Abstract
Purpose
Immunogenic causes of inflammation may be difficult to differentiate in the work-up of orbital inflammatory disease. The study aims to investigate the utility of autoimmune markers in the screening for orbital inflammation. Markers studied included angiotensin-converting enzyme (ACE), antinuclear antibody (ANA), anti-neutrophilic cytoplasmic autoantibodies (ANCA), extractable nuclear antigen (ENA), anti-cyclic citrullinated peptide (Anti-CCP) and anti-double stranded DNA antibody (Anti-dsDNA antibody).
Methods
A retrospective single-centre study of consecutive patients with non-infective orbital inflammation screened for autoimmune markers at presentation. Serology was interpreted alongside clinical course and other investigations (e.g. radiographic features and histopathology). Tabulated data and Pearson’s Chi-square allowed analysis of trends between serology, diagnosis and the decision to biopsy.
Results
79 patients, between 1999 and 2021, were included (50 females, mean age was 50.4 ± 17.4 years). 28 (34.6%) patients had specific orbital inflammation and 53 (65.4%) patients had non-specific orbital inflammation (NSOI). Of the 12 patients with positive serology and a specific diagnosis, only 5 (41.7%) patients had concordant serological results. There was no association between serology results and the patient undergoing biopsy (P = 0.651). Serology was unable to exclude nor differentiate NSOI from other specific conditions and ANA had limited discriminatory value between specific conditions and NSOI.
Conclusion
Serological testing alone may not provide a clear direction for further investigation of orbital inflammation and a biopsy may occur independently of the serological results. The value of autoimmune markers may lie in subsequent follow-up as patients may develop suggestive symptoms after an indeterminate positive result or initially seronegative disease.
Similar content being viewed by others
Introduction
Orbital inflammatory disease (OID) may be due to specific localised or systemic inflammatory diseases, such as sarcoidosis, granulomatosis with polyangiitis (GPA) and IgG4-related orbital disease (IgG4-ROD) [1,2,3,4]. If no identifiable underlying cause can be determined, a diagnosis of non-specific orbital inflammation (NSOI) can be made [5]. Serology can be used to help diagnose various immunogenic causes of orbital inflammation. However, in situations of diagnostic uncertainty, histopathological analysis may still be required to confirm or support the diagnosis [2, 3].
The aim of this study was to investigate the utility of various autoimmune markers in screening for immunogenic OID. Autoimmune markers studied included angiotensin-converting enzyme (ACE), antinuclear antibody (ANA), anti-neutrophilic cytoplasmic autoantibodies (ANCA), extractable nuclear antigen (ENA), anti-cyclic citrullinated peptide (Anti-CCP) and anti-double stranded DNA antibody (Anti-dsDNA antibody).
Methods
This was a single-centre cross-sectional retrospective study of patients with orbital inflammation. Inclusion criteria were patients >18 years of age with clinical, radiological and/or histologically confirmed OID, with autoimmune markers conducted at initial presentation. Exclusion criteria included patients without any autoimmune markers screened; patients with thyroid eye disease; and patients diagnosed with infectious orbital disease (e.g.: orbital cellulitis). Patients with infectious orbital disease were excluded based on suggestive clinico-radiological findings and response to systemic antibiotics. Patients included in this study were categorised as having a specific diagnosis (e.g.: sarcoidosis, orbital GPA) if there was clinico-radiological and/or histological evidence of a specific diagnosis. For example, a diagnosis of IgG4-ROD was assessed according to the Comprehensive diagnostic (CD) criteria for IgG4-related disease; and GPA was diagnosed as according to the 1990 American College of Rheumatology (ACR) criteria [6, 7]. Meanwhile, patients without any identifiable local or systemic cause were deemed NSOI (i.e. a diagnosis of exclusion). A diagnosis of NSOI was further evidenced by supporting clinico-radiological (including lack of radiologically-specific changes seen in specific diagnoses, such as sino-nasal involvement in GPA) and/or histopathological presentation and response to corticosteroid therapy [8].
