Abstract
Objective
To assess the relationship of retinal pigment clump (RPC) size and its location with visual acuity and retinal neovascularisation in eyes with type 2 macular telangiectasia (MacTel).
Methods
In this cross-sectional study, eyes diagnosed with type 2 MacTel showing RPC were included. Area occupied by pigment was measured on the multicolour image using the area tool on the Spectralis, Heidelberg machine. Pigment location within retinal layers was noted with OCT. Analysis was performed to identify factors associated with poor vision and proliferative disease.
Results
Sixty-two eyes of 42 patients diagnosed with type 2 MacTel and RPC were included. The mean age was 64.31 ± 10.19 years. There were 13 (31%) males and 29 (69%) females in the study. 74% of patients were diabetics and the mean logMAR visual acuity of the participants was 0.619 ± 0.359. Univariate and multivariate binary logistic regression analysis identified female gender (p = 0.026), increasing RPC size (p = 0.008) and its presence above the outer plexiform layer (p = 0.006) to be associated with poor vision and proliferative disease in type 2 MacTel.
Conclusion
Our data identified female gender, larger pigment size and its location above the OPL to be associated with poor vision and proliferative disease. This data may be useful for further improving the current system for staging disease severity in type 2 MacTel.
Similar content being viewed by others
Introduction
Type 2 macular telangiectasia (MacTel) is primarily a bilateral, retinal neurodegenerative and vascular disease of the elderly affecting the deep capillary network at the macula. It is associated with changes in inner and outer retinal structures, ultimately leading to photoreceptor layer atrophy, development of retinal pigment clumps (RPC) and abnormal neovascular complexes [1]. Gass and Blodi identified the different clinical features of type 2 MacTel and divided them into five different stages and later in 2006, Yannuzzi et al. simplified this classification further into two distinct stages: non-proliferative and proliferative [2, 3].
RPC are categorised in the stage 4 of the classification proposed by Gass and Blodi [2]. They are often noted at the tips of the dilated right-angled venules. They are associated with focal areas of photoreceptor layer atrophy. They originate from small foci of hyperplasia of retinal pigment epithelial (RPE) cells and these pigmented foci subsequently move into the neurosensory retina.
RPC can be detected using multiple imaging modalities. On clinical examination or on conventional colour fundus images, it appears as a jet-black pigment and can be distinguished from scar tissue or retinal blood based on its colour [1]. In fundus fluorescein angiography (FA) images, the pigment clump appears dark and it blocks underlying retinal or choroidal fluorescence or both based on its location in the retina [4]. The entire lateral extent of pigment plaques is very well demonstrated by fundus autofluorescence (FAF) imaging. On FAF images, the RPC deposited within the retinal layers internal to the RPE block autofluoroscent light and appear as localised areas of decreased FAF, usually surrounded by an area of increased FAF due to surrounding photoreceptor layer atrophy [5, 6]. It is highly reflective in optical coherence tomography (OCT) scans, blocking the path of light and thus casting ‘shadows’ on tissues underneath its location [7]. Our previous paper on Multicolour® (MC) imaging studied the morphological description of RPC on composite MC image [8]. The pigment appeared reddish-brown in colour on the MC image. As the MC® imaging uses lights of three different wavelengths, it carries the advantage of identifying RPC on blue, green and infrared reflectance channels depending upon its location within the retinal layers. Also, the complete extent of pigment clump is well-delineated on the MC image, thus, allowing an accurate measurement of area occupied by the pigment plaque.
Studies have shown that development of subretinal neovascular membrane (SRNVM) is most often preceded by the development of RPC [6, 8, 9]. However, literature is scarce on association between various characteristics of the RPC with vision and development of SRNVM in type 2 MacTel. In 2018, Leung et al. as a part of the MacTel study project reported that with an increase in size of the pigment plaque on FAF image, there is increased chances of developing SRNVM [6].
With this background, we aimed to study the relationship of pigment size measured on MC image and its location within the retinal layers on OCT with visual acuity and retinal neovascularisation (NV) in eyes with type 2 MacTel.
