Abstract
Background
Anti-vascular endothelial growth factor (VEGF) treatments are the first-line treatment for Retinal Vein Occlusion (RVO). Although effectiveness and safety of these treatments is well documented, knowledge regarding the effect of lapses in anti-VEGF treatment among RVO patients is lacking. The purpose of this study is to analyse the anatomic and visual outcomes from a lapse in anti-VEGF treatment in patients with RVO.
Methods
This retrospective case-control study evaluated 136 patients diagnosed with RVO and treated with anti-VEGF between January 2012 and June 2020 at Cole Eye Institute, Cleveland Clinic. Patients were divided into two cohorts: RVO patients with no lapse in anti-VEGF treatment (control group) and RVO patients with a lapse ≥3 months (lapse group). Central subfield thickness (CST) and best corrected visual acuity (BCVA) were collected pre-lapse, the first appointment post-lapse, and at 3-, 6-, and 12-month follow-up appointments.
Results
Lapse patients (n = 68) and control patients (n = 68) had similar pre-lapse CST (p = 0.466) and BCVA (p = 0.303). Lapse patients experienced a significant increase in CST after discontinuing anti-VEGF therapy (lapse: 400.6 ± 192.1 µm, control: 333.0 ± 111.1 µm, p = 0.024). This persisted 12 months post-lapse after re-initiation of anti-VEGF agents (lapse: 381.6 ± 161.1 µm, control: 307.5 ± 95.4 µm, p = 0.030). Lapse patients also experienced a decrease in BCVA after lapse (lapse: 54.3 ± 25.1 ETDRS, control: 64.4 ± 17.8 ETDRS, p < 0.001) that recovered after 6 months of anti-VEGF treatment.
Conclusions
RVO patients with any lapse of anti-VEGF treatment are at risk for poorer anatomic and visual outcomes. Though BCVA normalizes upon treatment resumption, patients experience a statistically significant increase in CST that does not recover.
Similar content being viewed by others
Introduction
Retinal vein occlusion (RVO) affects ~16.4 million people globally and is the second leading cause of vision loss due to retinal vascular disease [1]. The two major types of RVO are branch retinal vein occlusion (BRVO) and central retinal vein occlusion (CRVO), depending on the location of venous occlusion. When retinal veins are blocked by a thrombus, the retina becomes ischemic and upregulates vascular endothelial growth factor (VEGF). VEGF induces endothelial cell growth, leading to neovascularization. In pericyte-deprived vessels of the retina, vascular leakage and macular oedema can occur and manifest as vision loss.
Intravitreal anti-vascular endothelial growth factor injections have become the first-line therapy for macular oedema in the setting of RVO. Prior to the use of anti-VEGF injections, laser photocoagulation was the primary RVO macular oedema treatment. However, while laser photocoagulation has been shown to be effective in treating BRVO related macular oedema, there is no visual benefit in using laser photocoagulation in cases of CRVO related macular oedema [2, 3]. Anti-VEGF intravitreal injections of bevacizumab, ranibizumab, and aflibercept have supplanted laser in long-term efficacy and safety given the results from several clinical trials such as BRAVO, CRUISE, GALILEO and COPERNICUS [4,5,6,7].
A potential challenge for RVO patients is to maintain care with a high frequency of intravitreal injections required for anti-VEGF therapy. While some patients receive injections at extended intervals (e.g. on a pro re nata basis or treat-and-extend protocol), it is common for patients to require injections every 4–12 weeks [8]. With a demanding schedule as well as other healthcare barriers, many RVO patients are lost to follow up (LTFU) [9,10,11].
