Abstract
The majority of choroidal tumours are diagnosed accurately with clinical examination and the additional data obtained from non-invasive imaging techniques. Choroidal biopsies may be undertaken for diagnostic clarity in cases such as small melanocytic or indeterminate lesions, identifying the primary tumour in the case of choroidal metastases or the subclassification of rarer conditions such as uveal lymphoma. There is however an increasing use of biopsy techniques for prognostication in uveal melanoma. This review explores the main indications and surgical techniques for tumour acquisition, and the optimised approach utilised by the current authors to improve successful yield for histological and genetic analysis.
摘要
大多数脉络膜肿瘤通过临床检查和非侵入性成像技术所获得的额外数据可得到准确诊断。对于小的黑色素细胞或不确定的病变, 可进行脉络膜活检以明确诊断, 以在脉络膜转移或更罕见疾病 (如葡萄膜淋巴瘤) 的亚类的情况下确定原发肿瘤。但是目前已经越来越多地使用活检技术来预测葡萄膜黑色素瘤的预后。
本综述探索了脉络膜活检获取肿瘤组织的主要适应症和手术技术, 并总结了当前学者们为提高组织学和遗传分析学的成功率而使用的优化方法。
Similar content being viewed by others
Introduction
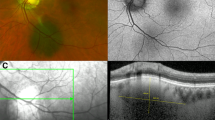
Unlike most oncological specialities, the majority of choroidal tumour diagnoses are made combining clinical features with non-invasive technology aimed at imaging in detail the deeper structures of the chorioretinal boundary. There have been comprehensive clinical descriptions of risk factors associated with melanocytic lesions and risk for malignant transformation [1,2,3,4] including symptomatology, overlying lipofuscin (a sign of cellular turnover), lesion dimensions (basal diameter and height), ultrasound characteristics (internal reflectivity, visible vascular pulsations), subretinal fluid, proximity to the optic nerve head and documented growth. Cumulative information from clinical examination, ocular coherence tomography (OCT), ultrasound biomicroscopy and fundus autofluorescence are usually sufficient to confirm the diagnosis [5,6,7,8,9,10]; other more invasive techniques such as fundus fluorescein angiography and indocyanine green, as well as radiation emitting imaging such as positron emission tomography and computerised tomography may assist in ruling out less common pathologies, or be utilised in individualised treatment planning [11,12,13,14,15,16,17,18]. The diagnostic accuracy in medium-sized and large tumours utilising clinical and imaging methods is >99% in experienced hands [19]. As such, histopathological verification is predominantly unnecessary. In this review, we describe the indications and considerations of choroidal biopsy.
Indications
Diagnostic biopsies
There are three main indications for a choroidal biopsy for diagnostic purposes.
-
1.
Diagnosis of small melanocytic or indeterminate lesions for observation or treatment. It was historically considered that micrometastases would have disseminated by the time of presentation of uveal melanoma, awaiting a positive environment for the development of macrometastases [20]; as such, traditional management of such patients was almost universally observation to monitor for progression. However, increasing evidence suggests that earlier diagnosis and treatment of these small but cancerous tumours may improve overall survival [21,22,23,24]. As such, there is a trend towards treating small melanomas earlier, with the aim to reduce the risk of metastatic disease. With the advent of more abundant imaging in the community and the wide availability of OCT scanning, more small lesions are being identified [25]. Although definitive small melanomas are often now treated at an earlier stage, the options for indeterminate melanocytic lesions (such as those with multiple risk factors for the diagnosis of choroidal melanoma but the absence of others) includes a period of observation, treatment with radiotherapy (or alternatives such as photodynamic therapy (PDT)) or diagnostic biopsy. The relative risks and advantages to each of these options can be discussed with the patient to enable a decision. Biopsy of these smaller lesions is therefore becoming more common. Should such biopsies demonstrate a malignant lesion, rapid and prompt treatment should be planned and recommended. In Liverpool, should there be a high suspicion of melanoma and the patient wishes for a diagnostic biopsy to confirm, we may put tantalum markers on at the time of biopsy to expedite proton radiation treatment following the histopathological analysis.
-
2.
Choroidal metastases are identifiable from clinical and imaging features such as the creamy colour, presence of subretinal fluid, often multiple/bilateral and classical uneven appearance on the OCT and on ultrasound biomicroscopy [26]. Although the most common causative primary tumours include those originating from the breast and lung, approximately 30% of patients with choroidal metastatic lesions have no known primary tumour at the time of presentation. Systemic screening to identify the primary tumour is common, however in Liverpool the trend is to undertake a choroidal biopsy to allow identification of the location of the primary tumour (within a few days) to enable quick and targeted investigation and treatment by the medical oncologists subsequently involved [27]. Larger tumour samples are required to enable multiple immunostaining, targeting various organ systems.
-
3.
The clinical diagnosis of uveal lymphoma is often challenging due to the classic masquerading nature of the disease. Biopsy is often required to enable subclassification [28,29,30,31,32]. Haemato-oncologists will base systemic therapies on the pathological subtype of lymphoma which can only be described on a histopathological level. Primary uveal lymphoma tends to be low-grade B-cell non-Hodgkin lymphoma [33,34,35,36,37,38,39]. Secondary manifestations of systemic lymphoma in the eye can also involve the uveal tract [38, 39]. Multiple samples are required with a high cellular yield to enable processing for cytology, immunocytology, immunohistochemistry and PCR [31]. In addition, the fragility of lymphocytic cells adds another dimension to the difficulty in obtaining and transporting biopsy samples, which need to be processed rapidly in the laboratory [40].