Patients were identified from the Oculoplastics Unit at the Royal Adelaide Hospital (Adelaide, Australia). Data were sourced from paper and electronic records. Data collected included patient demographics (age at presentation, gender, past medical history and relevant medications), diagnostic details (diagnosis and orbital structure involved) and autoimmune markers (ACE, ANA, ENA, ANCA, anti-dsDNA antibody and anti-CCP). Patients were classified as having positive or negative serology. Autoimmune markers were deemed positive if a positive marker or titre was reported at presentation. At our institution, ANA was reported positive at titres above 1/40. Patients were further categorised according to their specific or non-specific diagnosis (i.e. NSOI) and those with a biopsy were also noted.
Within the typical diagnostic work-up of OID, laboratory investigations included a combination of a complete blood count, erythrocyte sedimentation rate, C-reactive protein, thyroid function tests (including anti-thyroid antibodies) and any indicated autoimmune serology. Radiological and/or histological findings provided further diagnostic information [9, 10].
Statistical analysis was performed with SPSS (IBM corporation, New York). Where applicable, results are expressed as mean ± standard deviation (σ) and presented in relevant tables. Pearson’s chi-square test allowed analysis between the incidence of autoimmune marker results and the incidence of patients undergoing a biopsy (P < 0.05 deemed statistically significant). All research was conducted in accordance with the Declaration of Helsinki and was approved by our Institutional Review Board with a waiver of consent granted.
Results
Demographics
79 patients, between 1999 and 2021, were analysed in this study. 50 (63.3%) were females, 29 (36.7%) were males and the mean age was 50.4 ± 17.4 years (range: 18–86 years). 28 (35.4%) patients had a specific diagnosis. The remaining 51 (64.6%) patients had NSOI, with 20 (39.2%) classified as diffuse inflammation, 20 (39.2%) as dacryoadenitis and 11 (21.6%) as myositis. A summary of diagnoses is provided in Table 1. Mean age was similar between both groups (NSOI: 49.5 ± 17.1 years; specific inflammation: 52.1 ± 18.2 years). Of the 47 (59.3%) patients with a biopsy, 16 (34.0%) patients had specific OID and 31 (66.0%) patients had NSOI. Mean follow-up period was 18.5 ± 32.7 months (range: 1 week–192 months) for NSOI patients and 18.6 ± 20.6 months (range: 3 days–75 months) for specific conditions.
Association between serology and diagnosis
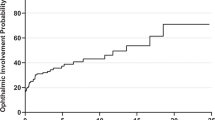
Figure 1 demonstrates the distribution of the 79 patients screened with autoimmune markers at presentation.
Biopsies were performed in patients where clinical and radiological findings were inconclusive, in patients with positive serology in the absence of known systemic involvement, and in patients with treatment-resistant disease. Overall, of the 27 patients with a positive serology, 17 (63.0%) underwent a biopsy. Meanwhile, of the 52 patients with negative serology, 30 (57.7%) patients underwent a biopsy. Chi-square analysis demonstrated no significant association between serology result (positive or negative) and the patient undergoing a biopsy (P = 0.651).
Of the 12 patients with positive serology and diagnosed with a specific condition, only 5 (41.7%) of these patients had serology supporting the final diagnosis. These included 2 (40%) patients with first onset sarcoidosis (no previously known systemic involvement), 1 (20%) patient later diagnosed with Sjogren’s syndrome (SS), 1 (20%) patient diagnosed with overlap syndrome at follow-up and 1 (20%) patient demonstrating ANA positivity in SLE-associated bilateral dacryoadenitis. Of these 5 patients with concordant serology, 3 (60%) proceeded to a biopsy, including the 2 patients with sarcoidosis and 1 patient with overlap syndrome.
Of the remaining 7 patients with positive serology and a specific diagnosis, 3 (42.9%) patients proceeded to a biopsy, including 2 patients with IgG4-ROD and 1 patient with RLH. The remaining 4 (57.1%) patients had a clinico-radiological diagnosis for their specific condition (e.g.: onset of myositis symptoms shortly after administration of bisphosphonates).
Analysis of individual markers
A summary of individual autoimmune marker findings is reported in Table 2. Only 2 patients had elevated ACE levels, representing 66.6% of those with sarcoidosis. 73 patients had ANA screening and 19 (26.0%) patients were positive. Of those with ANA positivity, 13 (68.4%) patients had NSOI, representing 26% of the 50 NSOI patients with a screening ANA. Within this NSOI group, there were no patients with a history of autoimmune connective tissue disease.
