Abstract
Objectives
To compare the long-term efficacy and safety of combined phacoemulsification, anterior vitrectomy, and sclerectomy (triple procedure surgery, TS); combined phacoemulsification and anterior vitrectomy (double procedure surgery, DS); and filtering surgery (FS) in nanophthalmos with angle-closure glaucoma (NACG).
Methods
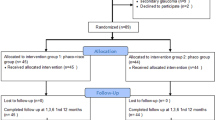
Retrospective cohort study. Forty patients (44 eyes) diagnosed with NACG who underwent TS, DS, and FS were included. All eyes in the TS group and seven (47%) eyes in the DS group also underwent goniosynechialysis during the surgery. The main outcome measures (intraocular pressure [IOP], best-corrected visual acuity, complications, and second surgeries) were recorded at the early- (within 1 week) and late-stage (>3 months) follow-up.
Results
The late-stage IOP was significantly lower in the TS (mean ± standard deviation: 13.29 ± 2.49 mm Hg) than in the DS (19.69 ± 6.97 mm Hg) and FS groups (27.57 ± 12.26 mm Hg, p < 0.001). More visual improvements were observed in the TS and DS groups than in the FS group at late-stage follow-up (p = 0.04). The complication rates in the TS, DS, and FS groups were 26%, 33%, and 70%, respectively (p = 0.046); the second surgery rates were 0%, 33%, and 60%, respectively (p < 0.001). In total, one, three, and six severe complications were observed in the TS, DS, and FS groups, respectively. The mean follow-up durations in the TS, DS, and FS groups were 18.89, 20.02, and 25.75 months, respectively.
Conclusions
NACG management remains challenging. TS presented relatively good clinical efficacy and safety with better postoperative IOP outcomes, lower complications, and second surgery rates among the three groups in eyes with NACG.
Similar content being viewed by others
Introduction
Nanophthalmos, a subtype of microphthalmia, is a rare disease characterized by short axial length (AL), thickened sclera, and shallow anterior chamber (AC) [1], with preserved ocular structure and no associated congenital malformations [2]. The anterior segment in nanophthalmos is crowded owing to the high lens-to-eye volume ratio, which results in eyes being highly susceptible to angle-closure glaucoma (ACG) [3]. ACG treatment becomes intractable as the disease progresses. Antiglaucoma agents have shown a poor response in intraocular pressure (IOP) control, and surgical treatment can be challenging owing to the high risk of postoperative complications [3, 4]. During the early stages of ACG, laser peripheral iridotomy (LPI) may be effective in alleviating pupillary block; however, intraocular surgery is usually necessary when patients had extensive peripheral anterior synechiae [5]. Filtering surgery is a traditional approach for glaucoma treatment; however, it has shown unsatisfactory results in nanophthalmic eyes owing to the high incidence of uveal effusion, retinal detachment, malignant glaucoma, and poor visual outcomes [4, 6, 7].
Recently, cataract surgery has become a popular procedure during the surgical treatment of nanophthalmos, especially with the advances in phacoemulsification and intraocular lens (IOL) implantation [8]. However, previous studies have also reported an increasing rate of intra- and postoperative complications associated with cataract surgery in nanophthalmos, including suprachoroidal hemorrhage, corneal endothelial decompensation, severe iritis, and cystoid macular edema [3, 9, 10]. Several measures have been proposed to prevent complications in cataract surgery performed on nanophthalmic eyes, such as prophylactic peripheral iridotomy, anterior vitrectomy, and scleral resection during surgery; however, the efficacy of these procedures remains inconclusive [3, 11]. Although some case reports and small case series have reported varying outcomes of cataract surgery in nanophthalmos, to the best of our knowledge, no studies to date have rigorously investigated the surgical results in a group of nanophthalmic eyes with ACG. When complicated with secondary ACG, the risk of operative complications, especially malignant glaucoma, has been proven to be much higher in nanophthalmic eyes [6, 12]. Therefore, it is imperative to explore relatively more beneficial surgical procedures for eyes with nanophthalmic ACG (NACG).