The effect of lapses in anti-VEGF treatment has not been studied in detail for RVO. A retrospective cohort study by Gao and Obeid found that 25.4% of 3400 RVO patients were LTFU [9]. The OCEAN study also found that after 12 months, 26% of RVO patients discontinued ranibizumab treatment and after 24 months, 44% discontinued treatment [10]. However, these studies did not investigate retinal or visual changes of patients who eventually returned for further anti-VEGF treatment. This study aims to examine retinal and visual changes resulting from discontinuation of anti-VEGF treatment (≥3 months) in macular oedema secondary to BRVO and CRVO. Retinal thickness and visual acuity (VA) are evaluated immediately post-lapse and after a year of follow up with anti-VEGF treatment to evaluate the effect of resumption of treatment.
Methods
This retrospective case-control study was approved by the Cleveland Clinic Investigational Review Board. Because of the retrospective nature of the study, written informed consent was not required. This study examined treatment-naïve patients 18 years or older receiving anti-VEGF injections for macular oedema secondary to BRVO or CRVO between January 2012 and June 2020. Patients with a lapse in evaluation and anti-VEGF treatment of 3 months or greater were included. Patient with a concomitant maculopathy (e.g. diabetic retinopathy, age-related macular degeneration, angioid streaks), treatment at an outside institution, lapse per provider recommendation (i.e. pro re nata or treat-and-extend protocol), or a complete loss to follow up were excluded. Only the earliest lapse and the first eligible eye per patient were evaluated.
A comprehensive chart review was performed. Baseline demographics including age, race, gender, and eye laterality were collected. Visual outcomes were measured by Snellen best corrected visual acuity (BCVA), and retinal thickness was measured by central subfield thickness (CST) on spectral domain optical coherence tomography. Snellen BCVA was converted to Early Treatment Diabetic Retinopathy Study (ETDRS) letters using the equation 85 + 50*log10 (Snellen BCVA). BCVA and CST were evaluated at five time points for each patient – pre-lapse (visit date preceding lapse in care/baseline visit), post-lapse (visit date following return from lapse), and follow-up visits at 3, 6 and 12 months. Additionally, the lapse length, reason for lapse, specific type of anti-VEGF injection, and total number of injections between the post-lapse and 12-month follow up were noted for each patient.
Patients with a lapse in anti-VEGF treatment were matched one-to-one with control patients who did not lapse in care but met all other inclusion and exclusion criteria. Importantly, control patients had a simulated lapse length equal to the lapse length of patients who experienced a true lapse. Thus, control patients were still assessed on visit dates that were consistent with lapse patients. Lapse and control patients were paired based on the following criteria: (1) Time between first ever anti-VEGF injection and pre-lapse visit; (2) Pre-lapse BCVA. Matching these values ensured similar baseline factors between groups including visual acuity and number of anti-VEGF injections as established in previous studies [12, 13]. The type of anti-VEGF injection and total number of injections were also recorded for controls. A total of 68 control patients were matched with 68 lapse patients. The selection process for patients included in the study is shown in Fig. 1.
Statistical analysis was conducted using R Statistical Software (version 3.6.1, Vienna, Austria). Continuous variables were expressed as means and standard deviations and compared using paired t tests and independent sample tests. Categorical variables were expressed as counts and percentages and compared using Pearson’s chi-squared (χ2) and Fisher’s exact test. Non-parametric equivalent tests (i.e. Wilcoxon signed rank test) were completed for continuous variables that did not demonstrate a normal distribution. Linear mixed model regressions were fitted to analyse the effect of individual variables on BCVA and CST among lapse and control patients. The variables in these models were: group (lapse vs control), time point, and group/time point interaction as fixed effects. Pre-lapse BCVA and CST were not used as independent terms but as additional time points, allowing for a more natural representation of the data. Patient identification numbers were included as a random effect to account for repeated measures among patients.
