Abstract
Background/Objectives
The aim of this study was to ascertain the use of ocular imaging and the updated screening criteria in the evaluation of choroidal nevus across the United States.
Methods
Sixty ophthalmologists completed an anonymous 21-question survey addressing their use of the screening criteria for evaluating choroidal nevi, as well as their use of ultrasonography (US), optical coherence tomography (OCT), and autofluorescence (AF) in daily practice.
Results
The majority of respondents were from the Northeast (55%), worked in private practice (83%), and practiced general ophthalmology (42%). The 2009 criteria TFSOM-UHHD was used by 39 (65%) respondents, while the 2019 criteria TFSOM-DIM was used by 29 (48%) respondents. Compared to anterior segment ophthalmologists, posterior segment ophthalmologists were more likely to use the TFSOM-UHHD criteria (94% vs. 53%, OR = 13.9, p = 0.014), the TFSOM-DIM criteria (88% vs. 33%, OR = 15.5, p < 0.001), fundus AF (82% vs. 19%, OR = 20.4, p < 0.001), and US (94% vs. 42%, OR = 22.2, p = 0.004) in daily practice.
Conclusions
From the survey of current practice patterns, we learned that there is a general trend of underutilization of the proper imaging modalities – and thus the criteria – in evaluating choroidal nevus. More education about ocular cancer and its screening could improve patient outcomes in the future.
Similar content being viewed by others
Introduction
Choroidal nevus is the most common intraocular tumor encountered in clinical practice [1]. The prevalence of choroidal nevus in a white population is 4–7%, with older individuals demonstrating a higher likelihood of possessing the lesion and demonstrating multifocal and thicker tumors, more often with overlying drusen, compared to younger patients [2,3,4]. While benign and often found incidentally, choroidal nevus does carry an estimated risk of 1/8845 for progression to malignant melanoma, and it is imperative that these lesions are screened and triaged as necessary to ocular oncology centers for evaluation of risk [5, 6].
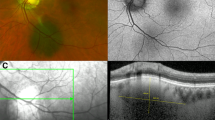
In 1995, Shields et al. published the initial clinical observations and risk factors for choroidal nevus transformation into melanoma in a cohort of 1329 patients using the mnemonic “To Find Small Ocular Melanoma”, representing T for thickness >2 mm on ultrasonography, F for fluid overlying the tumor clinically, S for symptoms, O for orange pigment seen clinically, and M for margin ≤3 mm from optic disc [7]. In 2009, Shields et al. reviewed a separate cohort of 2514 patients to further refine the risk factors for transformation of choroidal nevus to melanoma using the mnemonic “To Find Small Ocular Melanoma Using Helpful Hints Daily,” abbreviated TFSOM-UHHD [6]. The new mnemonic added UH for Ultrasonographic Hollowness, H for Halo absent, and D for Drusen absent. Most recently, in 2019, Shields et al. further studied the imaging findings of choroidal nevus in 3806 additional patients and updated the mnemonic to represent risk factors based on multimodal imaging including “To Find Small Ocular Melanoma Doing IMaging,” abbreviated TFSOM-DIM to represent T for tumor thickness greater than 2 mm on ultrasonography (US), F for subretinal fluid on optical coherence tomography (OCT), S for Symptomatic vision loss 20/50 or worse, O for Orange pigment on fundus autofluorescence (AF), M for Melanoma hollow on US, DIM for DIaMeter greater than 5 mm on fundus photography (shown in Fig. 1) [8]. These measures are crucial for screening choroidal nevus patients for early detection of choroidal melanoma transformation. However, these measures require access to multiple imaging modalities, including ocular ultrasonography, optical coherence tomography, fundus autofluorescence, and fundus photography.
Graphic demonstrating the 6 criteria for TFSOM-DIM: thickness greater than 2 millimeters (mm) on ultrasound (T), subretinal fluid on optical coherence tomography (F), symptomatic vision loss 20/50 or worse (S), orange pigment on autofluorescence (O), melanoma hollow on ultrasound (M), diameter greater than 5 mm (DIM). For each criterion, there is a fundus photo depicting the appearance of the lesion and an image in the appropriate modality to assess for each feature. For T, the thickness of the tumor is 3.75 mm. For F, the subretinal fluid is denoted by the arrow. For DIM, the diameter of the lesion is 8 mm.
Nevertheless, the understanding and use of these imaging modalities by ophthalmologists in daily practice can vary across the United States and by subspecialisation. Patients in areas where ophthalmologists have limited access to these imaging modalities may not receive adequate screening. The objective of the present study was to ascertain the use of imaging modalities for implementation of the updated TFSOM-DIM criteria in the evaluation of choroidal nevus across the United States.