Here, we observed that phacoemulsification combined with anterior vitrectomy and sclerectomy (triple procedure surgery) results in a good prognosis in some NACG cases. Moreover, the advantages of this surgery in nanophthalmos management have not been previously reported. Therefore, we retrospectively enrolled patients with NACG who had undergone combined cataract surgery and filtering surgery, with the aim to evaluate the postoperative efficacy and safety of the triple procedure surgery, and to investigate the treatment outcomes of different types of surgery in such intractable cases.
Subjects and methods
Participants
We retrospectively enrolled 40 patients (44 eyes) diagnosed with NACG who had undergone combined cataract surgery and filtering surgery at the Eye and ENT Hospital of Fudan University between March 2013 and May 2020. The participants were consecutive patients with diagnosis of nanophthalmos and ACG, those with uncontrolled high IOP, and those who required and were suitable for surgical treatments.
The inclusion criteria were as follows: (1) diagnosis of nanophthalmos and ACG; (2) treatment with phacoemulsification combined with anterior vitrectomy and sclerectomy, phacoemulsification combined with anterior vitrectomy, or filtering surgery. Eyes with associated congenital abnormalities were excluded from the study.
Patients were divided into three groups based on the performed surgical procedures. Among the included eyes, 19 were treated with phacoemulsification combined with anterior vitrectomy and sclerectomy (triple procedure surgery [TS]); 15 underwent phacoemulsification combined with anterior vitrectomy (double procedure surgery [DS]); and 10 underwent filtering surgery (FS), of whom six underwent trabeculectomy and the other four underwent aqueous drainage device implantation. IOL implantation was performed based on the capsular integrity and the risk of poor visual outcome. In total, 6 and 10 eyes in the TS and DS groups, respectively, received IOL implantations. Goniosynechialysis (GSL) was performed in all eyes in the TS group and in seven eyes in the DS group.
In this study, nanophthalmos was defined as an AL < 20.5 mm without congenital morphologic abnormality [3, 8, 13, 14]. ACG was defined as an IOP > 21 mm Hg, a shallow AC, narrow or closed AC angle, and glaucomatous optic nerve damage [4, 5].
Preoperative examinations
The following evaluations were performed in all enrolled patients: best-corrected visual acuity (BCVA) measurement, slit-lamp microscopy, fundus examination, IOP measurement using Goldmann applanation tonometry, ultrasound biomicroscopy (UBM), optical coherence tomography (OCT), A-scan ultrasound, Lenstar examination, B-scan ultrasound, and visual field. UBM was used to assess the AC depth (ACD) and AC angle. The Hoffer-Q formula was applied to evaluate the required IOL power in the TS and DS groups [13]. The following conversion was applied for cases wherein the visual acuity was worse than “counting fingers”: [15,16,17] “counting fingers,” 2.0 logMAR; “hand motion,” 2.3 logMAR; “light perception,” 2.6 logMAR; and “no light perception,” 2.9 logMAR.
Surgical procedures
The detailed surgical procedures of the combined cataract surgery are presented in Fig. 1. For triple procedure surgery, sclerectomy was performed at 7–8 mm posterior to the corneoscleral limbus, with a resection size of 3 × 3 mm2 and thickness of 90% sclera, in the inferotemporal and inferonasal quadrants of the eyeball. A 2-mm incision was made on the deep sclera, and the edges of the incision were cauterized to maintain patency. Vitreous puncture, prepared for anterior vitrectomy, was performed at 2 mm posterior to the corneoscleral limbus. Further, phacoemulsification and IOL implantation (when applicable) were performed, followed by GSL with viscoelastic agent and 360° gonioscopic examination to ensure open chamber angle. Anterior vitrectomy, including resection of the anterior hyaloid face and part of zonule, as well as capsulotomy and iridectomy, was performed to render communication between the anterior and posterior chambers. All intra-operative complications were recorded. The puncture to the vitreous chamber was performed at 2 mm posterior to the corneoscleral limbus in order to avoid operative retinal damage in anterior vitrectomy.