Results
A total of 136 eyes for 136 patients were included in this study with 68 lapse patients and 68 control patients. Baseline demographics for the two groups can be found in Table 1. The mean age of the patients was 69.3 ± 11.0 in the lapse group and 73 ± 12.4 in the control group (p = 0.035). In both groups, most patients were white and female. The total number of anti-VEGF injections post-lapse was significantly higher in the control group at 5.4 ± 3.2 than in the lapse group at 3.9 ± 2.6 (p = 0.003). The lapse length in the lapse group was 7.8 ± 10.5 months while the simulated lapse length in the control group was 7.8 ± 10.5 (p = 0.660). Lapse lengths experienced by patients in the lapse group are shown in Supplementary Fig. 1.
Confounding variables were controlled for by a one-to-one matching process of lapse and control patients. The two matching criteria included: time elapsed from their first anti-VEGF injection to their pre-lapse visit and pre-lapse BCVA. The time to lapse was 11.9 ± 14.1 months in both the lapse group and the control group (p = 1.00). The initial pre-lapse BCVA (ETDRS) in the lapse group was 63.3 ± 17.8 on average while it was 63.6 ± 19.1 (p = 0.303). CST values pre-lapse were not matched, but values were similar for both groups at the pre-lapse visit. The lapse group had an average retinal thickness of 346.6 ± 128.4 μm and the control group had an average of 364.2 ± 140.0 μm (p = 0.466).
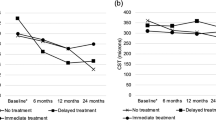
The mean values for BCVA and CST at each time point were compared between the lapse and control groups (Fig. 2, Supplementary Table 1). Immediately post-lapse, the lapse group had a BCVA of 54.3 ± 25.1 ETDRS while the control group had a BCVA of 64.4 ± 17.8 (P < 0.001). The difference was noted at 3 months (p = 0.004) but was no longer significant at 6 months or 12 months. There were also significant differences in CST between the two groups. The lapse group had an average retinal thickness of 400.6 ± 192.1 µm immediately post-lapse while the control group had an average retinal thickness of 333.0 ± 111.1 µm (p = 0.024). This increase persisted at 6 months (p = 0.042) and 12 months post-lapse (p = 0.030).
Linear mixed model regressions for BCVA and CST values matched raw analysis findings. According to the model, the lapse group had a −9.25 ± 5.49 ETDRS letter decrease at post-lapse compared to the control group (P < 0.001). However, although the raw analysis detected a difference in BCVA of −5.70 letters (p = 0.004) between the two groups at 3 months, the linear mixed model regression did not detect this. The model was consistent with the raw analysis demonstrating regained visual acuity 6 months and 12 months post-lapse in the lapse group (Table 2). For CST values, a mixed model validated the results seen in the raw analysis, displaying a significant increase in macular thickness immediately post-lapse that persisted at 6 months and 12 months in the lapse group but not in the control group (Table 3).
Secondary analysis assessed whether RVO type has an effect on BCVA and CST values. Patients were stratified by CRVO (n = 33) and BRVO (n = 35) diagnosis. Both CRVO and BRVO linear mixed model regressions show that both subgroups follow the trends of the pooled data. Lapse CRVO patients demonstrated a significant decrease in vision of −8.76 ± 8.69 ETDRS letters immediately post-lapse (p = 0.048) (Supplementary Table 2). This group also demonstrated an increase in CST at 6 months post-lapse (p = 0007) and 12 months post-lapse (P < 0.001) with a significant increase immediately post-lapse (p = 0.011) (Supplementary Table 3). Similarly, BRVO lapse patients demonstrated a significant decrease of −10.27 ETDRS letters immediately post-lapse (p = 0.001) (Supplementary Table 4). These patients showed no significant change in CST values at any time point (p = 0.015). (Supplementary Table 5).
Discussion
The results of this study demonstrate the negative impacts of an unintended lapse (≥3 months) in anti-VEGF treatment for macular oedema in RVO patients. Both the raw data analyses and mixed model regressions show significantly decreased visual acuity and increased retinal thickness immediately post-lapse. Nearly two lines of vision, −9.25 ± 5.49 ETDRS, are lost, and retinal thickness, on average, increases over 67 μm in patients who lapse in care. Upon resuming anti-VEGF treatment, visual acuity recovers although retinal thickness remains increased at 6 months and 12 months post-lapse.
