Abstract
Background/Objective
Management of concomitant cataract and glaucoma depends on the stage of glaucoma and the patient’s situation. There are different surgical options for handling visually significant cataract and mild-to-moderate open-angle glaucoma (OAG). We aimed to compare the one-year results of phacoemulsification alone versus phacoviscocanalostomy in these patients.
Subjects/Methods
This was a parallel-arm, single-masked, randomized-controlled trial, conducted at Farabi Eye Hospital, Tehran, Iran between January 2016 and January 2018. We enrolled 89 eyes from 89 patients with mild-to-moderate primary OAG or pseudoexfoliative glaucoma (PEXG) with visually significant age-related cataract. They randomly underwent phacoemulsification alone (n = 44) or combined phaco-viscocanalostomy (n = 45). All patients had a 12-month follow-up period, and the mean intraocular pressure (IOP), the number of antiglaucoma medications, and complete and qualified success rates were compared.
Results
After the 1st and 3rd months, the mean IOP showed significantly decreased in the phaco-visco group compared to the phaco group (P < 0001 and P = 0.004, respectively), but it was not statistically significant at 6th and 12th months (P = 0.540 and P = 0.530). The need for antiglaucoma medication and the complete and qualified success rates were significantly in favour of the phaco-visco group in all postoperative visits (P < 0.05).
Conclusions
Although both phacoemulsification alone and phacoviscocanalostomy procedures can be considered for patients with mild-to-moderate OAG, we found better success rates using phacoviscocanalostomy. Therefore, if the surgeon is an expert in performing this technique, this non-penetrating procedure can be applied in patients with visually significant cataract and earlier stages of OAG, especially in patients with PEXG.
Similar content being viewed by others
Introduction
Both glaucoma and cataract are age-related conditions, and their co-existence may be common in the same patient, especially in older ages, and the management of this issue can be a challenge for ophthalmologists. So, there may be many patients who are planned to undergo cataract surgery but may also require glaucoma interventions or vice versa [1,2,3].
The final outcome of glaucoma treatment is the preservation of the optic nerve from being damaged by high levels of intraocular pressure (IOP) [4, 5], and the final outcome of cataract surgery is improving visual acuity. While combined phacotrabeculectomy is currently the standard procedure for many surgeons, trabeculectomy is associated with serious short- and long-term postoperative complications such as choroidal effusion, hypotony with or without maculopathy, and bleb-related complications, as well as shallow or flat anterior chambers [4]. On the other hand, combined phacotrabeculectomy may induce much more inflammation than trabeculectomy alone, and the risk of bleb fibrosis and final failure increases during combined trabeculectomy and cataract surgery [6, 7]. Therefore, a paradigm shift from phacotrabeculectomy to other less invasive procedures has occurred in recent years for the management of concomitant cataracts and glaucoma [8].
Alternative techniques such as non-penetrating glaucoma surgery (NPGS) and minimally invasive glaucoma surgery (MIGS) attempted to lower IOP in a safer manner. For example, viscocanalostomy, which is a type of NPGS, introduced by Stegmann and colleagues [9] has fewer postoperative complications than standard trabeculectomy [6]. Compared with trabeculectomy, the IOP-lowering efficacy of visconalostomy is the same or less, depending on the study [10,11,12]. In different comparative studies, when phacoemulsification is combined with viscocanalostomy, it results in similar IOP reduction, with lower complication rates than phacotrabeculectomy [13,14,15]. In a study that compared some techniques of NPGS and MIGS for patients suffering from cataracts and OAG, the authors conducted that phacoviscocanalostomy led to the largest IOP drop and largest reduction of medication use compared to the other less invasive procedures [4].
Cataract surgery alone may independently lower IOP, which may allow for greater IOP control among patients with co-existing cataracts and glaucoma. The decision between undergoing combined glaucoma and cataract surgery versus cataract surgery alone is complex. The type and severity of glaucoma, the preoperative IOP, the systemic and ocular condition of the patient, and the impact of cataracts on visual acuity are all important to choose the best surgical option. Shoji and co-workers [16] compared the success rate of phacoemulsification alone and combined phacoviscocanalostomy in patients with cataract and normal-tension glaucoma (NTG) and showed a greater success rate and better IOP control after combined surgery.