Methods
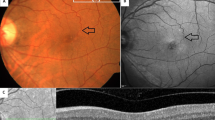
In this retrospective observational study, we reviewed the clinical records, MC and OCT images at presentation of patients diagnosed with type 2 MacTel attending the retina services at a tertiary eye care hospital in South India between January 2017 and December 2020. Type 2 MacTel was diagnosed based on clinical features as described by Gass and Blodi [2] and confirmed with the MC image obtained using the Spectralis machine (Spectralis, Heidelberg Engineering, Germany). The diagnosis was confirmed on the MC image based on the description provided in our previous publication on MC® imaging in type 2 MacTel [8]. Eyes with MC images showing RPC were only included in the study. Pigment clumps were seen as jet-black spots seen in the perifoveal region on the BR and GR reflectance images. MC images showed the pigment plaques as characteristic reddish-brown spots. The confirmation of the diagnosis and the decision to include in the study was made by RV (one of the authors in the study). The clinical details collected included age, gender, laterality and presence of diabetes mellitus. In addition to the presence of RPC, other features of type 2 MacTel like perifoveal greying and loss of retinal transparency, visible telangiectasias, superficial retinal crystals, dilated right-angled vessels and presence of SRNVM were noted. As per classification by Yannuzzi et al., eyes were divided into non-proliferative and proliferative stages [3]. Eyes with macular NV were considered in the proliferative disease stage while the remainder were considered in the non-proliferative stage. Macular NV was confirmed on FA which showed late intense focal and diffuse perifoveal leakage in all eyes. The total area occupied by the pigment clump in type 2 MacTel was called the “pigment area”, which was measured as the outer boundary of an area occupied by reddish-brown pigment as seen on the composite MC image. The “pigment area” was documented in square millimetres and was measured manually on the MC image using the area tool provided on the Heyex software of the Heidelberg, Spectralis machine (Fig. 1). The measurements were carried out by two independent trained retinal specialists (NR and SAM) who were masked to the clinical findings and OCT findings of the study subjects.
A Colour fundus photograph of the right eye obtained with Topcon TRC 50Dx fundus camera which shows a jet-black coloured pigment plaque occupying the foveal region. B Fundus autofluorescence image shows the pigment plaque as a hypoautofluorescent lesion with temporal perifoveal hyperautofluorescence in type 2 MacTel. C Late fluorescein angiography image showing blocked retinal and choroidal fluorescence the fovea due to the overlying pigment plaque. There is diffuse perifoveal hyperfluorescence due to leakage from the telangiectatic vessels in type 2 MacTel. D Multicolour image obtained on Heidelberg, Spectralis® machine delineates the pigment plaque as a reddish-brown structure. The area occupied by the pigment plaque is computed by using area measurement tool on the multicolour image. E Spectral domain optical coherence tomography shows the migration of the retinal pigment plaque to the inner retinal surface to posterior back shadowing.
OCT findings
All OCT scans were obtained using the Spectralis machine (Spectralis, Heidelberg Engineering, Germany). Macular volumetric assessments consisting of 512 A-scans per line with 30° scanning area and 25-line horizontal raster volume scans centred at the fovea were performed. OCT scans having a quality score ≥ 20 were used for analysis and interpretating the findings. All the images encompassing the macular area were analysed by a single masked observer (PRM) who was unaware of the clinical findings and the following features were noted from inner retina to outer retina in a sequential manner: (1) irregularity of the foveal contour, (2) internal limiting membrane (ILM) drape, (3) hyperreflectivity of the middle retinal layers i.e. between the inner plexiform to the outer plexiform layers (OPL), (4) identification of retinal crystals as superficial hyperreflective retinal dots, (5) hyporeflective inner retinal cavities, (6) hyporeflective outer retinal cavities, (7) outward bending of inner retinal layers, (8) subfoveal neurosensory detachment, (9) presence of external limiting membrane and inner segment – outer segment (IS-OS) layer discontinuity, (10) location of RPC either below or above the OPL, (11) macular hole either full-thickness or pseudohole or lamellar macular hole, (12) epiretinal neovascularisation (ERN), (13) SRNVM and (14) retino-choroidal anastomosis (RCA).
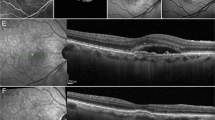
Supplemental Table 1 and Fig. 2 depict the different OCT abnormalities seen with type 2 MacTel.
A Increased reflectivity of the inner retina at the middle retinal layers at the temporal parafovea and asymmetric foveal contour are present. B Hyperreflective middle retinal layers temporal to the fovea with hyporeflective inner retinal cavity. C Inner and outer retinal hyporeflective cavities are noted. D Hyporeflective inner retinal cavity can be found at the foveal centre with an overlying ILM drape is present. E Superficial retinal crystals are seen as hyperreflective spots in the superficial layer of the retina without any back shadowing and outward turning of the inner retinal layers is noted with shallow sub foveal SRF. F Outward turning of the inner retinal layers is noted. G Pigment migration to the inner retinal layers with back shadowing is noted. H Hyperreflective middle retinal layers with subfoveal SRF is noted. I Foveal contour distortion with presence of ILM drape with outward turning of the middle retinal layers and presence of subretinal neovascular membrane is noted. J ILM drape with foveal contour irregularity is noted. There is a hyperreflective material noted in the retinal layers breaching the retinal pigment epithelium suggestive of retino-choroidal anastomosis. K ERN is noted as irregular hyperreflectivity on the retinal surface within the region of MacTel. Retinal pigment plaque is noted as dense hyperreflective lesion with back shadowing in the inner retinal layers.