The discrepancy between visual and anatomical recovery in retinal disease is not uncommon. Previous studies have found that retinal thickness only accounts for a small proportion of change in visual acuity post anti-VEGF therapy in other conditions. The results of a randomized control trial by Ying et al. evaluating the use of anti-VEGF for treatment of choroidal neovascularization secondary to age-related macular degeneration showed poor correlation between VA and CST [14]. Similarly, a study on anti-VEGF treatment of diabetic macular oedema noted no association between VA and CST [15]. Although VA and CST likely have little association due to the multifactorial nature of increased retinal thickness, their relationship in RVO remains to be investigated [16]. The results of this study indicate that while decreased visual acuity can be reversed by resuming anti-VEGF therapy, increased retinal thickness from a lapse in care can persist long-term.
Few studies examine how a lapse in anti-VEGF treatment affects visual outcomes when comparing treatment for macular oedema in the setting of CRVO and BRVO. While the two types of retinal vein occlusion present with a different pathophysiology, Brown et al. and Campochiaro et al. showed anti-VEGF effectively treats macular oedema and restores vision loss in both [4, 5]. BRVO and CRVO differ in that BRVO patients who delayed anti-VEGF treatment for six months were able to recover with additional follow up, but CRVO patients were not [17, 18]. There may be several reasons as to why our study differs from these findings. In previous studies, patient populations differed significantly in patient demographics including age, race, and baseline BCVA. The patients in this study had prior anti-VEGF injections while patients in Campochiaro et al. were excluded if they had anti-VEGF 3 months prior to the study. Previous studies have also found that patients with RVO who began anti-VEGF therapy 6 months after diagnosis were less likely to achieve clinically significant visual gains than patients who immediately began treatment [15, 16]. This observation likely results from prolonged oedema and irreversible retinal damage caused by undertreatment. This study’s results expand on previous literature in that lapses as short as 3 months resulted in ocular effects some of which persisted to the end of our study.
The number of anti-VEGF injections and average age of the two groups are potential confounding factors. The lapse group had an average age of 69.3 ± 11.0 years while the control group had an average age of 73 ± 12.4 years (p = 0.035). Despite the lapse group being 4 years younger on average than the control group, they experienced worse visual and anatomical outcomes than the control group. Within the 12-month post-lapse period, control patients received significantly more anti-VEGF injections than control patients (control = 5.4 ± 3.2, lapse = 3.9 ± 2.6, p = 0.003). While this would not affect the immediate post-lapse results, it could affect values obtained during follow-up visits at 3, 6, and 12 months. One would hypothesize that less intensive anti-VEGF therapy in the lapse patients may have contributed to the persistent increase in retinal thickness at 6 months and 12 months. However, when constrained mixed model regressions were constructed, the number of anti-VEGF injections were not found to have a significant effect on either CST or BCVA, further demonstrating that a lapse in treatment of 3 months has severe consequences in this routine clinical practice study.
This study helps provide a more complete picture of the variety and magnitude of the effects of a lapse in treatment of RVO patients with anti-VEGF injections. A 2019 retrospective cohort study by Gao et al. found that out of 3400 patients with RVO, 25.4% were LTFU, and of those, 8.6% eventually returned for treatment [9]. With 16.4 million patients with RVO worldwide, this translates to over 1.4 million potential patients experiencing a lapse in treatment. These results suggest that visual deficits and retinal damage result from a lapse in care and stress the importance of early intervention and frequent anti-VEGF treatment for RVO.
