Abstract
Purpose
To investigate retinal perfusion by optical coherence tomography (OCT)-angiography and choroidal vascular features using an OCT-based image binarization method in patients with Behçet’s disease (BD) without clinical ocular involvement.
Methods
This study included 38 patients with non-ocular BD and 35 healthy participants. Macular region was evaluated with OCT-angiography (Optovue, Inc., Fremont, CA). A 6.0 × 6.0 mm rectanglescan centred on the fovea was used to record the scans. The enhanced depth imaging OCT scans (Heidelberg Eye Explorer version 1.8.6.0; Heidelberg Engineering) of the macula and peripapillary scans of the optic nerve head were binarized using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The luminal area (LA), stromal area (SA), total choroidal area (TCA), and choroidal thickness (CT) were analysed. The choroidal vascularity index (CVI) was calculated as the ratio of the LA to the TCA.
Results
Vessel density values of the superficial and deep macular capillary plexus were similar between the two groups (all p > 0.05). There was no significant difference between the groups in terms of the CT and TCA values in the macula and in all peripapillary sectors (all p > 0.05). However, the macula and temporal, nasal, and inferior sectors of the peripapillary area had significantly lower CVI values in the BD group compared with controls (p = 0.009, p = 0.002, p = 0.010, and p = 0.008, respectively).
Conclusions
Retinal microperfusion deficit was not observed in non-ocular Behçet patients. CVI may be a more robust marker than CT to indicate choroidal perfusion. A reduced CVI may suggest subclinical ocular involvement and choroidal ischemia in these patients.
Similar content being viewed by others
Introduction
Behçet’s disease (BD) is a chronic, autoinflammatory, and multi-system disorder that affects the skin, mucous membranes, and ocular, articular, pulmonary, gastrointestinal, genital, and central nervous systems [1]. The main pathology in BD is a systemic occlusive and necrotizing vasculitis that may involve any size and type of blood vessel [2]. The condition was named after in 1937 by Hulusi Behçet as a syndrome characterized by recurrent aphthous ulcers in the oral and genital areas and also ocular inflammation [3].
The frequency of ocular involvement in BD ranges from 60–80% [4]. Ocular involvement occurs mostly in a bilateral asymmetric manner and develops within an average of five years of the disease onset [5]. The classical pattern of ocular involvement is a relapsing-remitting acute onset panuveitis and retinal vasculitis. Other ocular findings include non-granulomatous keratic precipitates, mobile hypopyon, posterior synechia, vitritis, retinitis, vasculitis, optic disc oedema, optic atrophy, disc or retinal neovascularization, and choroiditis [6, 7].
The choroid is the vascular layer of the eye and contains 85% of the eye’s total blood flow [8]. It plays a role in the blood supply of the prelaminar, laminar, and retrolaminar regions of the optic nerve head and the outer one-third of the retinal segments. Conditions that cause disruption in choroidal circulation may lead a predisposition to ocular disorders [9, 10]. Choroidal thickness (CT) alterations were detected in non-ocular BD cases [11,12,13,14,15]. Furthermore, optical coherence tomography (OCT)-angiography imaging also revealed impaired perfusion and decreased vessel density in the retinal capillary plexus and choriocapillaris of patients with non-ocular BD [12, 16, 17]. The choroidal vascularity index (CVI), first described by Agrawal et al., is a novel non-invasive marker of the choroid [18]. The choroidal region can be divided into a stromal area (SA) and a luminal area (LA) using an image binarization tool, which allows the vascular status to be analysed in detail. The CVI is currently being comprehensively investigated, particularly for its role in various retinal and choroid diseases [19, 20]. However, the macular and peripapillary CVI in patients with non-ocular BD has not yet been investigated. In the present study, our aim was to explore whether retinal and choroidal vascular changes took place during the period without clinically ocular involvement, and also to compare these results with those obtained from control participants.
Materials and methods
Study design and participants
This retrospective, cross-sectional, and comparative study evaluated patients with BD without ocular involvement as the patient group and similar healthy participants as the control group. This research was approved by the Ethics Committee of Yuksek Ihtisas University, Medical Park Hospital, Ankara, Turkey (Report number: 2020-06-02). All study procedures were conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all participants.
Inclusion-exclusion criteria and examinations
The diagnosis of BD was primarily based on the International Criteria for Behçet’s Disease [21]. According to these criteria, ocular lesions, genital aphthosis, or oral aphthosis were scored as two points, and skin lesions, vascular manifestations, or neurological manifestations were scored as one point. A total score greater than or equal to four points indicated BD. This study evaluated patients confirmed to have BD who were referred by a rheumatology clinic to our tertiary eye care centre for ocular involvement examinations. Only patients without ocular involvement were included in this study. The control participants were selected from age-matched and sex-matched individuals, who had visited our outpatient clinic for routine ocular examination and had no other ocular/systemic disease conditions. The right eye characteristics of all participants were included in the analysis.