Based on the identification of NV (i.e. ERN, SRNVM or RCA) on OCT, further analysis was performed between the NV group and non-NV group. Analysis was performed with the intention to identify the factors associated with proliferative disease in eyes with type 2 MacTel having RPC.
The study complied with the tenets of the Declaration of Helsinki and was approved by the local Institutional Review Board/Ethics Committee. Because the study was a retrospective analysis, waiver for informed consent was obtained.
Statistical analysis
All data were analysed using GraphPad Prism version 9.1.2 (226) for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com. The Shapiro-Wilk normality test was used to test the normality of the data sets. Snellen’s vision data was converted to logMAR vision for statistical analysis. Categorical variables such as gender and presence of diabetes were expressed as numbers and percentages while continuous variables such as age, logMAR visual acuity and pigment area were expressed as mean and standard deviation (SD). Quantitative variables between the two groups were analysed using the Mann–Whitney U test for non-parametric data and unpaired t-test for parametric data. Chi-square test was used to compare the categorical data between the two groups. Multiple variable binary logistic regression analysis was performed with presence of proliferative disease as the dependent variable and statistically significant clinical and OCT features as independent variables. P values < 0.05 were considered statistically significant.
Results
In this study, 62 eyes of 42 patients diagnosed with type 2 MacTel and having RPC were included. The mean age and SD of the study participants was 64.31 ± 10.19 years. There were 13 (31%) males and 29 (69%) females in the study. Right eye was affected in 33 (53%) eyes while left eye in 29 (47%) eyes. 74% of patients were diabetics and the mean logMAR visual acuity and SD of the participants was 0.619 ± 0.359 (Supplemental Table 2).
Other clinical features noted on MC image is described in Supplemental Table 3. The mean area and SD occupied by RPC was 1.34 ± 1.172 mm2. 20 (32%) eyes were categorised into proliferative disease group and remaining 42 (68%) eyes into non-proliferative disease group.
Supplemental Table 4 compares the demographic and clinical features such as pigment area and logMAR visual acuity between non-proliferative group and proliferative group in type 2 MacTel. More females were involved in the proliferative group (41% vs. 83%, p = 0.026). Eyes with proliferative disease showed a significantly poorer visual acuity (p = 0.029) and larger area of pigment involvement (p = 0.008) compared to non-proliferative group.
Using univariate and multivariate binary logistic regression analysis with presence of proliferative disease as the dependent variable, area under ROC curve (AUROC) was generated for age, female gender, logMAR visual acuity and pigment area (Fig. 3 and Supplemental Table 5). AUROC values for female gender, logMAR visual acuity and pigment area were >0.5 and were considered statistically significant in predicting the development of proliferative type 2 MacTel disease. Larger area of RPC involvement (OR = 1.579) and poorer visual acuity (OR = 2.808) had higher odds of predicting the development of proliferative disease.
Supplemental Table 6 compares the demographic, visual acuity, pigment area and other OCT features between eyes with NV and with no NV on OCT in patients with type 2 MacTel. Female gender (p = 0.033), poor visual acuity (p = 0.029), larger pigment area (p = 0.029) and presence of RPC between the OPL and ILM (p = 0.006) were commonly seen with eyes showing NV on OCT.
Univariate and multivariate binary logistic regression analysis was performed with identification of NV on OCT as the dependent variable (Fig. 4 and Supplemental Table 7). Factors such as female gender, poor visual acuity, larger pigment area and more superficial location of the pigment plaque between OPL and ILM (Fig. 5) were associated with higher chances of developing NV on OCT in type 2 MacTel.
A A case of non-proliferative type 2 macular telangiectasia showing the pigment clump with underlying shadowing below the outer plexiform layer. There is an associated discontinuity of external limiting membrane and inner segment – outer segment layers. Irregularity of foveal contour is noted as well. B A case of proliferative disease in type 2 macular telangiectasia showing the pigment clump with underlying shadowing above the outer plexiform layer. There is an associated discontinuity of external limiting membrane and inner segment – outer segment layers. Irregularity of foveal contour with presence of draping of internal limiting membrane drape is noted as well.