Strengths of this study include its one-to-one patient matching protocol and well-defined criteria for a lapse in treatment. The effects of confounding variables were reduced by matching lapse and control patients based on baseline visual acuity and treatment timeline. Additionally, this study defined a lapse as ≥3 months of discontinuation of anti-VEGF treatment against provider recommendation. A study by Greenlee et al. utilized similar methods in measuring visual acuity and CST outcomes in the discontinuation of anti-VEGF treatment in neovascular age-related macular degeneration [13]. They found patients who discontinued anti-VEGF treatment for ≥3 months experienced diminished VA and increased retinal thickness immediately post-lapse, as did this study in RVO patients. Unlike this study, they found that VA persisted 12-months post-lapse and that retinal thickness decreased upon resumption of treatment.
This study is limited by its retrospective nature, a relatively small sample size, and evaluation of only each patient’s first lapse. As a retrospective chart review, this study was unable to control for all possible confounding factors. The one-to-one matching process ensured that control and lapse patients had similar baseline characteristics and were assessed at similar time points, thereby eliminating possible confounders. However, this matching process also has the potential to introduce selection bias into the control group. These effects were reduced by randomly selecting control patients and solely using the three matching criteria while adhering to the same inclusion and exclusion criteria as lapse patients. Additionally, patients in the lapse group were not screened for a second lapse. However, given the normalization of VA after resuming treatment, it is unlikely that patients with multiple lapses skewed the data significantly.
Classification of type of RVO was conducted by investigator review of the chart. In a small number of cases, wide-field fluorescein angiography was used to determine the type of RVO as well as the ischemic status. Due to the small sample size, a comparison of ischemic status would likely not yield significant results nor was the study powered to show a difference between these groups. Nonetheless, from recent studies it appears that ischemic status does not influence response to treatment. The VIBRANT study did evaluate this is a prospective fashion and found little difference in the final visual acuity or anatomy in patients by perfusion status [19]. On the contrary, a study by Spooner et al. showed that anti-VEGF treatment resulted in greater VA gains in patients with non-ischemic CRVO than ischemic CRVO at 8 years post occlusion [20]. This data suggests that non-ischemic CRVO and ischemic CRVO may be affected differently by a lapse in anti-VEGF treatment. Thus, the true effect of perfusion status on lapse outcomes is not known and might result in either an over or underestimate of the impact of final vision and anatomic outcomes.
This retrospective case-control study in a routine clinical practice demonstrates RVO patients who experience a lapse in care are susceptible to an immediate decrease in visual acuity and a long-lasting increase in retinal thickness. Furthermore, the time required for a lapse in anti-VEGF treatment to cause a long-term change in retinal thickness can be short. These findings, in conjunction with previous literature, underscore the importance of creating treatment plans for RVO patients as well as educating them on this condition and its prognosis. Future studies could focus on the effects of treatment lapse on outcomes years after the initial lapse. Additionally, the effect of multiple lapses against a singular lapse and no lapse should be explored.
Summary
What was known before
-
Anti-vascular endothelial growth factors are the primary treatment for retinal vein occlusion.
-
The effectiveness and safety of these treatments is well documented, but knowledge about the effect of lapses in treatment is unknown.
What this study adds
-
This study looks at anatomical and visual outcomes after lapses in anti-VEGF treatment in patients with retinal vein occlusion.
-
This study will provide insight on the impact of lapses in treatment and resumption of treatment.
References
Rogers S, McIntosh R, Cheung N, Lim L, Wang J, Mitchell P, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–9.
Clarkson J, Gass J, Curtin V, Norton E, Blankenship G, Flynn H, et al. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 2018;196:xxx–viii. Online ahead of print.
Clarkson J, Chuang E, Gass D, Pedroso M, Cubillas T, Duria E, et al. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology. 1995;102:1425–33.
Brown D, Campochiaro P, Singh R, Li Z, Gray S, Saroj N, et al. Ranibizumab for macular edema following central retinal vein occlusion. Ophthalmology. 2010;117:1124–33.
Campochiaro P, Heier J, Feiner L, Gray S, Saroj N, Rundle A, et al. Ranibizumab for macular edema following branch retinal vein occlusion. Ophthalmology. 2010;117:1102–12.