A Separate conjunctival flap. B–D Sclerectomy was performed at 7–8 mm posterior to the corneoscleral limbus, with a resection size of 3 × 3 mm2 and thickness of 90% sclera. A 2-mm incision was made on the deep sclera, and the edges of the incision were cauterized to maintain patency. Sclerectomy was performed in the inferotemporal and inferonasal quadrants of the eyeball. E Vitreous puncture was performed at 2 mm posterior to the corneoscleral limbus, prepared for anterior vitrectomy. F–H Phacoemulsification and intraocular lens implantation (when applicable). I Goniosynechialysis with viscoelastic agent and J 360° gonioscopy examination were performed to ensure that the chamber angle was open. K Anterior vitrectomy, including resection of the anterior hyaloid face and part of zonule as well as capsulotomy and iridectomy, was performed to render communication between the anterior and posterior chambers. L Closure of all incisions.
Postoperative follow-up
Ocular examinations (IOP, BCVA, refraction, slit-lamp examination, Lenstar examination, and B ultrasound scan) were performed regularly at 1 day, 1 week, 1 month, and 3 months post operatively. Ultrasound scans were conducted to assess conditions of the vitreous body, choroid, and retina. From the third month post operation onwards, patients were followed up routinely every 3 to 6 months. Early-stage follow-up measurements were recorded within 1 week post operatively, and the patient’s examination results at the last visit were regarded as the late-stage follow-up.
All intra-operative and postoperative complications and the subsequent surgical treatments were recorded during the follow-up period. Complications developing within and after postoperative 3 months were defined as early-stage and late-stage complications, respectively. According to previous studies and observations in this study [13, 18], we regarded suprachoroidal hemorrhage, posterior capsule, corneal endothelial decompensation, retinal detachment, and malignant glaucoma as severe complications. Surgical success was defined as postoperative IOP 20% below the baseline and <21 mm Hg (>6 mm Hg) at the last visit, without severe complications or the need for further surgical treatments during the follow-up period.
Statistical analysis
Data are expressed as mean ± standard deviation (normally distributed variables) or median with range (non-normally distributed variables). One-way ANOVA or Kruskal–Wallis test was used to assess the differences between the three groups with regard to continuous variables for normally distributed and non-normally distributed data, respectively. Fisher’s exact test was used to compare the proportions. Paired t-tests and Wilcoxon signed rank sum test were used to assess the differences between the preoperative and postoperative values of IOP (normally distributed) and BCVA (non-normally distributed), respectively. We used STATA ver. 15.1 (College Station, TX, USA) to perform statistical analysis, and two-tailed between-group differences were considered significant at p < 0.05.
Results
Baseline characteristics
Table 1 presents the preoperative baseline characteristics of patients with NACG in the three groups. No significant differences were observed among the three groups regarding age, sex, right eye, cup/disc ratio, BCVA, IOP, angle closure, visual field, and follow-up period. The number of eyes with a history of previous surgery was comparable among the three groups (p = 0.68). The AL was shorter in eyes of the TS group than in those in the FS group (p = 0.04), in consistency with the results of the refractive error (p = 0.03). Additionally, the AC of eyes in the TS and DS groups was shallower than those in the FS group (p = 0.01). The mean and median follow-up durations in the TS, DS, and FS groups were 18.89 and 15, 20.02 and 15, and 25.75 and 19 months, respectively.
IOP and visual outcomes
The IOP and visual acuity outcomes are presented in Table 2. The mean preoperative IOP in the TS, DS, and FS groups was 37.29 ± 10.75, 29.41 ± 11.49, and 37.59 ± 12.48 mm Hg, respectively. The postoperative IOP in all three groups was significantly reduced, both in the early-stage (TS, DS, FS: p < 0 .001, p = 0.03, p = 0.002, respectively) and late-stage (TS, DS, FS: p < 0.001, p = 0.008, p = 0.04, respectively) follow-up periods. Early-stage IOP was comparable between the TS (18.34 ± 9.04 mm Hg), DS (21.86 ± 9.65 mm Hg), and FS (20.90 ± 9.06 mm Hg, p = 0.53) groups. However, at the late-stage follow-up examination, IOP in the TS group (13.29 ± 2.49 mm Hg) was significantly lower than those in the DS (19.69 ± 6.97 mm Hg) and FS groups (27.57 ± 12.26 mm Hg, p < 0.001). Moreover, the difference between the late-stage and initial IOP was significantly larger in the TS group (24.00 ± 11.40, 9.72 ± 13.82, and 10.02 ± 16.33 mm Hg in the TS, DS, and FS groups, respectively; p = 0.005). Further, there was no significant difference between the preoperative and postoperative BCVA among the three groups. However, more visual improvements were observed in the TS and DS groups than in the FS group, and the difference was statistically significant at the late-stage follow-up examination (p = 0.04). The BCVA of the eyes that underwent filtering surgery was worse at the late-stage follow-up examination than at the preoperative stage (p = 0.02), with visual improvement observed in only one (10%) eye.