Discussion
This study analysed the size of RPC on MC image and its location within the retinal layers on OCT in eyes with type 2 MacTel and correlated them with visual acuity and the development of stage 5 or proliferative disease. The results of this study suggest that in type 2 MacTel, female patients having large size pigment plaque and those located above the OPL are often associated with poor vision and proliferative disease.
The pathogenesis of RPC in type 2 MacTel is interesting. Type 2 MacTel is a neurodegenerative vascular retinal pathology which affects the retinal Müller glial cells at the macula and causes cell death [10, 11]. This degenerative disease causes structural damage to the retinal layers leading to the development inner and outer retinal degenerative cavities. This further leads to the outward bending of inner retinal layers towards the RPE on OCT. At the same time, dilated right-angled veins are seen on clinical examination [12, 13]. With further progression of the disease, there is atrophy of the photoreceptor layer which leads to migrations of the RPE cells towards its centre. When the pigment cells from the RPE come in contact with these abnormal vessels, the pigment cells use them as a scaffold and migrate along the vessels into the inner retina, where they proliferate and spread “sideways” away from the vessels along the retinal layers, approximately at the level of the OPL. Such growth of the pigment cells along the blood vessels is well known and documented with other retinal diseases like retinitis pigmentosa [14, 15]. The rate of growth of the pigment cells sideways is faster than its growth vertically along the vessels after the pigment cells reach the inner retinal layers [6, 12]. Thus, the horizontal spread is larger allowing us to accurately measure the size of the pigment plaque. Hence, the pigment plaques are noted clinically as dark-brown or black structures, enveloping the blood vessels, typically appearing located to the mid-retina, temporal to the foveal centre on clinical examination [2, 16].
In majority of the cases of type 2 MacTel, pigment plaques may precede SRNVM [6, 17]. In some cases, SRNVM may occur simultaneous with the pigment and it can occur even in the absence of MacTel-type pigment. In our series of cases, SRNVM was noted in 20 eyes having RPC on MC images in type 2 MacTel cases. Since, our study was a cross-sectional study, the data regarding SRNVM developing over a period of time in the remaining 42 eyes was not known. Furthermore, RPC may also be associated with the secondary SRNVM itself and differentiating them from the pigment clumps seen in type 2 MacTel may be challenging. Also, both types of pigments may co-exist. However, there may be some morphological features in pigment secondary to SRNVM itself such as more convex/round lesions, smoother convex edges, not immediately adjacent to the vessel which may help in differentiating it from the pigment associated with type 2 MacTel [6, 17, 18].
Meleth et al noted a linear pattern of growth rate of pigment plaque over time which was associated with poor visual acuity. They concluded that pigment clumping may be an important indicator of disease severity and a useful outcome measure for longitudinal studies [17]. A recent study by Leung et noted a non-linear progression in the pigment plaque size over time but there was a strong association with development of SRNVM [6]. In both these studies, only the lateral extent of pigment plaque was measured. The location of RPC in relation to the retinal layers was not taken into consideration. In the current study, we measured the lateral extent of pigment plaque and also noted its location in relation to the OPL. However, we did not look into the longitudinal changes in the pigment plaque over time.
Our study has a few limitations. Firstly, the study has a small sample size and is a retrospective study. Secondly, the study lacks longitudinal data which would have been useful to evaluate the progression of the pigment plaques and time taken for the development of SRNVM. Thirdly, due to the loss of retinal structure differentiation in type 2 MacTel, layer differentiation on OCT may possibly be difficult as well. Finally, the OCT angiography details were missing which might have helped in identifying the pre-proliferative disease stage and might have provided an additional dimension to the pathogenesis of pigment plaque.
In conclusion, vertical migration of RPE cells onto the neurosensory retina crossing the OPL followed by a horizontal spread in a female patient diagnosed with type 2 MacTel have a higher odd of developing poor vision and proliferative disease. We believe that the point regarding the location of pigment plaque above or below the OPL should be taken into consideration in refining the current system for staging disease severity in type 2 MacTel.
Summary
What was known before
-
Pigment plaques in type 2 MacTel are commonly seen in stage 4 disease.
-
Subretinal neovascularization in type 2 MacTel usually follows the pigment plaque formation.
What this study adds
-
This paper talks about the pigment characteristics in type 2 MacTel for predicting the development of subretinal neovascularization and visual acuity.