72 patients were tested for ANCA and 6 (8.3%) reported ANCA positivity. These 6 patients, including 4 patients which were diagnosed with NSOI, had weak ANCA positivity, and ultimately had a negative myeloperoxidase and proteinase 3 (PR3). All 6 patients with orbital GPA reported negative ANCA titres at presentation. However, 5 patients had previously known systemic disease and had decreasing or negative titres on maintenance therapy. 1 patient presented with new-onset sino-orbital disease, but seroconversion only occurred at 3 months follow-up. Negative ANCA was observed in the single patient with eosinophilic granulomatosis with polyangiitis (EGPA).
ENA was screened in 53 patients and was positive in 3 (5.7%) patients. Of these 3 patients, 1 (33.3%) patient with positive anti-Ku antibody developed systemic symptoms consistent with overlap syndrome. Both patients with SS had negative anti-Ro or anti-La antibodies. Anti-CCP was screened in 26 patients with 1 (3.8%) positive result, which was observed in a case of SS. This patient was subsequently diagnosed with rheumatoid arthritis and secondary SS.
Finally, none of the 38 patients tested for anti-dsDNA had positive results.
Association between serology and orbital structure
Figure 2 depicts the utility of autoimmune markers according to affected orbital structure. Within this study, 31 (39.2%) patients had diffuse inflammation, 15 (19.0%) had myositis and 33 (41.8%) had dacryoadenitis. 16 (51.6%), 8 (53.3%) and 23 (69.7%) patients within these groups, respectively, had a biopsy. Chi-square analysis demonstrated statistically insignificant associations between serology result and the patient undergoing a biopsy for diffuse inflammation (P = 0.320), myositis (P = 0.876) and dacryoadenitis (P = 0.775).
Discussion
The results show that only a small proportion of patients with specific immunogenic OID are supported by concordant serological results at time of presentation. The results of serological testing do not specify a diagnosis. They are an adjunctive investigation, used along with clinical findings and radiological tests, for diagnosing specific OID (e.g.: GPA is not solely diagnosed by ANCA positivity, nor is sarcoidosis diagnosed solely by elevated ACE levels). In addition, many of these patients may have previously known systemic involvement; have a diagnosis only confirmed at follow-up; or may still proceed to a biopsy for histopathological diagnosis. Serological testing was unable to exclude nor differentiate NSOI from other specific causes of orbital inflammation. Even after differentiation of the subtype of orbital inflammation (diffuse, myositis or dacryoadenitis), there was no association between serology and the patient undergoing a biopsy. Thus, serology alone was unlikely to influence the decision to undergo a biopsy, enforcing the point that the decision to biopsy remains multifactorial. For example, biopsy may be indicated in treatment-resistant disease, or less likely to be conducted in situations where a clinico-radiological diagnosis may be made (e.g.: a previous diagnosis of a systemic disease).
Regarding the individual markers, elevated ACE levels supported sarcoidosis, but normal levels did not exclude disease. ANA was a highly non-specific marker with limited utility. ANCA screening has limited value in patients with known systemic GPA on maintenance therapy, and seroconversion for first presentation may only be revealed at future follow-up. In patients with specific markers, such as anti-CCP or anti-Ku antibody, follow-up may reveal systemic manifestations confirming a diagnosis.
Within the literature, the limitations and diagnostic complexity when approaching suspected cases of immunogenic OID have been outlined [11, 12]. Mombaerts et al. has previously reported that serological studies have limited diagnostic value in orbital inflammation as results may be non-specific, disease is generally localised to the orbit and severe disease would be required to create a positive assay. Thus, as evidenced by our findings and the literature, positive markers are considered only as corroborative evidence [3]. This study further highlights the limitations of serological testing and suggests that in many circumstances such results do not strongly influence the diagnostic pathway and decision to proceed to a biopsy.