To the best of our knowledge, no studies have investigated the efficacy of phacoemulsification alone versus combined phacoviscocanalostomy in the management of patients with concurrent cataracts and mild-to-moderate OAG. Therefore, we aimed to compare the midterm success rate and efficacy of these two surgical techniques in eyes with co-existing cataracts and this stage of OAG. It is important to compare the effectiveness of these two interventions to aid clinicians and patients in choosing the better management approach.
Subjects and methods
We conducted a parallel-arm, single-masked, randomized-controlled trial at Farabi Eye Hospital, Tehran, Iran, between January 2016 and January 2018. The study was approved by the Ethics Committee of Tehran University of Medical Sciences. The study adhered to the principles of the Declaration of Helsinki.
We included 89 eyes from 89 patients that had mild to moderate primary OAG or pseudoexfoliative glaucoma (PEXG) with age-related cataracts. The sample size calculation formula was as follows:
Inclusion criteria
We included patients ≥40 years old at the time of surgery with POAG and age-related cataracts. The diagnosis of OAG was based on history, clinical examination, and typical glaucomatous visual field loss by Humphrey field analyser (HFA; Carl Zeiss Meditec Inc., Oberkochen, Germany). On examination, the presence of glaucomatous optic neuropathy (GON; loss of the neuroretinal rim with a vertical cup-to-disc ratio (VCDR) of >0.6 and/or notching with a nerve fibre layer defect that was attributable to glaucoma) with compatible Visual field loss was considered as glaucoma. The indications for surgery were the presence of a visually significant cataract (visual acuity ≤0.5) in the presence of primary OAG or PEXG. The diagnosis of pseudoexfoliation was according to the signs of exfoliative materials in the anterior chamber structures (e.g. target sign on the lens capsule and/or sampolesi line in the gonioscopy). Patients were included if the cataract was associated with uncontrolled glaucoma (IOP > 21 mmHg despite maximally tolerated medical therapy) or if the IOP was ≤21 mmHg with the use of at least two antiglaucoma drugs with medication intolerance, poor patient compliance or patients could not attend medical supervision.
Exclusion criteria
Patients were excluded if they had closed-angle glaucoma, other types of OAG (such as pigmentary glaucoma, inflammatory glaucoma, or neovascular glaucoma), previous ocular trauma or surgery, lens subluxation, or other eye diseases affecting the vision such as anterior uveitis and vitreoretinal or neuro-ophthalmological problems. Patients with high pre-op IOP (>25 mmHg despite maximally tolerated medical therapy) or those with advanced glaucoma (vertical cup-disc ratio larger than 0.9) were also excluded. Moreover, pregnant or breastfeeding women were excluded. Patients were also excluded if there was a large perforation of the Descemet’s membrane with iris prolapse during surgery (cases with microperforation, which is defined as small perforation with no associated iris prolapse, occurring during surgery were not excluded) or if they had other intraoperative complications that might affect IOP, such as vitreous loss or retained cortical materials.
Study protocol
Written informed consent was obtained from every patient after explaining the procedure and the possible consequences of the surgery.
Patient examinations
For all the patients recruited in the study, demographic details such as age, sex, highest baseline IOP, central corneal thickness (CCT), topical hypotensive medications, history of previous ocular surgeries, and history of drug allergy were recorded at baseline. Patients were evaluated preoperatively and postoperatively on day 1, and 1, 3, 6 and 12 months. Each preoperative and postoperative examination included best-corrected visual acuity (BCVA), comprehensive anterior segment evaluation, IOP, and dilated fundus evaluation. BCVA was measured using Snellen visual acuity chart at 20 feet and was converted into decimal fraction. IOP was measured using a Goldmann applanation tonometer and the average of 3 readings was noted.
Surgical technique
Operations were done by either standby or general anaesthesia according to the patient’s cooperation and condition by or under supervision of a single surgeon (RZ). In the phaco group, a temporal clear corneal phacoemulsification was performed by either stop and chop or horizontal chop techniques according to nuclear density.