Complications and reoperation
Table 3 presents the intra-operative and postoperative complications at different stages. Corneal edema was the most common complication at the early-stage follow-up examination. Except for one (10%) eye in the FS group that developed chronic endothelial decompensation, all other eyes with corneal edema recovered within 1–2 weeks. The postoperative AC inflammation reaction, hyphema, and fibrin membrane in the pupil were all absorbed within 2 weeks post operatively.
The total complications and subsequent surgeries are presented in Table 4. More eyes with shallow AC were observed in the FS group (p < 0.001). The proportion of uveal effusion in the FS and DS groups was almost four times higher than that in the TS group, and retinal detachment was only observed in the FS and DS groups. No malignant glaucoma was observed in the TS and DS groups. Mild corneal edema is relatively common in cataract and glaucoma surgery, and it might have been presented in the eyes with elevated IOP pre-operatively. Therefore, corneal edema was excluded from the total number of complications (Table 4). Overall, the proportion of eyes with operative complications was the smallest in the TS group (p = 0.046). The TS group also had the least number of severe complications; however, the difference was not statistically significant. Furthermore, the number of eyes that underwent subsequent surgery for complications were also the least in the TS group (p < 0.001). The surgical success rates of the TS, DS, and FS groups were 89% (17/19), 60% (9/15), and 20% (2/10), respectively (Table 4).
Postoperative elevated IOP
Postoperative IOP was elevated in four (21%), six (40%), and six (60%) eyes in the TS, DS, and FS groups at the early-stage follow-up examination, respectively; in addition, it was elevated in one (7%) eye in the DS group and in one (10%) eye in the FS group at the late-stage follow-up examination (Table 3). In total, four (21%), seven (47%), and seven (70%) eyes in the TS, DS, and FS groups, respectively, had postoperative elevated IOP (Table 3). The subsequent surgical treatment for elevated IOP is also presented in Table 4. More cases of postoperative elevated IOP were observed in the FS group compared with in the TS group (p = 0.03), in line with the IOP results shown in Table 2. Moreover, the elevated IOP in the TS group reduced to the normal range through treatment with IOP-lowering medication and remained stable during the follow-up period, except for one eye complicated with intra-operative suprachoroidal hemorrhage. In the DS group, the elevated IOP in three eyes at the early-stage follow-up examination became normal after treatment with IOP-lowering agents, and the other two eyes had to undergo cyclocryotherapy and EX-PRESS implantation, respectively (Table 4). The IOP in one eye in the DS group was elevated at the late-stage follow-up and was treated with LPI. Seven eyes had elevated IOP in the FS group, four of which were treated with trabeculectomy, and the other three were treated with aqueous drainage device implantation. Among the four eyes, one underwent filtration bleb needling, a repeated surgery through the initial surgical site of the trabeculectomy to remove the blockage of the initial filter passage caused by scar tissue; one underwent filtration bleb needling followed by combined phacoemulsification, IOL implantation, and anterior vitrectomy; and one underwent anterior chamber reformation with viscoelastics for shallow AC (Table 4). One eye did not undergo surgical treatment; however, IOP of this eye was unstable following treatment with IOP-lowering medication. Among the remaining three eyes that had aqueous drainage device implantation in the FS group, one eye had intra-operative suprachoroidal hemorrhage. The other two eyes developed malignant glaucoma, one of which recovered after treatment with medication. The other eye, which was also complicated with uveal effusion and retinal detachment, underwent sclerectomy combined with PPV, photocoagulation, and AC reformation. This was followed by drainage tube recanalization and AC reformation, subsequently followed by enucleation due to the uncontrolled IOP, severe pain, and loss of vision (Table 4).