Choice of autoimmune markers in the diagnostic approach of orbital inflammation should be guided by clinico-radiological presentation [2]. Elevated ACE levels reportedly occurs in 60–90% of active sarcoidosis, with levels generally proportional to severity [13,14,15]. Thus, normal ACE levels may not distinguish between less active and absent disease [14]. Furthermore, systemic disease may subsequently be revealed in 9–50% of patients, but ACE levels may remain normal [13, 16]. ACE may also be increased in other conditions, such as orbital lymphoma and NSOI [17, 18]. Elevated ACE levels are reportedly a predictor of positive yield on biopsy [19]. Tissue biopsy should be obtained in patients with supportive clinico-radiological features and elevated serum ACE, as was evident in our cases [11].
Within our study, ANA had limited discriminatory value between specific and non-specific disease [20]. Although ANA positivity is reported in 59–85% of SS patients, both SS patients in our study had negative ANA, albeit in a small represented sample [21]. ANA is a poor screening test for primary SS, as 23% have ANA negativity and 9% have a negative ANA and ENA [22]. Positive ANA may also occur in other autoimmune and non-rheumatic diseases, and are detectable in up to 20–30% of healthy adults, with significantly elevated levels observed in 2.5% of the general population [23,24,25]. Although, patients may exhibit positive ANA years before a diagnosis of autoimmune disease, studies have reported that many ANA-positive patients do not have autoimmune disease nor do they have an increased likelihood of future autoimmune disease [3, 24]. Furthermore, although thyroid eye disease was excluded from this study, interpretation of ANA should also consider autoimmune thyroid disease, as ANA positivity may also occur in these cases [26, 27]. Thus, thyroid function tests should be considered alongside any indicated autoimmune markers.
Positive cANCA may be observed in 90% of active generalised GPA [28,29,30,31,32]. However, ANCA testing is less sensitive in localised and isolated sino-orbital GPA, and titres can become negative after treatment, as was evident in our study [11, 32,33,34,35,36,37,38,39,40]. Patients with new-onset disease may present with negative ANCA, but may seroconvert at follow-up [39, 41, 42]. It has been estimated that only 32–35% of patients with known sino-orbital disease have cANCA-positivity at presentation, with an additional 10% converting 6–24 months after initial presentation [39, 41]. Although biopsy can help confirm the diagnosis, histopathology may reveal granulomatous inflammation without vasculitis, a non-specific finding which may also be indicative of sarcoidosis, NSOI or aspergillosis [33, 43, 44].
ENAs encompass a range of specific autoantibodies used to help diagnose autoimmune connective tissue diseases [45]. For example, anti-Ro antibodies are found in 50–75% and 15% of patients with primary and secondary SS, respectively [46,47,48]. However, positive anti-Ro antibodies may be observed in a range of other autoimmune disorders and in health individuals [46, 47]. Within our study, a patient with positive anti-Ku antibody developed symptoms suggestive of overlap syndrome.
Anti-CCP is typically a specific marker for rheumatoid arthritis. In addition, high anti-CCP levels are also observed in the minority of patients with primary SS [49,50,51]. Only 1 patient with SS had a positive anti-CCP, and follow-up revealed early rheumatoid arthritis. Anti-CCP in patients with arthritis may help predict progression to rheumatoid arthritis [52]. Anti-dsDNA antibody is specific for SLE, which remains a cause of orbital inflammation [53, 54]. However, unless there are clinical features supporting a high pre-test probability of SLE, anti-dsDNA antibody is not a recommended investigation in situations of ANA negativity [55].
The main limitation of this study is the small sample sizes represented for each specific inflammatory condition. Secondly, patients diagnosed with GPA were on maintenance therapy which are known to reduce or cause negative ANCA titres. Finally, although the heterogeneity of autoimmune markers tested at presentation and subsequent follow-up reflect the clinical pre-test probability of a specific diagnosis, various orbital conditions, such as limited GPA-related orbital disease, are known to present with seronegative disease [56]. There is limited literature regarding the characteristics of seroconversion of autoimmune markers in patients with orbital disease. Thus, future studies may involve longitudinal analysis of patients screened with autoimmune markers to investigate the utility of serial testing in initially seronegative disease.
In conclusion, autoimmune markers often have limited utility in screening for immunogenic OID. The choice of autoimmune markers in the initial diagnostic work-up of OID should be selective and rationalised by the clinico-radiological presentation, as a full panel approach may yield non-specific findings. In many circumstances, the decision to proceed with a biopsy occurs independently of the serological result. The value of autoimmune markers may lie in subsequent follow-up as patients may later develop suggestive clinical features after a non-specific positive result or in initially seronegative disease.