Prognostic biopsies
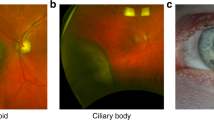
Due to the diagnostic accuracy with clinical and imaging features, the vast majority of choroidal biopsies are obtained for the process of prognostication for uveal melanoma. Although clinical features such as age, sex, tumour diameter, thickness and location (involvement of the ciliary body) and extraocular extension all enable a degree of prognostic estimation, cytogenetic analysis considerably increases the accuracy of these predictions [41,42,43]. Spindle-cell tumours grow in a compact cohesive fashion surrounded by a dense reticulin framework; epithelioid cells grow less cohesively and are not surrounded by a network of reticulin. Spindle cell melanomas have the most favourable prognosis [44,45,46]. Aberrations in chromosomes 1, 3, 6 and 8, in particular deletion of one copy of chromosome 3 (termed monosomy 3), have certainly been shown as the strongest predictors for metastatic disease. Not only is monosomy 3 associated with BAP-1 loss, monosomy 3 UM are more likely to be associated with increased inflammatory cell infiltration (macrophages and T-cells) and larger tumour sizes. When using transcriptional profiling based on 12 signature genes, UM can be classified into class 1 and class 2 categories [47,48,49,50,51], with class 1 tumours having a better prognosis and less risk of metastatic disease. Since the development of this classification, they have been subdivided into class 1 A and B and class 2 A and B, based on the presence or absence of a preferentially expressed antigen in melanoma (PRAME) mutation, which is also considered to be a negative prognostic indicator [52]. Individualised risk stratification based on all the molecular characteristics of the tumour better correlates with mortality when combined with all clinical, histological and genetic risk factors. With the analysis of a large number of tumours and the development of increasing numbers of prognostic indicators, it is clear that although there are clear high and low risk UM, there are also many with a combination of high and low risk characteristics that are more difficult to prognosticate. The Liverpool Uveal Melanoma Prognosticator Online (LUMPO) has been internally and externally validated as a very effective and accurate predictor of metastatic risk, and combines a number of key clinical, histopathological and genetic risk factors to produce an individualised outcome predictor [53, 54].
Processing these small samples requires analysis that can obtain information via amplification methods. A number of methods are practised at present including fluorescence in situ hybridization, comparative genomic hybridization, spectral karyotyping, microsatellite analysis, multiplex ligation-dependent probe amplification, gene expression profiling and single-nucleotide polymorphism arrays [42, 49, 55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89]. Next generation sequencing processes created in Liverpool are likely to become more established with the need for smaller tumour samples and a targeted array panel [90, 91]. All of these methods require experienced Pathologist and technician support in the handling and processing of these often very scanty cellular samples.
Methods of tumour sample acquisition
Intraocular biopsies may be taken either via a trans-scleral or a trans-retinal route. Both run risks of complications from the procedure and, in the case of uveal melanoma, the risk of tumour seeding on to the surface of the eye [92,93,94,95,96].
Trans scleral approach
Biopsies taken via the trans-scleral approach tend to be for tumours more anteriorly located (pre-equatorial). This procedure involves creating a scleral flap and cut down to the choroid and obtaining a sample blindly with a small gauge needle (fine needle aspirate, FNAB) usually 25–30 g [97,98,99]. The technique pioneered in Liverpool can also be undertaken with sampling under direct visualisation with Essen forceps pushed through the scleral cut withdrawing segments of tumour; this gives a much higher yield and therefore higher success rate of both cytological and genetic analysis (99% and 96%) [95]. The scleral tract is then closed with surgical glue, negating the risk of suture tract migration of cells (video supplementary file 1). For uveal melanoma, this technique is most commonly performed at the time of plaque brachytherapy treatment [97], where the plaque (either iodine121 or ruthenium106) is placed over the tumour base and therefore over the biopsy site, sterilising any potentially seeded cells and the biopsy tract. This can also be undertaken at the time of plaque removal to negate the risk of local seeding, although the tissues tend to be more oedematous and bleed more during the surgery.
Trans retinal approach
Choroidal samples taken via the trans-retinal route is much more variable in technique. FNAB (25–27 g needle) is also utilised for this approach with the needle inserted via the pars plana into the choroidal via the retina [98,99,100]. This process may involve no vitrectomy, partial (core) vitrectomy or full vitrectomy prior to sampling [101], usually via 25/27 g ports, to try and minimise non clearing vitreous haemorrhage and vitreous incarceration/traction. Some groups promote maintaining the intraocular pressure (IOP) with an infusion to reduce bleeding whilst others advise keeping the IOP relatively low to prevent egress of fluid and consequent tumour cell surface seeding. It is thought that bending the needle [60–90°] may increase yield due to the oblique nature of the needle track, and also reduce the risk of scleral perforation beneath the tumour base [102, 103]. Viewing techniques also vary from use of the indirect ophthalmoscope to the newer microscope wide field viewing systems.
FNAB has a low rate of complications but a notoriously low yield [97, 104,105,106,107,108] often giving rise to just a cytological result with insufficient sample for genetic analysis. This is certainly related to the tumours size (diameter <5 mm and thickness <2.5 mm) [98, 107, 109]. The Shields group however have reported a high yield with FNAB (84%) in tumours <3 mm in thickness [99].
Additional surgical methods to obtain a higher yield of tumour sample have since been developed. The use of the vitreous cutter has been pioneered in Liverpool [110] (video supplementary file 2) with good yield for both cytology and genetic analysis [25, 92,93,94, 96, 111, 112]. Although there are some centres that advocate this approach with full vitrectomy and tamponade to reduce the risk of rhegmatogenous retinal detachment from the biopsy site by reducing vitreous traction and incarceration [109, 113] the non-vitrectomy technique with no retinopexy seems to have a low rate of such secondary complications [93, 95], with reduced procedure time and procedure related patient morbidity. Vitrectomy seems to bear little benefit for this procedure, and it is likely that the formation of a small iatrogenic round hole, with no vitreous traction, supported by a posterior buckling effect of the choroidal tumour, closed by any blood at the time of sampling and a chorioretinal scar formed by any radiation treatment would be protective against such complications.
More invasive methods include retinal incisions with tissue acquisition with an Essen forceps [114]. This gives rise to a high yield of tissue but has a risk of the sample getting caught in the port, increasing the risk of tumour dissemination. In addition, haemostasis in the case of sudden subretinal and/or preretinal haemorrhage during tissue extraction can only be controlled by raising the intraocular pressure, quickest with a vitrectomy infusion set up. Tumour en bloc incisional biopsy has also been described following 20/23 g vitrectomy with a diamond knife retinotomy and direct excision of a 1–3 mm block of tumour extracted with forceps and a subsequent sulphur hexafluoride 20% tamponade [115]. This technique has a higher risk of retinal detachment than others described, but only in cases with a pre-existing exudative detachment. A multicentre study [116] demonstrated that the choice of biopsy technique by individual surgeons was determined by tumour location (anterior vs posterior), tumour size, and the experience of the surgeon in vitreoretinal procedures (more likely to use a direct viewing system and sample via a trans-retinal approach with a cutter).