Methods
The primary goal of this study was to survey ophthalmologists across the United States to assess the use of ophthalmic imaging and the TFSOM-DIM criteria in their evaluation of choroidal nevus. Qualtrics software (Provo, UT, USA) was used to construct an anonymous online survey consisting of 21 questions addressing demographics, practice characteristics, and knowledge (Supplementary Table 1). A “Yes” response to the questions regarding imaging modalities triggered follow-up questions about that corresponding imaging modality. The institutional review board at Wills Eye Hospital considered the current study exempt. The study adhered to the tenets of the Declaration of Helsinki.
Between March 2021 and July 2021, survey distribution requests were emailed to executive directors of the state ophthalmology societies. A follow-up email and phone call were made to state societies that did not respond to the initial email. Participants could skip any number of the questions while still completing the survey. The ophthalmologists completing the survey did not provide informed consent because the data were deidentified.
Demographic information was collected, including location of practice in the U.S., practice type, ophthalmology subspecialty, and number of years in practice. For practice characteristics, we assessed ophthalmologist use of the TFSOM-UHHD and the updated TFSOM-DIM criteria for evaluating choroidal nevi, as well as their use of US, OCT, and AF in their daily practice. Participants were also asked to estimate how many patients they treat yearly with choroidal nevus, choroidal melanoma, and if they ever had a patient progress from choroidal nevus to choroidal melanoma.
Using the online Qualtrics software, data was collected and anonymously exported using an Excel spreadsheet (Microsoft, Redmond, WA, USA). Frequencies of each survey response were determined using Excel. The data were further analyzed using IBM SPSS Statistics, version 28.0 (IBM Corp., Armonk, NY, USA). Binary logistic regression was performed to assess the relationship between use of imaging modalities and location, extent of experience, and type of subspecialisation. A p-value of <0.05 was considered statistically significant for the results of all analyses.
Results
Of the 50 state ophthalmology societies, 16 (32%) agreed to distribute the survey, 8 (16%) declined to distribute, and 26 (52%) did not respond to the email or follow-up phone call requests. Responses were received from ophthalmologists located in 10 different states. A total of 64 ophthalmologists practicing in the U.S. responded to the survey request. Four participants did not answer the majority of questions and were excluded from the study. Participants that completed at least half of the questions but did not answer some were analyzed.
Demographics are displayed in Table 1. Participants were most commonly from the Northeast (33 [55%]), and the majority of respondents work in private practice (50 [83%]), compared with academic centers (10 [17%]). The most common subspecialties among responders were general ophthalmology (25 [42%]) and retina (16 [27%]). Participants had varying duration of experience but most commonly 20–49 years (41 [68%]).
Knowledge and experience questions for choroidal nevus and choroidal melanoma are displayed in Table 2. Participants examined a varying number of choroidal nevus patients per year: 0–10 patients (8 [13%]), 10–20 patients (13 [22%]), 20–50 patients (14 [23%]), 50–100 (14 [23%]), and >100 (11 [18%]). Of those, there were 21 (35%) participants who had observed a choroidal nevus transformation into melanoma, and 20 (95%) of those participants referred the patient to ocular oncology or retina specialists.
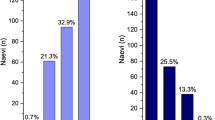
Practice characteristics and participant use of the criteria for choroidal nevus and melanoma are displayed in Table 3. The 2009 criteria TFSOM-UHHD was used by 39 (65%) respondents, while the 2019 criteria TFSOM-DIM was used by 29 (48%) respondents. However, neither set of criteria was used by 17 (28%) respondents. Ocular US was available to 46 (77%) respondents but was used routinely in practice by only 34 (57%). Moreover, US was used in every evaluation of choroidal nevus by only 3 (9%) respondents.
Optical coherence tomography was available to 59 (98%) respondents and was routinely used by 57 (95%). Of those 57 participants, OCT was used to image choroidal nevus in 37 (65%) respondents but was used on every examination in only 7 (12%).
Snellen visual acuity (S) was considered in the analysis of choroidal nevus by 45 (75%) participants, while photography was used to measure tumor diameter (DIM) by 43 (75%) participants. Finally, AF was available to 37 (62%) participants but was routinely used in practice by only 22 (37%). Of those 22 participants, AF was used to judge the presence of orange pigment (O) over a choroidal nevus by 15 (68%) participants and was used in this manner on every examination by only 1 (5%) participant.