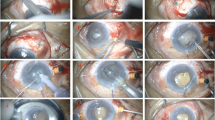
In the phaco-visco group, after a temporal phacoemulsification and intraocular lens implantation, a traction suture in the cornea (12 o, clock) was made using 7–0 Vicryl suture. A superior fornix-based conjunctival flap was fashioned about 6 millimetres (mm), and gentle cautery was applied. A superficial scleral flap of 5 × 5 mm was made, and dissection was done 2 mm into the clear cornea. A smaller, deeper flap of 4 × 4 mm was made until unroofing of Schlemm’s canal was achieved. Decompression of the eye was done through one of the paracentesis incisions to achieve an IOP of about 10 mmHg, then dissection with a microsponge and a crescent knife was done to create a trabeculo-descematic window that extended over Descemet’s membrane until 1 to 2 mm inside the clear cornea. Subsequently, the sides of the deep flap were freed from the adjacent sclera by Vannas scissors, and the deep flap was cut by the same scissors. Then high-viscosity sodium hyaluronate (Healon GV) was injected into the canal on both sides using a 30 gauge cannulated needle. After that, the superficial flap was sutured using two interrupted 10–0 nylon sutures, each one on one side of the flap.
Postoperative management
Postoperative management included administration of 0.3% ciprofloxacin eye drops for 1 week with a tapered schedule of 0.1% betamethasone eye drops. Antiglaucoma medication was discontinued after surgery in all cases. Postoperatively, follow-up examination was done on days 1 and 30, then at 3rd, 6th, and 12th months. The follow-up examination included assessment of IOP, CCT, VA, disc cupping (using a biconvex 90 lens), and slit-lamp examination. Any detected postoperative complication was recorded. Signs of inflammation, such as cell infiltration and flare, were recorded and graded from 0 to 4. Antiglaucoma drugs (latanoprost) were prescribed if the IOP was elevated above 21 mmHg. The IOP examiner did not know which group the patients were assigned to.
Outcome assessment
The main outcome measures were the IOP at the one-year follow-up and the overall success, which included complete success when IOP was ≤21 mmHg without any antiglaucoma drugs, and qualified success if IOP was ≤21 mmHg with the use of a single antiglaucoma medication. Failure was considered if an IOP ≤ 21 mmHg could not be reached even with the use of a single antiglaucoma medication at any follow-up visit or the presence of hypotony.
The optic nerve head was also examined, and VCDR of the optic disc was recorded in each visit.
Randomization and masking
The randomization scheme was simple randomization with allocation concealment. The participants were randomly assigned into 2 groups with the allocation ratio of 1:1. The randomization scheme was generated using computer-generated random numbers. The participants were masked to the intervention. The person recording IOP (M.N.) was masked to the intervention.
Statistical analysis
The data were analysed was using SPSS software, version 22 (SPSS Inc., Chicago, Illinois, USA). Continuous variables were presented as mean ± SD and compared using Student’s t test. A two-tailed test was used to detect significance between the two groups, and one-tailed t test was used to detect the significance before and after intervention in the same group. Mann-Whitney U test was used for nonparametric analysis. Comparison of categorical variables was made using the Chi-square test. P < 0.05 was considered statistically significant.
Results
Out of 100 eyes assessed for eligibility, 89eyes of 89 patients with mild-to-moderate OAG were recruited and randomized to undergo either phaco viscocanalostomy (n = 45) or phaco alone (n = 44) and completed the follow-up period (Fig. 1). There were no significant differences between the two groups regarding patient demographics or preoperative data (Table 1).
No postoperative complications were encountered during the 12-month follow-up.