Summary
What was known before
-
Immunogenic causes of orbital inflammation may be difficult to differentiate.
-
Autoimmune markers are commonly used to screen for causes of orbital inflammation.
-
In situations of diagnostic uncertainty, histopathological analysis may still be required to support or confirm the diagnosis.
What this study adds
-
Only a small proportion of patients with a specific diagnosis may have positive concordant serological results on initial assessment.
-
Serological testing alone may not provide a clear direction for further investigation of orbital inflammation.
-
The value of serological testing may lie in subsequent follow-up to detect initially indeterminate positive findings or seronegative disease.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to containing information that could compromise patient privacy but are available from the corresponding author on reasonable request.
References
Moore GrantH, Rootman, Daniel B. Orbital inflammatory disease management. Expert Rev Ophthalmol. 2016;11:415–28.
Gordon LK. Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye 2006;20:1196–206.
Mombaerts I, Rose GE, Garrity JA. Orbital inflammation: Biopsy first. Surv Ophthalmol. 2016;61:664–9.
Rootman J. Diseases of the orbit: a multidisciplinary approach. 2nd ed. Philadelphia, Pa.: Lippincott Williams & Wilkins; 2002. p. 586.
Rubin PA, Foster CS. Etiology and Management of Idiopathic Orbital Inflammation. Am J Ophthalmo. 2004;138:1041–3.
Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30.
Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33:1101–7.
Kapur R, Sepahdari AR, Mafee MF, Putterman AM, Aakalu V, Wendel LJ, et al. MR imaging of orbital inflammatory syndrome, orbital cellulitis, and orbital lymphoid lesions: the role of diffusion-weighted imaging. AJNR Am J Neuroradiol. 2009;30:64–70.
Kapur R, Sepahdari AR, Mafee MF, Putterman AM, Aakalu V, Wendel LJA, et al. MR Imaging of Orbital Inflammatory Syndrome, Orbital Cellulitis, and Orbital Lymphoid Lesions: The Role of Diffusion-Weighted Imaging. Am J Neuroradiol. 2009;30:64–70.
Ferreira TA, Saraiva P, Genders SW, Buchem MV, Luyten GPM, Beenakker JW. CT and MR imaging of orbital inflammation. Neuroradiology 2018;60:1253–66.
Srinivasan A, Kleinberg TT, Murchison AP, Jurij R. Bilyk. Laboratory Investigations for Diagnosis of Autoimmune and Inflammatory Periocular Disease: Part II. Ophthalmic Plast Reconstr Surg. 2017;33:1–8.
Srinivasan A, Kleinberg TT, Murchison AP, Bilyk JR. Laboratory Investigations for Diagnosis of Autoimmune and Inflammatory Periocular Disease: Part I. Ophthalmic Plast Reconstr Surg. 2016;32:321–8.
Mavrikakis I, Rootman J. Diverse clinical presentations of orbital sarcoid. Am J Ophthalmol. 2007;144:769–75.
Weinreb RN, Tessler H. Laboratory diagnosis of ophthalmic sarcoidosis. Surv Ophthalmol. 1984;28:653–64.
Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975;59:365–72.
Collison JM, Miller NR, Green WR. Involvement of orbital tissues by sarcoid. Am J Ophthalmol. 1986;102:302–7.
Tang SX, Lim RP, Al-Dahmash S, Blaydon SM, Cho RI, Choe CH, et al. Bilateral lacrimal gland disease: clinical features of 97 cases. Ophthalmology 2014;121:2040–6.
Bénéteau-Burnat B, Baudin B. Angiotensin-converting enzyme: clinical applications and laboratory investigations on serum and other biological fluids. Crit Rev Clin Lab Sci. 1991;28:337–56.
Rabinowitz MP, Halfpenny CP, Bedrossian EH Jr. The frequency of granulomatous lacrimal gland inflammation as a cause of lacrimal gland enlargement in patients without a diagnosis of systemic sarcoidosis. Orbit 2013;32:151–5.
Abeles AM, Abeles M. The clinical utility of a positive antinuclear antibody test result. Am J Med. 2013;126:342–8.