Whether utilising FNAB or the cutter technique, the treatment of ports also demonstrates variability between techniques, with some suturing sclerotomies, and some advocating cryotherapy in the sclerotomy region to eliminate any seeded cells. There is theoretically less risk of seeding with newer smaller gauge and valved ports.
Considerations of choroidal biopsies
Complications
Most disappointing for patients is the failure to obtain a diagnosis/prognostic result due to a poor yield of tumour tissue. The trans-scleral Essen forceps and the trans-retinal vitreous cutter techniques certainly provide more tissue with higher rates of success for histology, immunohistochemistry and cytogenetics than FNAB. The success is of course related to tumour size, although small tumour biopsies can reach high levels of success with experience [95].
The most common complication of choroidal biopsy is vitreous haemorrhage, especially in those utilising vitrectors as a sampling instrument in comparison to FNAB. [93, 94, 96, 107, 112, 117]. Rates of vitreous haemorrhage on day 1 have been reported up to >90%; the majority spontaneously resolve however, and the incidence of persistent haemorrhage requiring further surgery is low (1–3%) with both techniques, but particularly low with FNAB.
Retinal detachment is relatively uncommon via all methods, particularly with FNAB. When retinal detachments do occur, the breaks tend to be away from the biopsy site, suggesting either a mechanism of vitreous base traction during the sampling process, or the promotion of posterior vitreous detachment/vitreous base contraction following the procedure.
Other complications are rare, but include hyphaema, haemophthalmos, subretinal haemorrhage, cataract, endophthalmitis and tumour seeding. The more invasive methods have a higher rate of such complications. Another described complication is the development of scleral thinning/necrosis post plaque brachytherapy at the site of the biopsy [118, 119]. The risk of scleral compromise was higher in larger tumours, likely due to the higher scleral dose at the base.
Need for prognostication
Although the current systemic treatments for metastatic disease are suboptimal in this patient cohort with uveal melanoma, multiple clinical trials are underway and newer drugs are in development, targeting various points along the oncogenic and cell cycle pathways, as well as harnessing and manipulating the tumour microenvironment targeting inflammatory and complement pathways. Tebentafusp has recently been approved for the treatment of systemic dissemination of uveal melanoma [120] targeting patients with a HLA-A*02:01 subtype. It is clear that the future of medical oncology is patient tailored care and it is likely that tumour analysis will be at the forefront of that. Currently, inclusion into clinical trials would not be possible for patients with no cytogenetic evidence of their risk profile for metastatic development.
The prognostication of uveal melanoma into fairly definitive high and low risk groups is uncommon in other forms of malignancy. Despite the lack of definitive treatments, this information is often desired by patients, particularly in the younger age group, to enable ownership of their disease and plan for the future. Psychological analyses have demonstrated that the primary concerns of patients diagnosed with uveal melanoma are prognosis related; there do not seem to be any significant symptoms of anxiety or depression in patients who have undergone a prognostic biopsy in comparison to others also diagnosed with uveal melanoma [121,122,123,124,125,126,127]. Prognostication does not, however, completely resolve the level of uncertainty which accompanies a diagnosis of cancer, even for those with a ‘good’ result.
Prognostic biopsies also enable patient tailored screening and monitoring for systemic disease. There is no consensus on the screening regime for detection of metastatic disease in uveal melanoma patients; traditionally, all patients would undergo liver scans (ultrasound or MRI) twice annually for an indefinite period of time. The predictability of the onset of metastatic disease by the LUMPO system can be utilised with prognostic information to determine individualised screening protocols and duration [22]. This could lead up to £500,000/year of saved costs for unnecessary liver screening for patients at low risk in the UK (where the incidence of uveal melanoma is approximately 600/year [128]).
Tumour heterogeneity
The genetic instability of uveal melanoma cells encourages the accumulation of aberrant mutations. As such, as the tumour progresses and divides, it is likely that amongst the clonal cells, a non clonal subgroup arises with acquired genetic heterogeneity. The presence of genetic variation within a uveal melanoma has been demonstrated [25, 129]. Bagger et all demonstrated up to 13% of enucleated eyes for large uveal melanoma had heterogeneity of chromosome 3, and 46% for chromosome 8. The accuracy of detection will depend on the sample analysis utilised [72] with MLPA being able to detect monosomy 3 populations more frequently than FISH. The higher the tumour cell yield, the more likely any heterogeneity will be detected; this is also less likely with FNAB due to the low yield, and the single pass through a single track (resulting in a lower spatial diversity of sample than with alternate methods). Heterogeneity seems to also occur to a greater extent at the base of the tumour; as such, this would be the optimal place for tumour sampling. Trans retinal FNAB is unlikely to access this region due to the risk of perforation through the sclera adjacent to the tumour base. Heterogeneity is less important in the biopsy of small tumours where the proportion of representative cells in the sample is greater, and the chance for multiple cumulative mutations is less likely.
Ocular surface tumour seeding
Intravascular spread of tumour cells during biopsy has been theorised due to the disruption of tumoural blood vessels [130] thereby increasing the risk of systemic tumour spread. Bagger et al however could find no difference in mortality between uveal melanoma patients who have had various techniques of tumour biopsy (FNAB, vitreous cutter, Essen forceps) and those that have never underwent a biopsy procedure [25, 117].
Although ocular surface seeding does not occur with choroidal metastases or lymphoma, a serious consideration for surgeons undertaking melanoma tumour sampling is the risk of seeding of active tumour cells into the vitreous cavity, along the scleral tract, into the anterior chamber or on to the ocular surface. It is these areas where subsequent conservative treatments such as proton beam or plaque radiotherapy would not be involved in the treatment field. Extra ocular spread of tumour cells is in itself an independent risk factor for increased metastatic related mortality [131]. For this reason, measures have been suggested to reduce the risk of seeding; maintaining a low IOP to prevent fluid egress containing tumours cells via the scleral track/port, use of valved and smaller gauge ports, and suturing ± cryotherapy to the port sites. There seems to be less cells along the needle track via a trans retinal FNAB; however a trans-scleral FNAB is likely to have the protection of involvement in the field of subsequent radiotherapy [105, 132]. Extra ocular seeding has been described following both FNAB and trans-retinal 25 g cutter sampling [92, 133,134,135]. Interestingly, histological examination of subsequently enucleated eyes showed no evidence of tumour cells along the scleral track following FNAB [105]. Some authors advocate choroidal melanoma tumour sampling for prognostication only post radiotherapy, to reduce the risk of active tumour cell migration. This has been shown to be accurate and successful [136], but there may a time limiting factor before the extent of tumour cell DNA damage causes an alteration in the cytogenetic analysis [137].