Triple procedure surgery
From the aforementioned results, we observed that phacoemulsification combined with anterior vitrectomy, sclerectomy, and GSL had relatively better efficacy and safety. The detailed surgical procedures are presented in Fig. 1. Except corneal edema, other complications were observed in a total of five (26%) eyes in the TS group. Three eyes showed early postoperative inflammation, including severe AC reaction in one eye and fibrin membrane in two eyes, all of which resolved within 1 week of increasing the frequency of topical steroid usage. Uveal effusion was observed in one eye at 10 days postoperatively, which regressed within 3 weeks with tobramycin-dexamethasone eyedrop four times per day (Supplementary Fig. 1). The preoperative BCVA of this eye was 1 logMAR (Snellen 20/200), which decreased to “counting fingers” upon development of uveal effusion, and recovered to 0.40 logMAR (Snellen 20/50) at the late-stage follow-up examination. Intra-operative suprachoroidal hemorrhage was observed in one eye (Supplementary Fig. 2). This patient was treated with LPI 9 years ago. Preoperative IOP of this eye was >45 mm Hg for approximately half a year with the administration of multiple IOP-lowering agents, and the preoperative visual acuity was “light perception.” Suprachoroidal hemorrhage occurred toward the completion of the anterior vitrectomy procedure. The postoperative BCVA of this eye was “no light perception,” which unfortunately, was not improved during the follow-up period.
Discussion
The incidence of ACG in nanophthalmos is approximately 35.7−77% [9, 19]. In this study, we enrolled a large group of patients with ACG secondary to nanophthalmos and investigated treatment outcomes of combined cataract surgery and filtering glaucoma surgery. More importantly, we observed a higher efficacy and safety of a triple procedure surgery (phacoemulsification and GSL combined with anterior vitrectomy and sclerectomy) for treating eyes with NACG.
Although the preoperative AL and ACD of the eyes in the TS group were the smallest among the three groups, the postoperative IOP was lower and less complications were observed in this group. The baseline characteristics could better demonstrate the efficacy and safety of the triple procedure surgery, since a previous study has proven short AL to be an independent risk factor for postoperative complications in nanophthalmos [6].
Postoperative IOP in all three groups was significantly reduced. However, at the late-stage follow-up examination, the average IOP in the FS group was elevated beyond the normal range (>21 mm Hg), which indicated poor long-term IOP control following filtering surgery in the eyes with NACG. Late-stage IOP was the lowest in the TS group (13.29 ± 2.49 mm Hg), followed by the DS group (19.69 ± 6.97 mm Hg); it was the highest in the FS group (27.57 ± 12.26 mm Hg). A previous study reported that cataract surgery is effective in decreasing the IOP in nanophthalmos, which may have been associated with the deeper AC and wider anterior angle observed postoperatively [20]. The difference between the TS and DS groups regarding IOP outcomes might have been associated with the GSL. All eyes in the TS group and less than half in the DS group underwent GSL during the surgery. During the follow-up period, four (21%) eyes in the TS group, seven (47%) eyes in the DS group (five without GSL), and seven (70%) eyes in the FS group had elevated IOP postoperatively. These results suggested that cataract surgery combined with GSL had a relatively satisfactory effect in the eyes with NACG considering long-term IOP control.
Previous studies have reported extremely limited visual improvement in eyes with nanophthalmos following cataract surgery, of which only 40–66% showed improved BCVA [3, 8]. Zheng et al. observed significant improvement of BCVA in nanophthalmos following cataract surgery; however, few eyes had preoperative glaucoma, and baseline IOP of the group in that study was within the normal range [13]. Another study reported a combined PPV and lensectomy in NACG management, and only 14% of eyes had a postoperative improvement in BCVA [5]. In our study, we observed that 58% and 67% of eyes in the TS and DS groups, respectively, had BCVA improvement. Moreover, 70% of eyes in the FS group had a decreased BCVA at the late-stage follow-up examination, and the postoperative BCVA was significantly worse than at the baseline (p = 0.02). These results revealed that combined cataract surgery was significantly better than filtering surgery considering visual acuity improvement in the eyes with NACG. The difficulty in obtaining visual improvement in the eyes with NACG could have been related to preoperative amblyopia, damage to the optic nerve caused by glaucoma, and under correction of postoperative refractive error [10].