Nardi N, Brito-Zerón P, Ramos-Casals M, Aguiló S, Cervera R, Ingelmo M, et al. Circulating auto-antibodies against nuclear and non-nuclear antigens in primary Sjögren’s syndrome: prevalence and clinical significance in 335 patients. Clin Rheumatol. 2006;25:341–6.
Lyne SA, Downie-Doyle S, Lester SE, Quinlivan A, Toby Coates P, Gordon TP, et al. Primary Sjögren’s syndrome in South Australia. Clin Exp Rheumatol. 2020;38:57–63.
Tan EM, Smolen JS, McDougal JS, Butcher BT, Conn D, Dawkins R, et al. A critical evaluation of enzyme immunoassays for detection of antinuclear autoantibodies of defined specificities. I. Precision, sensitivity, and specificity. Arthritis Rheum. 1999;42:455–64.
Grygiel-Górniak B, Rogacka N, Puszczewicz M. Antinuclear antibodies in healthy people and non-rheumatic diseases - diagnostic and clinical implications. Reumatologia 2018;56:243–8.
Nosal RS, Superville SS, Varacallo M. Biochemistry, Antinuclear Antibodies (ANA). StatPearls.(Treasure Island (FL): StatPearls Publishing; 2021).
Segni M, Pucarelli I, Truglia S, Turriziani I, Serafinelli C, Conti F. High prevalence of antinuclear antibodies in children with thyroid autoimmunity. J Immunol Res. 2014;2014:150239.
Petri M, Karlson EW, Cooper DS, Ladenson PW. Autoantibody tests in autoimmune thyroid disease: a case-control study. J Rheumatol. 1991;18:1529–31.
Muller K, Lin JH. Orbital granulomatosis with polyangiitis (Wegener granulomatosis): clinical and pathologic findings. Arch Pathol Lab Med. 2014;138:1110–4.
Ahmed M, Niffenegger JH, Jakobiec FA, Ben-Arie-Weintrob Y, Gion N, Androudi S, et al. Diagnosis of limited ophthalmic Wegener granulomatosis: distinctive pathologic features with ANCA test confirmation. Int Ophthalmol. 2008;28:35–46.
Holle JU, Gross WL. Neurological involvement in Wegener’s granulomatosis. Curr Opin Rheumatol. 2011;23:7–11.
Pakrou N, Selva D, Leibovitch I. Wegener’s granulomatosis: ophthalmic manifestations and management. Semin Arthritis Rheum. 2006;35:284–92.
Rao JK, Weinberger M, Oddone EZ, Allen NB, Landsman P, Feussner JR. The role of antineutrophil cytoplasmic antibody (c-ANCA) testing in the diagnosis of Wegener granulomatosis. A literature review and meta-analysis. Ann Intern Med. 1995;123:925–32.
Bijlsma WR, Hené RJ, Mourits MP, Kalmann R. Orbital mass as manifestation of Wegener’s granulomatosis: an ophthalmologic diagnostic approach. Clin Exp Rheumatol. 2011;29:S35–9.
Knoch DW, Lucarelli MJ, Dortzbach RK, Smith ME. Limited Wegener granulomatosis with 40 years of follow-up. Arch Ophthalmol. 2003;121:1640–2.
Finkielman JD, Lee AS, Hummel AM, Viss MA, Jacob GL, Homburger HA, et al. ANCA are detectable in nearly all patients with active severe Wegener’s granulomatosis. Am J Med. 2007;120:643.e9–14.
Holle JU, Wu QJ, Moosig F, Gross WL, Csernok E. Membrane proteinase 3 (mPR3) expression on neutrophils is not increased in localized Wegener’s granulomatosis (WG) and Churg-Strauss syndrome (CSS). Clin Exp Rheumatol. 2010;28:46–50.
Schmidt J, Pulido JS, Matteson EL. Ocular manifestations of systemic disease: antineutrophil cytoplasmic antibody-associated vasculitis. Curr Opin Ophthalmol. 2011;22:489–95.
Tarabishy AB, Schulte M, Papaliodis GN, Hoffman GS. Wegener’s granulomatosis: clinical manifestations, differential diagnosis, and management of ocular and systemic disease. Surv Ophthalmol. 2010;55:429–44.