Liverpool experience of prognostic biopsies
The Liverpool Ocular Oncology Centre is the first to routinely offer prognostic biopsies to all choroidal melanoma patients at presentation, regardless of tumour size or location. As such, our practice is to offer patients prognostic uveal melanoma biopsies during the insertion of their brachytherapy plaque via a trans-scleral route utilising a scleral flap and cut down and sample acquisition with Essen forceps and closure with tissue glue, enabling us to place the plaque over the biopsy site, with the aim to treat any cells inadvertently seeded onto the ocular surface. The yield of both cytology and genetics has increased following change to this methodology [95]. However, if the lesion is too posterior to safely access via this surgical method, or proton beam radiotherapy was the method of radiotherapy treatment, we offer prognostic biopsy after completion of their radiotherapy, ideally on the last day or within 4 weeks of their radiotherapy [136]. For this predominantly trans-retinal approach, 25–27 g valved sutureless ports are utilised and the vitrector passed through the tumour, a full vitrectomy, tamponade or retinopexy is not required in these cases with an intact posterior vitreous face. Ports are not sutured or treated with cryotherapy. Complication rates are low with this method, with no cases of tumour seeding onto the ocular surface in a recently analysed series [93]. Our experience in small tumours has increased over the years. We have recently undertaken a retrospective analysis of prospectively collected data on all patients who underwent a prognostic biopsy for a choroidal melanoma of <2 mm thickness over a 5-year period between January 2016-December 2021. During the study period, 320 prognostic choroidal biopsies were undertaken; 68 cases were of a tumour thickness ≤2 mm (0.7–2.0 mm, mean 1.4 mm). Longitudinal base diameter ranged from 4.1 to 15.6 mm (AJCC classification 64 cases T1a, 4 cases T2a). Treatment included ruthenium-106 plaque brachytherapy in 21 patients (31%) and proton beam radiotherapy in 47 (69%). Biopsies were taken 0-98 days post treatment. All cases were May Grunewald Giemsa stained and histologically confirmed as melanoma (8 epithelioid, 4 mixed cell, 56 spindle); chromosome 3 status was determined by multiplex ligation-dependent probe amplification (MLPA) or microsatellite analysis (MSA). One case had insufficient material for genetic analysis; of the remaining 67 cases, 19 (28%) had complete or partial loss of a copy of chromosome 3, 48 (72%) were disomy 3. There were no cases of non-clearing vitreous haemorrhage or retinal detachment.
Conclusion
Choroidal biopsies are necessary where additional histopathological information may influence patient care and outcome. The ability to locate the primary tumour in the case of choroidal metastases influences patient onward care and management without the need for prolonged systemic screening. The concept of early treatment of small melanomas to reduce the risk of metastatic disease has also increased the use of these biopsy techniques in small melanocytic lesions. However, in Liverpool, most choroidal biopsies are undertaken for prognostic purposes in the context of a clinically diagnosed uveal melanoma. Although this procedure remains controversial, the familiarity with newer and more controlled equipment, and the increasing yield from these small samples with various techniques is enabling an exponential rise in the use of prognostication in the standard treatment and counselling of choroidal melanoma patients. Certainly, in our experience, Essen forceps trans scleral samples and 25 g trans retinal biopsies result in an excellent yield sufficient for prognostic analysis of chromosome 3 status (predominantly with MSA) in 99% of cases, even in small (<2 mm) tumours. Despite the clinically low risk by TNM staging, over one quarter of these demonstrated high risk monosomy 3 mutations, challenging the concept of low risk ‘small benign melanomas’. An increasing understanding of patient tailored care and its potential in the future will drive prognostication biopsies further, and it likely that any development in systemic treatments will rely on an individualised approach based on these parameters.
References
Shields CL, Furuta M, Berman EL, Zahler JD, Hoberman DM, Dinh DH, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127:981–7.
Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102:1351–61.
Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology. 2005;112:1784–9.
van Hees CL, de Boer A, Jager MJ, Bleeker JC, Kakebeeke HM, Crijns MB, et al. Are atypical nevi a risk factor for uveal melanoma? A case-control study. J Invest Dermatol. 1994;103:202–5.
Shields CL, Dalvin LA, Ancona-Lezama D, Yu MD, Di Nicola M, Williams BK Jr, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39:1840–51.
Shields CL, Demirci H, Materin MA, Marr BP, Mashayekhi A, Shields JA. Clinical factors in the identification of small choroidal melanoma. Can J Ophthalmol. 2004;39:351–7.
Shields CL, Dalvin LA, Yu MD, Ancona-Lezama D, Di Nicola M, Williams BK, et al. Choroidal nevus transformation into melanoma per millimeter increment in thickness using multimodal imaging in 2355 cases: The 2019 Wendell L. Hughes Lecture. Retina. 2019;39:1852–60.
Sayanagi K, Pelayes DE, Kaiser PK, Singh AD. 3D Spectral domain optical coherence tomography findings in choroidal tumors. Eur J Ophthalmol. 2011;21:271–5.
Tarlan B, Kiratli H. Uveal melanoma: current trends in diagnosis and management. Turk J Ophthalmol. 2016;46:123–37.
Kivela T. Diagnosis of uveal melanoma. Dev Ophthalmol. 2012;49:1–15.
Mueller AJ, Bartsch DU, Folberg R, Mehaffey MG, Boldt HC, Meyer M, et al. Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol. 1998;116:31–9.
Bakri SJ, Sculley L, Singh AD. Imaging techniques for uveal melanoma. Int Ophthalmol Clin. 2006;46:1–13.
Ferreira TA, Jaarsma-Coes MG, Marinkovic M, Verbist B, Verdijk RM, Jager MJ, et al. MR imaging characteristics of uveal melanoma with histopathological validation. Neuroradiology 2022;64:171–84.
Tang MCY, Jaarsma-Coes MG, Ferreira TA, Zwirs-Grech Fonk L, Marinkovic M, Luyten GPM, et al. A comparison of 3 T and 7 T MRI for the clinical evaluation of uveal melanoma. J Magn Reson Imaging. 2022;55:1504–15.