A randomized controlled trial investigated the cataract surgery outcomes with or without sclerostomy in eyes with nanophthalmos, demonstrating that prophylactic sclerostomy might reduce the incidence of uveal effusion [10]. Wu et al. also suggested the performance of lamellar sclerectomy in eyes with nanophthalmos in anticipation of uveal effusion [12]. Consistent with the aforementioned findings, the uveal effusion rate was lower in the eyes that underwent sclerectomy (TS group); however, there was no statistical significance, which could have been attributed to the small sample size. The effect of the scleral window surgery on preventing uveal effusion may result from the prepared drainage pathway for the fluid accumulating in the suprachoroidal space intra- and postoperatively.
Postoperative malignant glaucoma is also a severe complication in NACG cases. Anterior vitrectomy is a common surgical treatment for malignant glaucoma [21]. A previous study reported development of postoperative malignant glaucoma in 18% of eyes with NACG after filtering surgery [4], which was similar to our findings (20%). Moreover, no postoperative malignant glaucoma was observed in the eyes that underwent anterior vitrectomy during the cataract surgery. In this study, all cases of malignant glaucoma occurred within 2 weeks post operatively, and one of them eventually underwent eye enucleation. Therefore, we considered that performing prophylactic anterior vitrectomy during surgery may be more beneficial to eyes with NACG than performing it after postoperative malignant glaucoma has developed. There are two critical points that were regarded as indispensable while performing anterior vitrectomy in the eyes with NACG: (1) the puncture to the vitreous chamber should be performed at 2 mm rather than at 3.5−4 mm posterior to the corneoscleral limbus to avoid operative retinal damage and (2) the anterior vitreous membrane, posterior capsule, part of the zonules, and part of the peripheral iris should also be resected to render communication between the AC and vitreous cavity.
Rajendrababu et al. previously reported the cases of 11 eyes with NACG, all of which had complications after filtering surgery, including suprachoroidal hemorrhage, malignant glaucoma, and uveal effusion [4]. Yalvac et al. examined 20 eyes with NACG that underwent combined trabeculectomy and inferior sclerotomy [22]. Despite performing prophylactic sclerotomy, postoperative uveal effusion was still observed in 75% of eyes in their study [22]. Consistent with these findings, our study also indicated a high incidence of postoperative complications in the eyes with NACG undergoing filtering surgery. Therefore, we suggest that filtering surgery may be unsuitable for eyes with NACG.
Some researchers have shown that with the advent of phacoemulsification, a lower complication rate has been observed in eyes with nanophthalmos following cataract surgery [3, 13, 23]. However, the majority of nanophthalmic eyes included in those studies did not have preoperative ACG. Moreover, high preoperative IOP (>22 mm Hg) is an independent risk factor in nanophthalmos following cataract surgery [6]. In our study, we still observed a relatively high complication rate in the eyes with NACG.
Among all three groups, the least number of complications and severe complications were observed in the TS group. Three (16%) eyes with early postoperative severe AC reaction and fibrin membrane resolved within 1 week, and uveal effusion in one eye also recovered within 3 weeks without any surgical treatment. However, intra-operative suprachoroidal hemorrhage, which was a severe complication, developed in one patient (5%) in the TS group. This eye had poor visual acuity and a high IOP of 45 mm Hg for nearly half a year. The occurrence of suprachoroidal hemorrhage may be related to the persistently high preoperative IOP and poor visual acuity. Therefore, we deemed that for such patients, there is still a high risk of suprachoroidal hemorrhage even with triple procedure surgery. Moreover, IOP should be reduced as much as possible pre-operatively.
Our study has certain limitations. First, owing to the rare occurrence of nanophthalmos, the sample size in this study was too small to detect a significant difference in some operative complications between the three types of surgery. This may hide greater benefits or problems in triple procedure surgery. Second, the diagnosis criteria of nanophthalmos vary widely across the literature, and recent studies tended to include thickened sclera and choroid when defined nanophthalmos. Although some previous studies have only included AL in the definition of nanophthalmos similar to our study [3, 8, 14], considering one parameter may be a little simplistic. Further, our study was retrospective; a larger-scale, multicentre, prospective study with a longer follow-up period is warranted to confirm the efficacy and safety of triple procedure surgery and other surgical procedures in eyes with NACG.