The comparison by subspecialty is displayed in Table 4. Compared to anterior segment ophthalmologists (cataract, cornea, general ophthalmology, glaucoma, oculoplastics, and paediatrics), posterior segment ophthalmologists (surgical retina, medical retina, ocular oncology) were more likely to use the 2009 TFSOM-UHHD criteria (94% vs. 53%, OR = 13.9, p = 0.014), the 2019 TFSOM-DIM criteria (88% vs. 33%, OR = 15.5, p < 0.001), fundus AF (82% vs. 19%, OR = 20.4, p < 0.001), and US (94% vs. 42%, OR = 22.2, p = 0.004) in their daily practice. There was no difference in use of OCT, and there were no relationships between location (Northeast vs. South) or number of years in practice (<20 vs. ≥20) and use of any imaging modality or criteria.
Discussion
Choroidal nevus is the most common intraocular tumor, and the highest likelihood of growth into melanoma is linked to a combination of risk factors [1]. The 5-year Kaplan–Meier estimate of nevus growth to melanoma with no risk factors was 1% compared to those with 1 factor (11%), 2 factors (22%), 3 factors (34%), and 4 or more factors (>50%) [8]. The use of the TFSOM-DIM criteria and the proper imaging modalities is imperative in assessing the risk of these lesions so tumors undergoing transformation into melanoma can be caught and treated early. Multimodal imaging including fundus photography, OCT, AF, and US all play an important role in the noninvasive detection of factors predictive of nevus growth into melanoma.
From the survey of current practice patterns, we learned that there is a general trend of underutilization of the criteria and the proper imaging modalities during evaluation of choroidal nevus. Less than half of the participants (48.3%) use the TFSOM-DIM criteria in their evaluation of choroidal nevus. OCT is most commonly used by participants in daily practice (95%), but only 57% and 37% of participants use US and AF routinely in practice, respectively. The results of the survey highlight an underutilization of the helpful diagnostic imaging of these lesions, which could lead to delayed detection and care for patients with suspicious lesions or early melanoma. Although the majority of participants referred their melanoma patients to ocular oncology or retina specialists (95%), earlier detection and referral could improve patient outcomes.
Use of the 2009 TFSOM-UHHD criteria, 2019 TFSOM-DIM criteria, and specific testing with fundus AF and ophthalmic US were found to have a significant relationship with subspecialty, when comparing anterior and posterior segment ophthalmologists. Posterior segment ophthalmologists, including retina specialists and ocular oncologists, were 14 times more likely to use the 2009 diagnostic criteria and 15 times more likely to use the 2019 criteria, compared to anterior segment physicians. Similarly, posterior segment ophthalmologists were 20 times more likely to use fundus AF, and 22 times more likely to use ophthalmic US. Because posterior segment surgeons use these imaging modalities more frequently and routinely for all pathologies, compared to anterior segment surgeons, their practices are more likely to have these specific imaging modalities rapidly available for use in evaluation of choroidal nevus. However, for all subspecialties, readily available ophthalmic imaging equipment may be underutilized in the evaluation of choroidal nevus, potentially leading to an inaccurate assessment for a tumor’s malignant potential.
Lack of appropriate screening of choroidal nevi could lead to both under-referral and over-referral, which can have important consequences: under-referral may result in patients being diagnosed too late for effective treatment while over-referral places an increased burden on subspecialty care services. Damato et al. found that 23.1% of uveal melanoma patients had tumors that were initially missed by the practitioner they first consulted, which resulted in significantly longer time to treatment [9]. Such patients tended to have a more advanced tumor by the time they reached an ocular oncologist and were more likely to require enucleation [9]. A better understanding of the risk factors for the progression of nevus to melanoma could allow earlier detection and more prompt treatment, leading to improved visual acuity, globe salvage, metastatic rate, and mortality.
The underutilization of these diagnostic imaging modalities may also result in over-referral to specialists when treatment is not necessary. While the majority of choroidal nevi are benign, the potential for missing an early melanoma prompts urgent over-referrals to ophthalmology subspecialists and ocular oncologists. This may lead to an unnecessary burden on the patient, including time, travel expenses, and cost of evaluation, all at the expense of specialist resources. Because specialists tend to use more resources than general practitioners, there is concern that the overuse of referrals wastes resources and unnecessarily drives up costs [10]. Law et al. demonstrated that only 38.3% of patients were correctly diagnosed with uveal melanoma before referral to an ocular oncology center [11]. There is room for improvement and a need for increased knowledge in ocular oncology and improvement of eye cancer care facilities at the referral base.