Ferreira TA, Grech Fonk L, Jaarsma-Coes MG, van Haren GGR, Marinkovic M, Beenakker JM. MRI of uveal melanoma. Cancers (Basel). 2019;11:377.
Singh AD, Bhatnagar P, Bybel B. Visualization of primary uveal melanoma with PET/CT scan. Eye (Lond). 2006;20:938–40.
Papastefanou VP, Islam S, Szyszko T, Grantham M, Sagoo MS, Cohen VM. Metabolic activity of primary uveal melanoma on PET/CT scan and its relationship with monosomy 3 and other prognostic factors. Br J Ophthalmol. 2014;98:1659–65.
Marko M, Lesko P, Jurenova D, Furda R, Gregus M. Importance of PET/CT examination in patients with malignant uveal melanoma. Cesk Slov Oftalmol. 2020;76:37–44.
COMS. Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS Rep. no 1 Arch Ophthalmol. 1990;108:1268–73.
Singh AD. Uveal melanoma: implications of tumor doubling time. Ophthalmology. 2001;108:829–31.
Hussain R, Czanner G, Taktak A, Damato B, Praidou A, Heimann H. Mortality of patients with uveal melanoma detected by diabetic retinopathy screening. Retina. 2020;40:2198–2206.
Hussain RN, Coupland SE, Kalirai H, Taktak AFG, Eleuteri A, Damato BE, et al. Small high-risk uveal melanomas have a lower mortality rate. Cancers. 2021;13.
Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997;115:886–93.
Boldt HC, Binkley E. Treating small choroidal melanoma: smaller is better. JAMA Ophthalmol. 2018;136:1333–4.
Bagger MM. Intraocular biopsy of uveal melanoma risk assessment and identification of genetic prognostic markers. Acta Ophthalmol. 2018;96:1–28.
Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian J Ophthalmol. 2015;63:122–7.
Konstantinidis L, Damato B. Intraocular metastases-a review. Asia Pac J Ophthalmol (Philos). 2017;6:208–14.
Sugita S, Takase H, Sugamoto Y, Arai A, Miura O, Mochizuki M. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn J Ophthalmol. 2009;53:209–14.
Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol. 2007;27:241–50.
Karma A, von Willebrand EO, Tommila PV, Paetau AE, Oskala PS, Immonen IJ. Primary intraocular lymphoma: improving the diagnostic procedure. Ophthalmology 2007;114:1372–7.
Coupland SE, Bechrakis NE, Anastassiou G, Foerster AM, Heiligenhaus A, Pleyer U, et al. Evaluation of vitrectomy specimens and chorioretinal biopsies in the diagnosis of primary intraocular lymphoma in patients with Masquerade syndrome. Graefes Arch Clin Exp Ophthalmol. 2003;241:860–70.
Mastropasqua R, Thaung C, Pavesio C, Lightman S, Westcott M, Okhravi N, et al. The role of chorioretinal biopsy in the diagnosis of intraocular lymphoma. Am J Ophthalmol. 2015;160:1127–32.e1.
Coupland SE, Foss HD, Hidayat AA, Cockerham GC, Hummel M, Stein H. Extranodal marginal zone B cell lymphomas of the uvea: an analysis of 13 cases. J Pathol. 2002;197:333–40.
Cockerham GC, Hidayat AA, Bijwaard KE, Sheng ZM. Re-evaluation of “reactive lymphoid hyperplasia of the uvea”: an immunohistochemical and molecular analysis of 10 cases. Ophthalmology 2000;107:151–8.
Grossniklaus HE, Martin DF, Avery R, Shields JA, Shields CL, Kuo IC, et al. Uveal lymphoid infiltration. Report of four cases and clinicopathologic review. Ophthalmology. 1998;105:1265–73.
Ciulla TA, Bains RA, Jakobiec FA, Topping TM, Gragoudas ES. Uveal lymphoid neoplasia: a clinical-pathologic correlation and review of the early form. Surv Ophthalmol. 1997;41:467–76.
Ben-Ezra D, Sahel JA, Harris NL, Hemo I, Albert DM. Uveal lymphoid infiltrates: immunohistochemical evidence for a lymphoid neoplasia. Br J Ophthalmol. 1989;73:846–51.
Qualman SJ, Mendelsohn G, Mann RB, Green WR. Intraocular lymphomas. Natural history based on a clinicopathologic study of eight cases and review of the literature. Cancer. 1983;52:878–86.
Coupland SE, Foss HD, Bechrakis NE, Hummel M, Stein H. Secondary ocular involvement in systemic “memory” B-cell lymphocytic leukemia. Ophthalmology 2001;108:1289–95.
Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol. 2004;242:901–13.
Ewens KG, Kanetsky PA, Richards-Yutz J, Al-Dahmash S, De Luca MC, Bianciotto CG, et al. Genomic profile of 320 uveal melanoma cases: chromosome 8p-loss and metastatic outcome. Invest Ophthalmol Vis Sci. 2013;54:5721–9.
Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudleman E, et al. Collaborative ocular oncology group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012;119:1596–603.
Seider MI, Mruthyunjaya P. Molecular prognostics for uveal melanoma. Retina 2018;38:211–9.
McLean IW, Foster WD, Zimmerman LE, Gamel JW. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983;96:502–9.
Coleman K, Baak JP, van Diest PJ, Mullaney J. Prognostic value of morphometric features and the callender classification in uveal melanomas. Ophthalmology 1996;103:1634–41.
Folberg R, Chen X, Boldt HC, Pe’er J, Brown CK, Woolson RF, et al. Microcirculation patterns other than loops and networks in choroidal and ciliary body melanomas. Ophthalmology 2001;108:996–1001.
Parrella P, Sidransky D, Merbs SL. Allelotype of posterior uveal melanoma: implications for a bifurcated tumor progression pathway. Cancer Res. 1999;59:3032–7.
Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol. 2014;25:234–9.
Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–9.
Onken MD, Ehlers JP, Worley LA, Makita J, Yokota Y, Harbour JW. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006;66:4602–9.
Harbour JW. The genetics of uveal melanoma: an emerging framework for targeted therapy. Pigment Cell Melanoma Res. 2012;25:171–81.
Field MG, Decatur CL, Kurtenbach S, Gezgin G, van der Velden PA, Jager MJ, et al. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Cancer Res. 2016;22:1234–42.