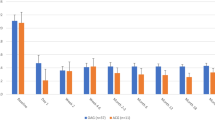
With respect to the preoperative and 12 months postoperative results in each group, improvement regarding visual acuity, decrease in IOP-lowering drug use, and decrease in IOP and VCDR change are shown in Table 2. As shown, in all postoperative visits, the mean IOP was decreased compared to baseline in both groups. Although after 1st and 3rd months, the mean IOP showed a statistically significant decrease in the phaco-visco group compared with the phaco group (P < 0001 and P = 0.004 respectively), it was not statistically significant after the 6th and 12th months visits (P = 0.540, P = 0.530). Figure 2A shows the trends of changes in mean IOP. On the other hand, the number of IOP-lowering drugs was significantly lower at all measurements in the phaco-visco group (P < 0.05 for all groups). The pattern of change in the number of antiglaucoma drops is shown in Fig. 2B. Over time, there was no change in the VCDR in either group (P < 0.05 for all groups).
A Trend of IOP changes in the 12-month follow-up period. In both groups, the IOP decreased significantly after the operation, but the slope of IOP reduction was slower in the phaco group. B Changes in the number of anti-glaucoma drops in the 12-month follow-up period. The drop numbers diminished after the operation in both groups, and the decrease was more significant in the phaco-visco group. After 1 month, the number of drops increased, which was more significant in the phaco group.
The complete and qualified success rates were significantly in favour of the phaco-visco group in all postoperative visits, although in both groups, the complete success rate had a descending trend while the qualified success had an ascending trend over time. There was no case of failure according to our definition in this follow-up period (Table 3). Supplemental Figs. 1 and 2 illustrate the trend of success rates during the follow-up period.
Linear mixed-model analysis was applied for comparing patients with PEXG and primary OAG. The results showed that the only difference was the number of antiglaucoma drops, which was lower in the phaco-visco group, was more pronounced and more stable in patients with PEXG compared with those with primary OAG. In PEXG group who underwent phaco-viscocanalostomy, after the 1st month visit, the need for IOP-lowering drugs was significantly less than those who underwent phacoemulsification alone, but in patients with primary OAG, during the 12-month follow-up period, the difference in the number of antiglaucoma medications was not statistically significant between the two types of surgeries (P > 0.05 for all, Supplemental Table 1).
Discussion
In primary OAG, the primary outflow resistance is located within the trabecular meshwork (TM), and therefore, non-penetrating glaucoma surgeries, based on the selective microsurgical dissection of TM, have become the focus of attention among glaucoma surgeons [16]. The smooth reduction in the intraocular pressure obtained without penetrating into the anterior chamber prevents most trabeculectomy-related complications such as hypotony leading to a shallow anterior chamber, hyphema, choroidal haemorrhage and detachment, bleb-related complications, and increased risk of developing cataract [17,18,19].
We found that although the phaco-visco group had greater IOP reduction than the phaco group in the first post-op month (43.2% vs. 24.4%), and this reduction was also pronounced in the third month (43.2% vs. 36%), with the extension of the follow-up period to 12 months, the mean IOP did not differ between the phaco and phaco-visco groups. We showed that phacoviscocanalostomy decreased the need for antiglaucoma medications much more than phacoemulsification alone during the 12-month follow-up (64.4% vs. 47.1% decrease in the number of medications at the end of follow-up). This means that although both groups reached a similar mean IOP at the end of the follow-up period, the IOP control in the phaco group was more dependent on antiglaucoma medications administration.
All our patients had visually significant cataracts, and mild-to-moderate glaucoma, and the baseline IOPs were not too high. Therefore, both procedures can decrease the IOP and provide appropriate IOP control, although more antiglaucoma medications may be needed in patients undergone phacoemulsification alone.
The effect of phacoemulsification in IOP reduction in normal and in eyes with glaucoma is well established in the previous studies. In patients with OAG, about 2–4 mmHg IOP reduction has been shown by cataract extraction, and this amount varies according to baseline IOP and the type and natural course of glaucoma [16, 20,21,22,23,24]. As our patients had a baseline IOP of around 22 mmHg, phacoemulsification alone had a good IOP control during the 12-month follow-up.