In conclusion, we observed that triple procedure surgery had better efficacy and safety than double procedure and filtering surgeries. Additionally, the visual improvement was relatively limited, and the intra- and postoperative complication rate was high when nanophthalmos was complicated with ACG. As a traditional surgical procedure for the treatment of glaucoma, filtering surgery may not be an appropriate method in nanophthalmos, which are fraught with poor IOP and visual outcome, and presents a high rate of complications and subsequent surgical interventions. The triple procedure surgery had preferable efficacy and safety; however, further rigorous studies are required to verify this finding.
Supplemental information is available at Eye’s website
Summary
What was known before
-
The treatment of nanophthalmos is a challenge. Nanophthalmos is highly susceptible to angle-closure glaucoma due to the crowded anterior segment.
-
Cataract surgery is commonly performed in nanophthalmos. Filtering surgery is usually applied in eyes complicated with glaucoma.
-
Intra- and postoperative complication incidence is high in nanophthalmic glaucoma.
What this study adds
-
Combined phacoemulsification, goniosynechialysis, anterior vitrectomy, and sclerectomy presents good clinical efficacy and safety in nanophthalmic glaucoma.
-
Prophylactic anterior vitrectomy in combined surgery reduces the risk of postoperative malignant glaucoma. Prophylactic sclerectomy minimizes the incidence of uveal effusion.
-
Filtering surgery has a poorer visual and long-term IOP outcomes, higher postoperative complications and second surgery rate than combined cataract surgery.
Data availability
The data that support the findings of this study are available from the corresponding author, XK, upon reasonable request.
References
Singh OS, Simmons RJ, Brockhurst RJ, Trempe CL. Nanophthalmos: a perspective on identification and therapy. Ophthalmology. 1982;89:1006–12.
Carricondo PC, Andrade T, Prasov L, Ayres BM, Moroi SE. Nanophthalmos: a review of the clinical spectrum and genetics. J Ophthalmol. 2018;2018:2735465 https://doi.org/10.1155/2018/2735465.
Steijns D, Bijlsma WR, Van, der Lelij A. Cataract surgery in patients with nanophthalmos. Ophthalmology. 2013;120:266–70. https://doi.org/10.1016/j.ophtha.2012.07.082.
Rajendrababu S, Shroff S, Uduman MS, Babu N. Clinical spectrum and treatment outcomes of patients with nanophthalmos. Eye. 2020. https://doi.org/10.1038/s41433-020-0971-4.
Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018; 361. https://doi.org/10.1126/science.aan8821.
Day AC, MacLaren RE, Bunce C, Stevens JD, Foster PJ. Outcomes of phacoemulsification and intraocular lens implantation in microphthalmos and nanophthalmos. J Cataract Refract Surg. 2013;39:87–96. https://doi.org/10.1016/j.jcrs.2012.08.057.
Schmoll C, Devlin H, Foster P. Uveal effusion syndrome as a complication of cyclodiode therapy in nanophthalmos glaucoma. Eye. 2011;25:963–4. https://doi.org/10.1038/eye.2011.67.
Jung KI, Yang JW, Lee YC, Kim SY. Cataract surgery in eyes with nanophthalmos and relative anterior microphthalmos. Am J Ophthalmol. 2012;153:1161–8 e1. https://doi.org/10.1016/j.ajo.2011.12.006.
Wladis EJ, Gewirtz MB, Guo S. Cataract surgery in the small adult eye. Surv Ophthalmol. 2006;51:153–61. https://doi.org/10.1016/j.survophthal.2005.12.005.
Rajendrababu S, Babu N, Sinha S, Balakrishnan V, Vardhan A, Puthuran GV, et al. A randomized controlled trial comparing outcomes of cataract surgery in nanophthalmos with and without prophylactic sclerostomy. Am J Ophthalmol. 2017;183:125–33. https://doi.org/10.1016/j.ajo.2017.09.008.
Brockhurst RJ. Cataract surgery in nanophthalmic eyes. Arch Ophthalmol. 1990;108:965–7. https://doi.org/10.1001/archopht.1990.01070090067041.
Wu W, Dawson DG, Sugar A, Elner SG, Meyer KA, McKey JB, et al. Cataract surgery in patients with nanophthalmos: results and complications. J Cataract Refract Surg. 2004;30:584–90. https://doi.org/10.1016/j.jcrs.2003.07.009.