Eleuteri A, Taktak AFG, Coupland SE, Heimann H, Kalirai H, Damato B. Prognostication of metastatic death in uveal melanoma patients: A Markov multi-state model. Comput Biol Med. 2018;102:151–6.
DeParis SW, Taktak A, Eleuteri A, Enanoria W, Heimann H, Coupland SE, et al. External validation of the liverpool uveal melanoma prognosticator online. Invest Ophthalmol Vis Sci. 2016;57:6116–22.
White JS, Becker RL, McLean IW, Director-Myska AE, Nath J. Molecular cytogenetic evaluation of 10 uveal melanoma cell lines. Cancer Genet Cytogenet. 2006;168:11–21.
Naus NC, van Drunen E, de Klein A, Luyten GP, Paridaens DA, Alers JC, et al. Characterization of complex chromosomal abnormalities in uveal melanoma by fluorescence in situ hybridization, spectral karyotyping, and comparative genomic hybridization. Genes Chromosomes Cancer. 2001;30:267–73.
Singh AD, Aronow ME, Sun Y, Bebek G, Saunthararajah Y, Schoenfield LR, et al. Chromosome 3 status in uveal melanoma: a comparison of fluorescence in situ hybridization and single-nucleotide polymorphism array. Invest Ophthalmol Vis Sci. 2012;53:3331–9.
Damato B, Duke C, Coupland SE, Hiscott P, Smith PA, Campbell I, et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology 2007;114:1925–31.
Sisley K, Tattersall N, Dyson M, Smith K, Mudhar HS, Rennie IG. Multiplex fluorescence in situ hybridization identifies novel rearrangements of chromosomes 6, 15, and 18 in primary uveal melanoma. Exp Eye Res. 2006;83:554–9.
Patel KA, Edmondson ND, Talbot F, Parsons MA, Rennie IG, Sisley K. Prediction of prognosis in patients with uveal melanoma using fluorescence in situ hybridisation. Br J Ophthalmol. 2001;85:1440–4.
McNamara M, Felix C, Davison EV, Fenton M, Kennedy SM. Assessment of chromosome 3 copy number in ocular melanoma using fluorescence in situ hybridization. Cancer Genet Cytogenet. 1997;98:4–8.
Sisley K, Rennie IG, Parsons MA, Jacques R, Hammond DW, Bell SM, et al. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer. 1997;19:22–8.
Petrausch U, Martus P, Tonnies H, Bechrakis NE, Lenze D, Wansel S, et al. Significance of gene expression analysis in uveal melanoma in comparison to standard risk factors for risk assessment of subsequent metastases. Eye (Lond). 2008;22:997–1007.
Kilic E, van Gils W, Lodder E, Beverloo HB, van Til ME, Mooy CM, et al. Clinical and cytogenetic analyses in uveal melanoma. Invest Ophthalmol Vis Sci. 2006;47:3703–7.
Hughes S, Damato BE, Giddings I, Hiscott PS, Humphreys J, Houlston RS. Microarray comparative genomic hybridisation analysis of intraocular uveal melanomas identifies distinctive imbalances associated with loss of chromosome 3. Br J Cancer. 2005;93:1191–6.
Aalto Y, Eriksson L, Seregard S, Larsson O, Knuutila S. Concomitant loss of chromosome 3 and whole arm losses and gains of chromosome 1, 6, or 8 in metastasizing primary uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:313–7.
Ghazvini S, Char DH, Kroll S, Waldman FM, Pinkel D. Comparative genomic hybridization analysis of archival formalin-fixed paraffin-embedded uveal melanomas. Cancer Genet Cytogenet. 1996;90:95–101.
Speicher MR, Prescher G, du Manoir S, Jauch A, Horsthemke B, Bornfeld N, et al. Chromosomal gains and losses in uveal melanomas detected by comparative genomic hybridization. Cancer Res. 1994;54:3817–23.
Gordon KB, Thompson CT, Char DH, O’Brien JM, Kroll S, Ghazvini S, et al. Comparative genomic hybridization in the detection of DNA copy number abnormalities in uveal melanoma. Cancer Res. 1994;54:4764–8.
Abi-Ayad N, Kodjikian L, Couturier J. [Genomic techniques used in uveal melanoma: a literature review]. J Fr Ophtalmol. 2011;34:259–64.
McCannel TA, Burgess BL, Nelson SF, Eskin A, Straatsma BR. Genomic identification of significant targets in ciliochoroidal melanoma. Invest Ophthalmol Vis Sci. 2011;52:3018–22.
Lake SL, Coupland SE, Taktak AF, Damato BE. Whole-genome microarray detects deletions and loss of heterozygosity of chromosome 3 occurring exclusively in metastasizing uveal melanoma. Invest Ophthalmol Vis Sci. 2010;51:4884–91.
Trolet J, Hupe P, Huon I, Lebigot I, Decraene C, Delattre O, et al. Genomic profiling and identification of high-risk uveal melanoma by array CGH analysis of primary tumors and liver metastases. Invest Ophthalmol Vis Sci. 2009;50:2572–80.
Onken MD, Worley LA, Person E, Char DH, Bowcock AM, Harbour JW. Loss of heterozygosity of chromosome 3 detected with single nucleotide polymorphisms is superior to monosomy 3 for predicting metastasis in uveal melanoma. Clin Cancer Res. 2007;13:2923–7.
Thomas S, Putter C, Weber S, Bornfeld N, Lohmann DR, Zeschnigk M. Prognostic significance of chromosome 3 alterations determined by microsatellite analysis in uveal melanoma: a long-term follow-up study. Br J Cancer. 2012;106:1171–6.
Shields CL, Ganguly A, Materin MA, Teixeira L, Mashayekhi A, Swanson LA, et al. Chromosome 3 analysis of uveal melanoma using fine-needle aspiration biopsy at the time of plaque radiotherapy in 140 consecutive cases: the Deborah Iverson, MD, Lectureship. Arch Ophthalmol. 2007;125:1017–24.
Hausler T, Stang A, Anastassiou G, Jockel KH, Mrzyk S, Horsthemke B, et al. Loss of heterozygosity of 1p in uveal melanomas with monosomy 3. Int J Cancer. 2005;116:909–13.
Tschentscher F, Prescher G, Zeschnigk M, Horsthemke B, Lohmann DR. Identification of chromosomes 3, 6, and 8 aberrations in uveal melanoma by microsatellite analysis in comparison to comparative genomic hybridization. Cancer Genet Cytogenet. 2000;122:13–7.