-
Vertical and horizontal spread of the pigment in a female patient diagnosed with type 2 MacTel have a higher odd of developing poor vision and proliferative disease.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Charbel Issa P, Gillies MC, Chew EY, Bird AC, Heeren TFC, Peto T, et al. Macular telangiectasia type 2. Prog Retin Eye Res. 2013;34:49–77.
Gass JD, Blodi BA. Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology. 1993;100:1536–46.
Yannuzzi LA, Bardal AMC, Freund KB, Chen K-J, Eandi CM, Blodi B. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124:450–60. Chic. Ill 1960.
Narayanan R, Majji AB, Hussain N, Hussain A, Jalali S, Mathai A, et al. Characterization of idiopathic macular telangiectasia type 2 by fundus fluorescein angiography in Indian population. Eur J Ophthalmol. 2008;18:587–90.
Wong WT, Forooghian F, Majumdar Z, Bonner RF, Cunningham D, Chew EY. Fundus autofluorescence in type 2 idiopathic macular telangiectasia: correlation with optical coherence tomography and microperimetry. Am J Ophthalmol. 2009;148:573–83.
Leung I, Sallo FB, Bonelli R, Clemons TE, Pauleikhoff D, Chew EY, et al. Characteristics of pigmented lesions in type 2 idiopathic macular telangiectasia. Retin Phila Pa. 2018;38:S43–S50.
Kim YH, Chung Y-R, Oh J, Kim S-W, Lee CS, Yun C, et al. Optical coherence tomographic features of macular telangiectasia type 2: Korean Macular Telangiectasia Type 2 Study—Report No. 1. Sci Rep. 2020;10:16594.
Venkatesh R, Pereira A, Bavaharan B, Jain K, Aseem A, Sangai S, et al. Relevance of Multicolor Imaging in Type 2 Macular Telangiectasia. J Curr Ophthalmol. 2020;32:375–80.
Marsonia K, Kiran Chandra K, Ali MH, Chhablani J, Narayanan R. Long term follow-up of visual acuity and incidence of subretinal neovascularization in Mactel Type 2 in 82 Eyes. Semin Ophthalmol. 2022;37:136–41.
Zhao M, Andrieu-Soler C, Kowalczuk L, Paz Cortés M, Berdugo M, Dernigoghossian M, et al. A new CRB1 rat mutation links Müller glial cells to retinal telangiectasia. J Neurosci J Soc Neurosci. 2015;35:6093–106.
Powner MB, Gillies MC, Zhu M, Vevis K, Hunyor AP, Fruttiger M. Loss of Müller’s cells and photoreceptors in macular telangiectasia type 2. Ophthalmology 2013;120:2344–52.
Wu L. Multimodality imaging in macular telangiectasia 2: a clue to its pathogenesis. Indian J Ophthalmol. 2015;63:394–8.
Tzaridis S, Heeren T, Mai C, Thiele S, Holz FG, Charbel Issa P, et al. Right-angled vessels in macular telangiectasia type 2. Br J Ophthalmol. 2019: bjophthalmol-2018-313364.
Li ZY, Possin DE, Milam AH. Histopathology of bone spicule pigmentation in retinitis pigmentosa. Ophthalmology 1995;102:805–16.
Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205.
Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100:769–80. Chic. Ill 1960.
Meleth AD, Toy BC, Nigam D, Agrón E, Murphy RP, Chew EY, et al. Prevalence and progression of pigment clumping associated with idiopathic macular telangiectasia type 2. Retin Phila Pa. 2013;33:762–70.
Lavin MJ, Eldem B, Gregor ZJ. Symmetry of disciform scars in bilateral age-related macular degeneration. Br J Ophthalmol. 1991;75:133–6.
Author information
Authors and Affiliations
Contributions
RV, JC—conceptualising the study, data acquisition, analysing the data, interpreting the findings, writing & reviewing the paper. PRM, NR, SAM, DSM —Data acquisition and analysing the data. NKY—analysing the data, interpreting the findings, reviewing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Venkatesh, R., Agrawal, S., Reddy, N.G. et al. Characteristics of retinal pigment clumps in Type 2 macular telangiectasia (MacTel). Eye 37, 1061–1066 (2023). https://doi.org/10.1038/s41433-022-02065-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02065-8
This article is cited by
-
Baseline demographic, clinical and multimodal imaging features of young patients with type 2 macular telangiectasia
International Journal of Retina and Vitreous (2023)
-
Vitreomacular interface abnormalities in type 2 macular telangiectasia (MacTel)
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)