On the other hand, our results showed that the effect of phacoviscocanalostomy in terms of IOP reduction might be decreased over time. During the viscocanalostomy procedure, we did not use any tissue spacer below the superficial flap, and this may be an important reason for the mentioned trend in IOP changes which is consistent with most reports in the literature. It may be due to the healing process and fibrosis, which result in adhesion formation and collapse of the superficial flap and a decrease in the intra-scleral lake volume. The peak time of this healing process is about 3 months after the operation, compatible with the pattern of IOP changes in our patients [25,26,27].
A complete success with the definition of IOP < 21 mmHg without antiglaucoma medications was achieved in 61.6% of the phaco-visco eyes and 20% of the phaco ones. Also, qualified success, defined as IOP < 21 with a single antiglaucoma medication, was attained in 79.9% and 20% of the phaco-visco and phaco eyes, respectively. Shoji and colleagues [16] designed a study on 70 eyes of patients with NTG to compare phacoemulsification alone and phaco-viscocanalostom. In line with our study, they found that phacoviscocanalostomy has a greater success rate and more consistent IOP control than phacoemulsification alone (up to three years) and the success rates of phacoviscocanalostomy decreased over time (78% in the 24th month vs 58% in the 48th month). They concluded that Phacoviscocanalostomy lowered IOP and maintained postoperative visual outcomes. In another study [28], phacoviscocanalostomy was performed on eyes with co-existing cataracts and medically uncontrolled glaucoma. Complete success, defined as an IOP reduction of more than 30% from the preoperative level without medications, was achieved in 50% of the eyes, with 40% having an IOP lower than 16 mmHg. The more success rate of our study may have three reasons: First of all, is the stage of glaucoma. All our patients had mild-to-moderate glaucoma without too high baseline IOPs. So, it is more likely to have a greater success rate after phacoviscocanalostomy in earlier stages of OAG. The second reason may be the shorter follow-up period in our study than the mentioned studies. As mentioned earlier, the effect of phacoviscocanalostomy may be decreased because of fibrosis. In the latter study, the patients have been followed for seven years. Third, the definitions of success rate are different in our study than the mentioned one. We defined IOP < 21 mmHg as the success rate.
In another study [29], the researchers found that phacoviscocanalostomy was more effective in eyes with PEXG than with primary OAG. In their study, at the last visit, the mean percentage of IOP reduction was 50% in the PEXG group and 30% in the POAG group. Our patients had PEXG or POAG, and we found that the number of antiglaucoma drops, which was lower in the phaco-visco group, was more pronounced and more stable in patients with PEXG. The more efficacy of phacoviscocanalostomy in patients with PEXG may be related to the removal of pseudoexfoliative material from the anterior lens during cataract extraction. Also, the clearance of these materials from trabecular meshwork will be accelerated with anti-inflammatory effect of haelan when injected to Schlemm canal during viscocanalostomy [29]. These factors can also provide better access of aqueous to the trabeculo-descemet membrane and consequently increase the aqueous outflow facility through both trabecular and non-trabecular pathways in PEXG. These multiple mechanisms elucidate why viscocanalostomy is more effective when combined with phacoemulsification [30, 31].
To the best of our knowledge, there is no report comparing the outcomes of phacoemulsification alone with phacoviscocanalostomy in mild-to-moderate OAG. Phacoviscocanalostomy has received great interest among ophthalmic surgeons and may be done in earlier stages of glaucoma. So, it seems to be necessary to compare this procedure with phacoemulsification in an earlier stage of OAG.
Our study had some limitations. The most important one was the short follow-up period. While our goal was comparing the mid-term outcome of the two procedures, further prospective randomized controlled studies are needed to compare these procedures as well as their long-term effects on IOP stability and glaucoma progression. Also, although the technique was similar for all phaco-visco group, we did not use any tissue spacer during the operation. Previous studies show that using different spacers may improve the outcome of phacoviscocanalostomy by preventing adhesions and collapse of the superficial flap [18, 31,32,33]. Therefore, future studies using any biocompatible spacer are suggested for comparing the outcomes of phacoviscocanalostomy with phacoemulsification alone in earlier stages of OAG especially PEXG. In conclusion, although both phacoemulsification alone and phacoviscocanalostomy procedures can be considered for patients with mild-to-moderate OAG, we demonstrated that greater success rates are achieved using phacoviscocanalostomy. So, if the surgeon is an expert in performing this technique, this non-penetrating procedure can be applied in patients with visually significant cataracts and earlier stages of OAG, especially in patients with PEXG.