Shields CL, Ganguly A, Bianciotto CG, Turaka K, Tavallali A, Shields JA. Prognosis of uveal melanoma in 500 cases using genetic testing of fine-needle aspiration biopsy specimens. Ophthalmology. 2011;118:396–401.
Scholes AG, Damato BE, Nunn J, Hiscott P, Grierson I, Field JK. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003;44:1008–11.
Coupland SE, Kalirai H, Ho V, Thornton S, Damato BE, Heimann H. Concordant chromosome 3 results in paired choroidal melanoma biopsies and subsequent tumour resection specimens. Br J Ophthalmol. 2015;99:1444–50.
Vaarwater J, van den Bosch T, Mensink HW, van Kempen C, Verdijk RM, Naus NC, et al. Multiplex ligation-dependent probe amplification equals fluorescence in-situ hybridization for the identification of patients at risk for metastatic disease in uveal melanoma. Melanoma Res. 2012;22:30–7.
Damato B. Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye (Lond). 2012;26:1157–72.
Coupland SE, Lake SL, Zeschnigk M, Damato BE. Molecular pathology of uveal melanoma. Eye (Lond). 2013;27:230–42.
Damato B, Dopierala J, Klaasen A, van Dijk M, Sibbring J, Coupland SE. Multiplex ligation-dependent probe amplification of uveal melanoma: correlation with metastatic death. Invest Ophthalmol Vis Sci. 2009;50:3048–55.
O’Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–24.
Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin Cancer Res. 2010;16:6083–92.
Harbour JW. Molecular prognostic testing and individualized patient care in uveal melanoma. Am J Ophthalmol. 2009;148:823–9.e1.
Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010;12:461–8.
Smit KN, van Poppelen NM, Vaarwater J, Verdijk R, van Marion R, Kalirai H, et al. Combined mutation and copy-number variation detection by targeted next-generation sequencing in uveal melanoma. Mod Pathol. 2018;31:763–71.
Thornton S, Coupland SE, Olohan L, Sibbring JS, Kenny JG, Hertz-Fowler C, et al. Targeted next-generation sequencing of 117 routine clinical samples provides further insights into the molecular landscape of uveal melanoma. Cancers (Basel). 2020;12:1039.
Raja V, Russo A, Coupland S, Groenewald C, Damato B. Extraocular seeding of choroidal melanoma after a transretinal biopsy with a 25-gauge vitrector. Retin Cases Brief Rep. 2011;5:194–6.
Grixti A, Angi M, Damato BE, Jmor F, Konstantinidis L, Groenewald C, et al. Vitreoretinal surgery for complications of choroidal tumor biopsy. Ophthalmology. 2014;121:2482–8.
Grewal DS, Cummings TJ, Mruthyunjaya P. Outcomes of 27-gauge vitrectomy-assisted choroidal and subretinal biopsy. Ophthalmic Surg Lasers Imaging Retina 2017;48:406–15.
Angi M, Kalirai H, Taktak A, Hussain R, Groenewald C, Damato BE, et al. Prognostic biopsy of choroidal melanoma: an optimised surgical and laboratory approach. Br J Ophthalmol. 2017;101:1143–6.
Nagiel A, McCannel CA, Moreno C, McCannel TA. Vitrectomy-assisted biopsy for molecular prognostication of choroidal melanoma 2 Mm or less in thickness with a 27-gauge cutter. Retina. 2017;37:1377–82.
Midena E, Bonaldi L, Parrozzani R, Tebaldi E, Boccassini B, Vujosevic S. In vivo detection of monosomy 3 in eyes with medium-sized uveal melanoma using transscleral fine needle aspiration biopsy. Eur J Ophthalmol. 2006;16:422–5.
Singh AD, Medina CA, Singh N, Aronow ME, Biscotti CV, Triozzi PL. Fine-needle aspiration biopsy of uveal melanoma: outcomes and complications. Br J Ophthalmol. 2016;100:456–62.
Shields CL, Materin MA, Teixeira L, Mashayekhi A, Ganguly A, Shields JA. Small choroidal melanoma with chromosome 3 monosomy on fine-needle aspiration biopsy. Ophthalmology. 2007;114:1919–24.
Rishi P, Dhami A, Biswas J. Biopsy techniques for intraocular tumors. Indian J Ophthalmol. 2016;64:415–21.
Afshar AR, Damato BE, Stewart JM, Heimann H, Coupland SE, Reddy RE, et al. Vitrectomy and vitrector port needle biopsy of choroidal melanoma for gene expression profile testing immediately before brachytherapy. (Ophthalmology. 2017;124:1377-1382). Ophthalmology. 2018;125:e28–e9.
McCannel TA. Fine-needle aspiration biopsy in the management of choroidal melanoma. Curr Opin Ophthalmol. 2013;24:262–6.
Eide N, Syrdalen P, Walaas L, Hagmar B. Fine needle aspiration biopsy in selecting treatment for inconclusive intraocular disease. Acta Ophthalmol Scand. 1999;77:448–52.
Eide N, Walaas L. Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: a review. Acta Ophthalmol. 2009;87:588–601.
Kim RS, Chevez-Barrios P, Bretana ME, Wong TP, Teh BS, Schefler AC. Histopathologic analysis of transvitreal fine needle aspiration biopsy needle tracts for uveal melanoma. Am J Ophthalmol. 2017;174:9–16.
Augsburger JJ, Correa ZM, Trichopoulos N. Prognostic implications of cytopathologic classification of melanocytic uveal tumors evaluated by fine-needle aspiration biopsy. Arq Bras Oftalmol. 2013;76:72–9.
Cohen VM, Dinakaran S, Parsons MA, Rennie IG. Transvitreal fine needle aspiration biopsy: the influence of intraocular lesion size on diagnostic biopsy result. Eye. 2001;15:143–7.
McCannel TA, Chang MY, Burgess BL. Multi-year follow-up of fine-needle aspiration biopsy in choroidal melanoma. Ophthalmology 2012;119:606–10.
Chang MY, McCannel TA. Comparison of uveal melanoma cytopathologic sample retrieval in trans-scleral versus vitrectomy-assisted transvitreal fine needle aspiration biopsy. Br J Ophthalmol. 2014;98:1654–8.