Ogura Y, Roider J, Korobelnik J, Holz F, Simader C, Schmidt-Erfurth U, et al. Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the phase 3 GALILEO study. Am J Ophthalmol. 2014;158:1032.
Heier J, Clark W, Boyer D, Brown D, Vitti R, Berliner A, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion. Ophthalmology. 2014;121:1414–20.
Heier J, Campochiaro P, Yau L, Li Z, Saroj N, Rubio R, et al. Ranibizumab for macular edema due to retinal vein occlusions. Ophthalmology. 2012;119:802–9.
Gao X, Obeid A, Adam M, Hyman L, Ho A, Hsu J. Loss to follow-up in patients with retinal vein occlusion undergoing intravitreal anti-VEGF injections. Ophthalmic Surg, Lasers Imaging Retin. 2019;50:159–66.
Callizo J, Ziemssen F, Bertelmann T, Feltgen N, Voegeler J, Koch M, et al. <p>Real-world data: ranibizumab treatment for retinal vein occlusion in the OCEAN Study</p>. Clin Ophthalmol. 2019;13:2167–79.
Hogg H, Talks S, Pearce M, Di Simplicio S. Real-world visual and neovascularisation outcomes from anti-VEGF in central retinal vein occlusion. Ophthalmic Epidemiol. 2020;28:70–6.
Yalamanchili S, Maatouk C, Enwere D, Conti T, Hom G, Briskin I, et al. The short-term effect of a single lapse in anti–vascular endothelial growth factor treatment for diabetic macular edema within routine clinical practice. Am J Ophthalmol. 2020;219:215–21.
Greenlee T, Wang V, Kang H, Ohlhausen M, Chen A, Hom G, et al. Consequences of lapses in treatment with vascular endothelial growth factor inhibitors in neovascular age-related macular degeneration in routine clinical practice. Retina. 2020;41:581–7.
Ying G, Maguire M, Daniel E, Ferris F, Jaffe G, Grunwald J, et al. Association of baseline characteristics and early vision response with 2-year vision outcomes in the comparison of AMD treatments trials (CATT). Ophthalmology. 2015;122:2523–31.
Bressler N, Odia I, Maguire M, Glassman A, Jampol L, MacCumber M, et al. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab. JAMA Ophthalmol. 2019;137:977.
Danis RP, Sadda S, Jiao J, Li X-Y, Whitcup SM. Relationship between retinal thickness and visual acuity in eyes with retinal vein occlusion treated with dexamethasone implant. Retina.2016;36:1170–6.
Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–602.
Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–9.
Campochiaro PA, Clark WL, Boyer DS, Heier JS, Brown DM, Vitti R, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015;122:538–44.
Spooner KL, Fraser-Bell S, Hong T, Wong JG, Chang AA. Long-term outcomes of anti-VEGF treatment of retinal vein occlusion. Eye. 2021.
Acknowledgements
Special thanks to Justin Muste for editing and Catherine Urbano for data collection.
Author information
Authors and Affiliations
Contributions
PA and JCL were responsible for writing the report, conducting the search, screening potentially eligible patients, extracting and analysing data, interpreting results, updating reference lists, and creating figures and tables. AII contributed to conducting the search and analysing data. PMK was responsible for writing the protocol. RPS provided feedback on the report.
Corresponding author
Ethics declarations
Competing interests
RPS reports personal fees from Genentech/Roche, Alcon/Novartis, Zeiss, Bausch + Lomb, Ophthea, and Regeneron Pharmaceuticals, Inc., grants from Apellis and Graybug. Other authors have no financial disclosures to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J.C., Alsaloum, P., Iyer, A.I. et al. Consequences of anti-vascular endothelial growth factor treatment lapse in patients with retinal vein occlusion. Eye 37, 453–458 (2023). https://doi.org/10.1038/s41433-022-01960-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-01960-4