This study has several limitations. First, this study had a small sample size due to a low response rate. Nevertheless, on a state level, the response rate reported here is comparable in both percent and total number of respondents to similar published ocular oncology surveys [12, 13]. Another limitation is the use of surveys to gauge practice patterns in general, which could include self-reporting, response bias, and voluntary participation and self-selection of participants that could skew the results of the survey.
Conclusion
In conclusion, this study provides insight into the practice patterns of ophthalmologists in their assessment of choroidal nevus and choroidal melanoma in the United States and provides critical information regarding the evaluation and diagnosis of patients with these lesions. It is imperative that ophthalmologists, regardless of subspecialty, be diligent in their screening of these lesions and use the adequate imaging modalities to properly assess risk of malignant transformation. More education about ocular cancer and its screening could improve patient outcomes in the future.
Summary
What was known before
-
Choroidal nevus is the most common intraocular tumor encountered in clinical practice. The clinical observations and risk factors for choroidal nevus transformation into melanoma requires the use of multimodal imaging including fundus photography, optical coherence tomography, fundus autofluorescence, and ophthalmic ultrasound.
What this study adds
-
From the survey of current practice patterns, we learned that there is a general trend of underutilization of the proper imaging modalities; and thus the criteria; in evaluating choroidal nevus. More education about ocular cancer and its screening could improve patient outcomes in the future.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request (email: JosephDDeSimone@gmail.com).
References
Shields JA, Shields CL. Intraocular tumors. An atlas and textbook. 3rd ed. Philadelphia, PA: Lippincott Wolters Kluwers; 2016. p. 69–80.
Sumich P, Mitchell P, Wang JJ. Choroidal nevi in a white population: the Blue Mountains Eye Study. Arch Ophthalmol. 1998;116:645–50.
Qiu M, Shields CL. Choroidal nevus in the United States adult population: racial disparities and associated factors in the national health and nutrition examination survey. Ophthalmology. 2015;122:2071–83.
Shields CL, Furuta M, Mashayekhi A, Berman EL, Zahler JD, Hoberman DM, et al. Clinical spectrum of choroidal nevi based on age at presentation in 3422 consecutive eyes. Ophthalmology. 2008;115:546–52.
Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of choroidal nevus. Ophthalmology. 2005;112:1784–9.
Shields CL, Furuta M, Berman EL, Zahler JD, Hoberman DM, Dinh DH, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127:981–7.
Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102:1351–61.
Shields CL, Dalvin LA, Ancona-Lezama D, Yu MD, Di Nicola M, Williams BK Jr., et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39:1840–51.
Damato EM, Damato BE. Detection and time to treatment of uveal melanoma in the United Kingdom: an evaluation of 2,384 patients. Ophthalmology. 2012;119:1582–9.
Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39–68.
Law C, Krema H, Simpson ER. Referral patterns of intraocular tumour patients to a dedicated Canadian ocular oncology department. Can J Ophthalmol. 2012;47:254–61.
Eiger-Moscovich M, Eagle RC Jr, Shields CL, Racher H, Lally SE, Silkiss RZ, et al. Muir-Torre Syndrome associated periocular sebaceous neoplasms: screening patterns in the literature and in clinical practice. Ocul Oncol Pathol. 2020;6:226–37.
Shah SN, Kogachi K, Correa ZM, Schefler AC, Aronow ME, Callejo SA, et al. Trends in radiation practices for female ocular oncologists in North America: a collaborative study of the international society of ocular oncology. Ocul Oncol Pathol. 2019;5:54–59.
Funding
Support provided in part by the Eye Tumor Research Foundation, Philadelphia, PA (CLS). The funders had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, and in the preparation, review or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
CLS had full access to the data and takes responsibility for the integrity of the data and data analysis. Concept and design: JDD, CLS. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: JDD. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: JDD, PWD. Obtained funding: CLS. Supervision: CLS, RRS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The Wills Eye Hospital Institutional Review Board deemed that this study was exempted from IRB approval. This study adhered to the tenets of the declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
DeSimone, J.D., Dockery, P.W., Kreinces, J.B. et al. Survey of ophthalmic imaging use to assess risk of progression of choroidal nevus to melanoma. Eye 37, 953–958 (2023). https://doi.org/10.1038/s41433-022-02110-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02110-6
This article is cited by
-
Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases
Signal Transduction and Targeted Therapy (2024)
-
Multimodal imaging risk factors predictive of small choroidal melanocytic lesion growth to melanoma: An educational study and pictorial guide
Eye (2024)
-
Ocular oncology demystified
Eye (2023)