Sen J, Groenewald C, Hiscott PS, Smith PA, Damato BE. Transretinal choroidal tumor biopsy with a 25-gauge vitrector. Ophthalmology 2006;113:1028–31.
Tang PH, Shields RA, Schefler AC, Mruthyunjaya P. Biopsy of a choroidal melanoma using transvitreal pars plana vitrectomy. Ophthalmic Surg Lasers Imaging Retina. 2018;49:645–7.
Abi-Ayad N, Grange JD, Salle M, Kodjikian L. Transretinal uveal melanoma biopsy with 25-gauge vitrectomy system. Acta Ophthalmol. 2013;91:279–81.
Reddy DM, Mason LB, Mason JO 3rd, Crosson JN, Yunker JJ. Vitrectomy and vitrector port needle biopsy of choroidal melanoma for gene expression profile testing immediately before brachytherapy. Ophthalmology 2017;124:1377–82.
Akgul H, Otterbach F, Bornfeld N, Jurklies B. Intraocular biopsy using special forceps: a new instrument and refined surgical technique. Br J Ophthalmol. 2011;95:79–82.
Seregard S, All-Ericsson C, Hjelmqvist L, Berglin L, Kvanta A. Diagnostic incisional biopsies in clinically indeterminate choroidal tumours. Eye (Lond). 2013;27:115–8.
Seider MI, Berry DE, Schefler AC, Materin M, Stinnett S, Mruthyunjaya P, et al. Multi-center analysis of intraocular biopsy technique and outcomes for uveal melanoma: Ocular Oncology Study Consortium report 4. Graefes Arch Clin Exp Ophthalmol. 2020;258:427–35.
Bagger M, Smidt-Nielsen I, Andersen MK, Jensen PK, Heegaard S, Andersen KK, et al. Long-Term Metastatic Risk after Biopsy of Posterior Uveal Melanoma. Ophthalmology 2018;125:1969–76.
Correa ZM, Huth B, Augsburger JJ. Scleral necrosis in patients with posterior uveal melanomas evaluated by transcleral fine needle aspiration biopsy and treated by 125I plaque. Arq Bras Oftalmol. 2018;81:330–5.
Siegel DT, Szalai E, Wells JR, Grossniklaus HE. Scleral thinning after transscleral biopsy for uveal melanoma using lamellar scleral flap. Ocul Oncol Pathol. 2018;4:381–7.
Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl J Med. 2021;385:1196–206.
Hope-Stone L, Brown SL, Heimann H, Damato B, Salmon P. How do patients with uveal melanoma experience and manage uncertainty? A qualitative study. Psychooncology 2015;24:1485–91.
Damato B, Hope-Stone L, Cooper B, Brown SL, Salmon P, Heimann H, et al. Patient-reported outcomes and quality of life after treatment of choroidal melanoma: a comparison of enucleation versus radiotherapy in 1596 patients. Am J Ophthalmol. 2018;193:230–51.
Damato B, Hope-Stone L, Cooper B, Brown S, Heimann H, Dunn L. Patient-reported outcomes and quality of life after treatment for choroidal melanoma. Ocul Oncol Pathol. 2019;5:402–11.
Brown SL, Fisher PL, Hope-Stone L, Hussain RN, Heimann H, Damato B, et al. Predictors of long-term anxiety and depression in uveal melanoma survivors: A cross-lagged five-year analysis. Psychooncology 2020;29:1864–73.
Brown SL, Fisher P, Hope-Stone L, Damato B, Heimann H, Hussain R, et al. Is accurate routine cancer prognostication psychologically harmful? 5-year outcomes of life expectancy prognostication in uveal melanoma survivors. J Cancer Surviv. 2022;16:408–20.
Cook SA, Damato B, Marshall E, Salmon P. Psychological aspects of cytogenetic testing of uveal melanoma: preliminary findings and directions for future research. Eye (Lond). 2009;23:581–5.
Beran TM, McCannel TA, Stanton AL, Straatsma BR, Burgess BL. Reactions to and desire for prognostic testing in choroidal melanoma patients. J Genet Couns. 2009;18:265–74.
Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114:2309–15.
Mensink HW, Vaarwater J, Kilic E, Naus NC, Mooy N, Luyten G, et al. Chromosome 3 intratumor heterogeneity in uveal melanoma. Invest Ophthalmol Vis Sci. 2009;50:500–4.
Glasgow BJ, Brown HH, Zargoza AM, Foos RY. Quantitation of tumor seeding from fine needle aspiration of ocular melanomas. Am J Ophthalmol. 1988;105:538–46.
Ophthalmic Oncology Task F. Local recurrence significantly increases the risk of metastatic uveal melanoma. Ophthalmology. 2016;123:86–91.
Char DH, Miller T. Accuracy of presumed uveal melanoma diagnosis before alternative therapy. Br J Ophthalmol. 1995;79:692–6.
Caminal JM, Sanz S, Carreras M, Catala I, Arruga J, Roca G. Epibulbar seeding at the site of a transvitreal fine-needle aspiration biopsy. Arch Ophthalmol. 2006;124:587–9.
Schefler AC, Gologorsky D, Marr BP, Shields CL, Zeolite I, Abramson DH. Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA Ophthalmol. 2013;131:1220–4.
Mashayekhi A, Lim RP, Shields CL, Eagle RC Jr, Shields JA. Extraocular extension of ciliochoroidal melanoma after transscleral fine-needle aspiration biopsy. Retin Cases Brief Rep. 2016;10:289–92.
Hussain RN, Kalirai H, Groenewald C, Kacperek A, Errington RD, Coupland SE, et al. Prognostic biopsy of choroidal melanoma after proton beam radiation therapy. Ophthalmology 2016;123:2264–5.
Dogrusoz M, Kroes WG, van Duinen SG, Creutzberg CL, Versluis M, Bleeker JC, et al. Radiation treatment affects chromosome testing in uveal melanoma. Invest Ophthalmol Vis Sci. 2015;56:5956–64.
Author information
Authors and Affiliations
Contributions
All authors (RNH, HH, BD) contributed to the conceptualisation, content and review of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hussain, R.N., Damato, B. & Heimann, H. Choroidal biopsies; a review and optimised approach. Eye 37, 900–906 (2023). https://doi.org/10.1038/s41433-022-02194-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02194-0
This article is cited by
-
Ocular oncology demystified
Eye (2023)