Woo TL, Francis IC, Wilcsek GA, Coroneo MT, McNab AA, Sullivan TJ. Australasian orbital and adnexal Wegener’s granulomatosis. Ophthalmology 2001;108:1535–43.
Perry SR, Rootman J, White VA. The clinical and pathologic constellation of Wegener granulomatosis of the orbit. Ophthalmology 1997;104:683–94.
Tan LT, Davagnanam I, Isa H, Taylor SR, Rose GE, Verity DH, et al. Clinical and imaging features predictive of orbital granulomatosis with polyangiitis and the risk of systemic involvement. Ophthalmology 2014;121:1304–9.
Kopstein AB, Kristopaitis T, Gujrati TM, Blake KA, Bouchard CS. Orbital Wegener granulomatosis without systemic findings. Ophthalmic Plast Reconstr Surg. 1999;15:467–9.
Sfiniadaki E, Tsiara I, Theodossiadis P, Chatziralli I. Ocular manifestations of granulomatosis with polyangiitis: a review of the literature. Ophthalmol Ther. 2019;8:227–34.
Kalina PH, Lie JT, Campbell RJ, Garrity JA. Diagnostic value and limitations of orbital biopsy in Wegener’s granulomatosis. Ophthalmology 1992;99:120–4.
Damoiseaux JG, Tervaert JW. From ANA to ENA: how to proceed? Autoimmun Rev. 2006;5:10–7.
Locht H, Pelck R, Manthorpe R. Diagnostic and prognostic significance of measuring antibodies to alpha-fodrin compared to anti-Ro-52, anti-Ro-60, and anti-La in primary Sjögren’s syndrome. J Rheumatol. 2008;35:845–9.
Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol. 2012;2012:606195.
Bowman SJ. Primary Sjögren’s syndrome. Lupus 2018;27:32–5.
Gottenberg JE, Mignot S, Nicaise-Rolland P, Cohen-Solal J, Aucouturier F, Goetz J, et al. Prevalence of anti-cyclic citrullinated peptide and anti-keratin antibodies in patients with primary Sjögren’s syndrome. Ann Rheum Dis. 2005;64:114–7.
Atzeni F, Sarzi-Puttini P, Lama N, Bonacci E, Bobbio-Pallavicini F, Montecucco C, et al. Anti-cyclic citrullinated peptide antibodies in primary Sjögren syndrome may be associated with non-erosive synovitis. Arthritis Res Ther. 2008;10:R51.
Goëb V, Salle V, Duhaut P, Jouen F, Smail A, Ducroix JP, et al. Clinical significance of autoantibodies recognizing Sjögren’s syndrome A (SSA), SSB, calpastatin and alpha-fodrin in primary Sjögren’s syndrome. Clin Exp Immunol. 2007;148:281–7.
Ryu YS, Park SH, Lee J, Kwok SK, Ju JH, Kim HY, et al. Follow-up of primary Sjogren’s syndrome patients presenting positive anti-cyclic citrullinated peptides antibody. Rheumatol Int. 2013;33:1443–6.
Chan AJ, Rai AS, Lake S. Orbital myositis in systemic lupus erythematosus: a case report and literature review. Eur J Rheumatol. 2020;7:135–7.
Grimson BS, Simons KB. Orbital inflammation, myositis, and systemic lupus erythematosus. Arch Ophthalmol. 1983;101:736–8.
Kavanaugh AF, Solomon DH. Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-DNA antibody tests. Arthritis Rheum. 2002;47:546–55.
Lee MJ, Planck SR, Choi D, Harrington CA, Wilson DJ, Dailey RA, et al. Non-specific orbital inflammation: current understanding and unmet needs. Prog Retin Eye Res. 2020;81:100885.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
TA was responsible for the writing the protocol, extracting and analysing data, interpreting results and preparation of the paper. VJ was responsible designing and reviewing the protocol and providing feedback on the paper. DS provided feedback on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ang, T., Juniat, V. & Selva, D. Autoimmune markers in screening for orbital inflammatory disease. Eye 37, 1088–1093 (2023). https://doi.org/10.1038/s41433-022-02068-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02068-5