Summary
What was known before
-
Both phaco alone and viscocanalostomy combined with phaco can lower the IOP in patients with open angle glaucoma.
What this study adds
-
The phacoviscocanalostomy procedure results in larger IOP reduction and the need for antiglaucoma medication than phaco alone in mild-to-moderate OAG.
-
Considering the higher complete and qualified success rates with phacoviscocanalostomy procedure, this technique is advised in patients with cataract and earlier stages of OAG.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Zhang ML, Hirunyachote P, Jampel H. Combined surgery versus cataract surgery alone for eyes with cataract and glaucoma. Cochrane Database Syst Rev. 2015;2015:CD008671.
Marchini G, Ceruti P, Vizzari G, Berzaghi D, Zampieri A. Management of concomitant cataract and glaucoma. Glaucoma Surg. 2017;59:155–64.
Rabin RL, Rabin AR, Zhang AD, Burney EN, Rhee DJ. Co-management of cataract and glaucoma in the era of minimally invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29:88–95.
Weiner AJ, Weiner Y, Weiner A. Intraocular pressure after cataract surgery combined with ab interno trabeculectomy versus trabecular micro-bypass stent: An intrasubject same-surgeon comparison. J Glaucoma. 2020;29:773–82.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79.
Murthy SK, Damji KF, Pan Y, Hodge WG. Trabeculectomy and phacotrabeculectomy, with mitomycin-C, show similar two-year target IOP outcomes. Can J Ophthalmol. 2006;41:51–9.
Cillino S, Di Pace F, Casuccio A, Calvaruso L, Morreale D, Vadalà M, et al. Deep sclerectomy versus punch trabeculectomy with or without phacoemulsification: a randomized clinical trial. J Glaucoma. 2004;13:500–6.
Vinod K, Gedde SJ, Feuer WJ, Panarelli JF, Chang TC, Chen PP, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society. J Glaucoma. 2017;26:687.
Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refractive Surg. 1999;25:316–22.
Ambresin A, Shaarawy T, Mermoud A. Deep sclerectomy with collagen implant in one eye compared with trabeculectomy in the other eye of the same patient. J Glaucoma. 2002;11:214–20.
Anand N. Deep sclerectomy with mitomycin C for glaucoma secondary to uveitis. Eur J Ophthalmol. 2011;21:708–14.
Carassa RG, Bettin P, Fiori M, Brancato R. Viscocanalostomy versus trabeculectomy in white adults affected by open-angle glaucoma: a 2-year randomized, controlled trial. Ophthalmology 2003;110:882–7.
Kobayashi H, Kobayashi K. Randomized comparison of the intraocular pressure–lowering effect of phacoviscocanalostomy and phacotrabeculectomy. Ophthalmology 2007;114:909–14.
Park M, Hayashi K, Takahashi H, Tanito M, Chihara E. Phaco-viscocanalostomy versus phaco-trabeculotomy: a middle-term study. J Glaucoma. 2006;15:456–61.
Tanito M, Park M, Nishikawa M, Ohira A, Chihara E. Comparison of surgical outcomes of combined viscocanalostomy and cataract surgery with combined trabeculotomy and cataract surgery. Am J Ophthalmol. 2002;134:513–20.
Shoji T, Tanito M, Takahashi H, Park M, Hayashi K, Sakurai Y, et al. Phacoviscocanalostomy versus cataract surgery only in patients with coexisting normal-tension glaucoma: midterm outcomes. J Cataract Refractive Surg. 2007;33:1209–16.
Narayanaswamy A, Perera SA, Htoon HM, Hoh ST, Seah SK, Wong TT, et al. Efficacy and safety of collagen matrix implants in phacotrabeculectomy and comparison with mitomycin C augmented phacotrabeculectomy at 1 year. Clin Exp Ophthalmol. 2013;41:552–60.