Zheng T, Chen Z, Xu J, Tang Y, Fan Q, Lu Y. Outcomes and prognostic factors of cataract surgery in adult extreme microphthalmos with axial length <18 mm or corneal diameter <8 mm. Am J Ophthalmol. 2017;184:84–96. https://doi.org/10.1016/j.ajo.2017.09.028.
Lemos JA, Rodrigues P, Resende RA, Menezes C, Goncalves RS, Coelho P. Cataract surgery in patients with nanophthalmos: results and complications. Eur J Ophthalmol. 2016;26:103–6. https://doi.org/10.5301/ejo.5000656.
Tomkins-Netzer O, Talat L, Bar A, Lula A, Taylor SR, Joshi L, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 2014;121:2387–92. https://doi.org/10.1016/j.ophtha.2014.07.007.
Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247:137–42. https://doi.org/10.1007/s00417-008-0926-0.
Kwon YH, Kim CS, Zimmerman MB, Alward WL, Hayreh SS. Rate of visual field loss and long-term visual outcome in primary open-angle glaucoma. Am J Ophthalmol. 2001;132:47–56. https://doi.org/10.1016/s0002-9394(01)00912-6.
Carifi G, Safa F, Aiello F, Baumann C, Maurino V. Cataract surgery in small adult eyes. Br J Ophthalmol. 2014;98:1261–5. https://doi.org/10.1136/bjophthalmol-2013-304579.
Ritch R, Chang BM, Liebmann JM. Angle closure in younger patients. Ophthalmology. 2003;110:1880–9. https://doi.org/10.1016/s0161-6420(03)00563-3.
Seki M, Fukuchi T, Ueda J, Suda K, Nakatsue T, Tanaka Y, et al. Nanophthalmos: quantitative analysis of anterior chamber angle configuration before and after cataract surgery. Br J Ophthalmol. 2012;96:1108–16. https://doi.org/10.1136/bjophthalmol-2012-301496.
Wang J, Du E, Tang J. The treatment of malignant glaucoma in nanophthalmos: a case report. BMC Ophthalmol. 2018;18:54 https://doi.org/10.1186/s12886-018-0714-5.
Yalvac IS, Satana B, Ozkan G, Eksioglu U, Duman S. Management of glaucoma in patients with nanophthalmos. Eye. 2008;22:838–43. https://doi.org/10.1038/sj.eye.6702742.
Faucher A, Hasanee K, Rootman DS. Phacoemulsification and intraocular lens implantation in nanophthalmic eyes: report of a medium-size series. J Cataract Refract Surg. 2002;28:837–42. https://doi.org/10.1016/s0886-3350(01)01161-0.
Acknowledgements
We would like to thank Shizhen Li from Fudan University, Shanghai, for her kindly assistance in the statistical analysis; and Ting Zhai from Harvard University, Boston, for her kindly help in English language editing.
Author contributions
XF designed the study; collected data; performed data analysis and interpretation; and wrote the manuscript. JW, QS, RZ contributed to data interpretation and discussion. XK designed the study; contributed to data collection, management, and discussion; and reviewed the manuscript. All authors have approved the manuscript.
Funding
The authors were supported by the Surface Project of National Natural Science Foundation of China under Grant 81770922 and 82070957; the Western Medicine Guidance Project of Shanghai Committee of Science and Technology under Grant 19411961600; the Experimental Animal Research Project of Shanghai Committee of Science and Technology under Grant 201409006600; the Double Excellent Project of EENT Hospital under Grant SYB202003. The funders had no role in study design, data collection, analysis and interpretation, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This retrospective cohort study was approved by the Institutional Review Board of the Eye and ENT Hospital of Fudan University, Shanghai, China (2020129-1), in accordance with the Tenets of the Declaration of Helsinki. Informed consent was obtained from all patients included in this study, after explanation of the nature and purpose of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fan, X., Wang, J., Sheng, Q. et al. Outcomes of combined phacoemulsification, anterior vitrectomy, and sclerectomy in nanophthalmic eyes with glaucoma. Eye 37, 751–759 (2023). https://doi.org/10.1038/s41433-022-02039-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02039-w