accumulation of amyloid-β plaques [41]. This postulated that pathophysiological mechanism can explain to a certain degree the reduced retinal vessel density found to AD patients compared to healthy individuals.
One of the most important concern of our meta-analyses is the variation in how vessel density is assessed among different OCTA machines. Moreover, a specific OCTA machine can utilize various OCTA metrics to quantify retinal vessel density. In a recent systematic review [42], investigating the use of OCTA in cerebral small-vessel disease SVD, stroke and dementia, this was one of the main reasons for not performing a meta-analysis of the included studies. We addressed this concern by using SMD as the summary statistic in our meta-analyses, giving us the possibility to summarize OCTA metrics that assess the same outcome (vessel density) on a different scale. In most of our included studies, vessel density was defined either as the percentage of perfused retinal area (unit of measurement was %) or as the ratio of total retinal vessels’ length per unit area in the region of measurement (unit of measurement was /mm). Two studies [14, 32] that they were not included in the meta-analyses, assessed vessel density through fractal analysis using the box counting method (unit of measurement was Dbox). Moreover, our systematic review and meta-analysis included two additional studies [30, 32], which were identified from searching the grey literature, compared to the most recent systematic review [42].
Our findings have important clinical implications with regards to AD screening and diagnosis. Current diagnostic methods for AD e.g. magnetic resonance imaging, positron emission tomography, cerebral spinal fluid examination, genetic markers and serum amyloid, can be characterized as costly, time-consuming, invasive, with suboptimal specificity and sensitivity, as well as limited access to specialists [43]. For example, although positron emission tomography scanning has brough upon a breakthrough in visualization of amyloid-β plagues presence in vivo in cognitively healthy individuals, it is currently not suitable for large-scale screening, since It is a costly diagnostic procedure that is only available in larger hospitals. Most of the methods mentioned above are not presently scalable for population screening of individuals at risk of AD. Faster, more reliable, more accessible and less invasive diagnostic techniques comprise a large unmet need for efficient screening of those at risk. In this context, quantitative OCTA metrics may constitute promising biomarkers for monitoring progression of pathological neural degeneration associated with AD. These biomarkers can meet the urgent scientific and clinical need for a patient-friendly, early, inexpensive AD biomarker, that preferably detects AD pathology before severe neurodegeneration.
Despite including only studies with moderate-to-high quality according to NOS score in our meta-analyses, our results should be considered in the context of several potential limitations. First, the methodological heterogeneity among studies regarding the OCTA machine and metrics that were used, the way that the eyes of participants were selected and the covariates included in analysis, may have affected the pooled estimates of our meta-analyses. Second, the cognitive status of AD patients varied among the studies, with the mean MMSE score ranging from 16.92 to 24.9. As a result, the variability in disease severity in individual studies, could have led to over- or under-estimation of the true effect sizes. Third, although most of the studies have controlled in the analysis for the age of participants as a strong potential confounder on the association estimates, other potential confounding variables like chronic diseases, were not taken into account. This may also be a result of the small sample size of most studies, which limits the number of covariates in the analysis and comprises an additional limitation of our meta-analysis. Last, since all meta-analysed studies are cross-sectional, we cannot establish temporal ordering of the OCTA metrics and AD.
In conclusion, this systematic review and meta-analysis provides evidence on inverse associations of whole and parafoveal SVP vessel density with AD. These results have important clinical implications, since OCTA metrics may have the potential to be utilized as biomarkers of AD. Due to several limitations, causal associations cannot be established and thus, future longitudinal studies, with larger sample sizes, more potential confounders controlled in their analysis and more homogenous assessments of retinal microvasculature are warranted to support our results.
Summary
What was known before
-
Retinal measurements of OCT have been found to be associated with AD.
What this study adds
-
This study highlights the potential usefulness of OCTA measurements as biomarkers of AD.
References
Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006239.
Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet 2009;374:1821–30.
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet 2006;368:387–403.
Lewis F. Estimation of future cases of dementia from those born in 2015. Consulting Report. Office of Health Economics, 2015.
Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement. 2014;10:135–42.
Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–48.
London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53.
Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab. 2013;33:1685–95.
Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315:485–7.
Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology 2019;126:497–510.
Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT27–36.
Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–36.
Bulut M, Kurtuluş F, Gözkaya O, Erol MK, Cengiz A, Akıdan M, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol. 2018;102:233–7.
Jiang H, Wei Y, Shi Y, Wright CB, Sun X, Gregori G, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol. 2018;38:292–8.
Yoon SP, Grewal DS, Thompson AC, Polascik BW, Dunn C, Burke JR, et al. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmol Retin. 2019;3:489–99.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12.
Wells GSB, Shea BJ, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. www.ohri.ca/programs/clinical_epidemiology/. Accessed 10 Nov 2019.
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0147601.
IntHout J, Ioannidis JPA, Borm GF. The Hartung–Knapp–Sidik–Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25.
Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79.
Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140–4.
Mathur MB, VanderWeele TJ. Sensitivity analysis for unmeasured confounding in meta-analyses. J Am Stat Assoc. 2020;115:163–72.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Chichester (UK): John Wiley & Sons. Cochrane, 2019. Available from: www.training.cochrane.org/handbook.
R Core Team. R: a language and environment for statistical computing. Austria: R Foundation for Statistical Computing V; 2018. https://www.R-project.org/.
Salobrar-García E, de Hoz R, Ramírez AI, López-Cuenca I, Rojas P, Vazirani R, et al. Changes in visual function and retinal structure in the progression of Alzheimer’s disease. PLoS ONE. 2019;14:e0220535.
Lahme L, Esser EL, Mihailovic N, Schubert F, Lauermann J, Johnen A, et al. Evaluation of ocular perfusion in Alzheimer’s disease using optical coherence tomography angiography. J Alzheimers Dis. 2018;66:1745–52.
Querques G, Borrelli E, Sacconi R, De Vitis L, Leocani L, Santangelo R, et al. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2019;9:63.
Zabel P, Kaluzny JJ, Wilkosc-Debczynska M, Gebska-Toloczko M, Suwala K, Zabel K, et al. Comparison of retinal microvasculature in patients with Alzheimer’s disease and primary open-angle glaucoma by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2019;60:3447–55.
den Haan J, van de Kreeke JA, van Berckel BN, Barkhof F, Teunissen CE, Scheltens P, et al. Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease? Alzheimers Dement. 2019;11:383–91.
Li ZB, Lin ZJ, Li N, Yu H, Wu YL, Shen X. Evaluation of retinal and choroidal changes in patients with Alzheimer’s type dementia using optical coherence tomography angiography. Int J Ophthalmol. 2021;14:860–8.
Wu J, Zhang X, Azhati G, Li T, Xu G, Liu F. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer’s disease by optical coherence tomography angiography. Acta Ophthalmol. 2020;98:e781–7.
Chua, J., Hu, Q., Ke, M. et al. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alz Res Therapy 2020;12:161. https://doi.org/10.1186/s13195-020-00724-0.
Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M. Optical coherence tomography angiography using the Optovue device. Dev Ophthalmol. 2016;56:6–12.
Rosenfeld PJ, Durbin MK, Roisman L, Zheng F, Miller A, Robbins G, et al. ZEISS Angioplex™ spectral domain optical coherence tomography angiography: technical aspects. Dev Ophthalmol. 2016;56:18–29.
Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Chichester (UK): John Wiley & Sons. Cochrane, 2020. Available from: www.training.cochrane.org/handbook.
Higgins JPT, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Chichester (UK): John Wiley & Sons. Cochrane, 2020. Available from: www.training.cochrane.org/handbook.
Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clin Inter Aging. 2007;2:347–59.
Klohs J. An integrated view on vascular dysfunction in Alzheimer’s disease. Neurodegenerative Dis. 2019;19:109–27.
Govindpani K, McNamara LG, Smith NR, Vinnakota C, Waldvogel HJ, Faull RL, et al. Vascular dysfunction in Alzheimer’s disease: a prelude to the pathological process or a consequence of it? J Clin Med. 2019;8:651.
Joyal J-S, Gantner ML, Smith LEH. Retinal energy demands control vascular supply of the retina in development and disease: the role of neuronal lipid and glucose metabolism. Prog Retin Eye Res. 2018;64:131–56.
Tsokolas G, Tsaousis KT, Diakonis VF, Matsou A, Tyradellis S. Optical coherence tomography angiography in neurodegenerative diseases: a review. Eye Brain. 2020;12:73–87.
Zhang J-F, Wiseman S, Valdés-Hernández MC, Doubal FN, Dhillon B, Wu Y-C, et al. The application of optical coherence tomography angiography in cerebral small vessel disease, ischemic stroke, and dementia: a systematic review. Front Neurol. 2020;11:1009.
Thal LJ, Kantarci K, Reiman EM, Klunk WE, Weiner MW, Zetterberg H, et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:6–15.
Author information
Authors and Affiliations
Contributions
Andreas K conceived and designed the presented study and performed the analysis. All authors wrote and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Katsimpris, A., Karamaounas, A., Sideri, A.M. et al. Optical coherence tomography angiography in Alzheimer’s disease: a systematic review and meta-analysis. Eye 36, 1419–1426 (2022). https://doi.org/10.1038/s41433-021-01648-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01648-1
This article is cited by
-
Enhancing foveal avascular zone analysis for Alzheimer’s diagnosis with AI segmentation and machine learning using multiple radiomic features
Scientific Reports (2024)
-
Potential Ocular Biomarkers for Early Detection of Alzheimer’s Disease and Their Roles in Artificial Intelligence Studies
Neurology and Therapy (2023)