Johnson MS, Sarkisian SR Jr. Using a collagen matrix implant (Ologen) versus mitomycin-C as a wound healing modulator in trabeculectomy with the Ex-PRESS mini glaucoma device: a 12-month retrospective review. J Glaucoma. 2014;23:649–52.
Hondur A, Onol M, Hasanreisoglu B. Nonpenetrating glaucoma surgery: meta-analysis of recent results. J Glaucoma. 2008;17:139–46.
Solano MM, Lin SC. Cataract, phacoemulsification and intraocular pressure: is the anterior segment anatomy the missing piece of the puzzle? Prog Retinal Eye Res. 2018;64:77–83.
Slabaugh MA, Chen PP. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol. 2014;25:122–6.
Huang G, Gonzalez E, Lee R, Chen Y-C, He M, Lin SC. Association of biometric factors with anterior chamber angle widening and intraocular pressure reduction after uneventful phacoemulsification for cataract. J Cataract Refractive Surg. 2012;38:108–16.
Poley BJ, Lindstrom RL, Samuelson TW, Schulze R Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refractive Surg. 2009;35:1946–55.
Siegel MJ, Boling WS, Faridi OS, Gupta CK, Kim C, Boling RC, et al. Combined endoscopic cyclophotocoagulation and phacoemulsification versus phacoemulsification alone in the treatment of mild to moderate glaucoma. Clin Exp Ophthalmol. 2015;43:531–9.
Smit BA, Johnstone MA. Effects of viscoelastic injection into Schlemm’s canal in primate and human eyes: potential relevance to viscocanalostomy. Ophthalmology. 2002;109:786–92.
Tamm ER, Carassa RG, Albert DM, B’Ann TG, Patel S, Rasmussen CA, et al. Viscocanalostomy in rhesus monkeys. Arch Ophthalmol. 2004;122:1826–38.
Shaarawy T, Mansouri K, Schnyder C, Ravinet E, Achache F, Mermoud A. Long-term results of deep sclerectomy with collagen implant. J Cataract Refractive Surg. 2004;30:1225–31.
Wishart MS, Dagres E. Seven-year follow-up of combined cataract extraction and viscocanalostomy. J Cataract Refractive Surg. 2006;32:2043–9.
Awadalla MA, Hassan KM. Phacoviscocanalostomy in pseudoexfoliation glaucoma versus primary open-angle glaucoma. Can J Ophthalmol. 2011;46:77–82.
Xin C, Wang H, Wang N. Minimally invasive glaucoma surgery: what do we know? Where should we go? Transl Vis Sci Technol. 2020;9:15.
Merkur A, Damji KF, Mintsioulis G, Hodge WG. Intraocular pressure decrease after phacoemulsification in patients with pseudoexfoliation syndrome. J Cataract Refractive Surg. 2001;27:528–32.
Ateş H, Üretmen Ö, Andaç K, Azarsiz SS. Deep sclerectomy with a nonabsorbable implant (T-Flux): preliminary results. Can J Ophthalmol. 2003;38:482–8.
Devloo S, Deghislage C, Van Malderen L, Goethals M, Zeyen T. Non-penetrating deep sclerectomy without or with autologous scleral implant in open-angle glaucoma: medium-term results. Graefe’s Arch Clin Exp Ophthalmol. 2005;243:1206–12.
Acknowledgements
The authors would like to thank committee members for their efforts and contributions to this work.
Author information
Authors and Affiliations
Contributions
RZ was responsible for the scientific writing/data gathering/data analysis. AA was responsible for the scientific writing/data gathering/ data analysis. GF was responsible for the data gathering/scientific writing. YE and MN were responsible for the data gathering. KN-M and JC were responsible for the scientific writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zarei, R., Azimi, A., Fakhraei, G. et al. Combined phacoviscocanalostomy versus phacoemulsification alone in patients with coexisting cataract and mild-to-moderate open-angle glaucoma; a randomized-controlled trial. Eye 37, 1390–1396 (2023). https://doi.org/10.1038/s41433-022-02152-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02152-w