Participants with the following situations were not included in the current study: past findings of a uveitis attack, including inflammatory cells in the anterior chamber, keratic precipitates, posterior synechia, iris pigments on the anterior lens capsule, vitreous opacities, optic disc pallor or optic atrophy, retinal vascular sheathing or sclerosis, and neovascularization of the disc or elsewhere; a history of ocular/orbital trauma; any previous intraocular operation or laser intervention; a prior history of any types of optic neuropathies; any type of retinal or choroidal diseases; a spherical and/or cylindrical refractive error >3 diopters (D); an axial length (AL) >24 mm and <22 mm; a best-corrected visual acuity (BCVA) <20/20; any systemic hematological or immunological disorders; using medication; and smoking or alcohol/drug abuse that might have induced vascular dysfunction.
The BD and control cases underwent a detailed ophthalmologic examination, including an anterior segment and a dilated fundus examination using a +90 D lens. The demographic data of participants, their BCVA values as measured with a Snellen chart (20 ft), and intraocular pressure (IOP) values obtained using Goldmann applanation tonometry were recorded. The AL and central corneal thickness (CCT) were measured with a Lenstar LS 900 (Hagg-Streit AG, Koeniz, Switzerland). The spectral domain (SD)-OCT (Spectralis HRA + OCT, Heidelberg Engineering GmbH, Heidelberg, Germany) was applied to all participants. When needed, standard automated perimetry analysis (Humphrey Visual Field Analyzer 750i; Carl Zeiss Meditec Inc., Dublin, CA, USA), fundus autofluorescence (Spectralis HRA + OCT, Heidelberg Engineering GmbH, Heidelberg, Germany), and fluorescein angiography (FA; KOVA imaging systems, Japan) were performed in patients with a suspected diagnosis to exclude ocular involvement.
Optical coherence tomography angiography imaging
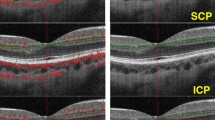
Optical coherence tomography angiography (OCT-A; RTVue XR Avanti, AngioVue software, version 2017.1.0.151; Optovue, Inc., Fremont, CA) was conducted by the same experienced physician. A 6.0 × 6.0 mm rectangle scan centered on the fovea was used to record the OCTA scans. All scans were reviewed in terms of image quality. Patients with poor image features, such as a low signal strength index (<8/10), blink artifacts, poor fixation causing residual motion or doubling artifacts, poor clarity, media opacity obscuring the view of the vasculature, and segmentation errors, were scanned again. Only images with signal strength indexes ≥8/10 were used for the quantitative assessment. The device automatically inserted three fovea-centered circles on the macula via a density assessment tool in both the superficial-capillary (SCP) and deep-capillary plexus (DCP).
Optical coherence tomography imaging
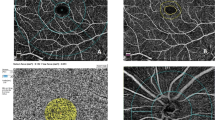
The enhanced depth imaging (EDI) mode of SD-OCT was performed by the same experienced medical technician. All OCT measurements were conducted at the same time of day (between 09:00 and 11:00 a.m.) to avoid diurnal fluctuations. All scans were obtained following pupil dilation. The macular images were acquired using a horizontal scan centred on the central foveal region (Fig. 1B). The peripapillary images were acquired using a 3.4 mm diameter, 360-degree circle scan centred on the optic nerve head (Fig. 2A). Only high-quality scans (>25 Q) were evaluated. The images were viewed and measured using Heidelberg Eye Explorer software (Heidelberg Eye Explorer version 1.8.6.0; Heidelberg Engineering).
A Horizontal macular scan centered on the fovea. B Submacular choroidal imaging on the scan, submacular choroidal imaging on the scan. C The binarized image to view the choroid-scleral junction using the autolocal threshold tool (Niblack method, 8-bit type) and the circumscribed total choroidal area using the polygon tool. D Yellow lines representing the luminal area (dark pixels) using the color threshold tool.
Choroidal thickness (CT) measurements were obtained manually from the macula and peripapillary scans. In the macular region, these dimensions were calculated from the subfovea and also 1000 µm temporal, 2000 µm temporal, 1000 µm nasal, and 2000 µm nasal to the fovea [22]. In the peripapillary area, these measurements were assessed using the midlines of the temporal, superior, nasal, and inferior sectors (0°, 90°, 180°, and 270° positions on TSNIT graph, respectively) [23]. The CT was measured as the vertical distance between the outer border of the hyperreflective retinal pigment epithelium and the inner surface of the choroid-scleral junction. Finally, the OCT scans were evaluated with an image-processing program for advanced analysis.
Image acquisition and processing
Image processing was performed using open-source software (http://fiji.sc/). OCT images were binarized using the protocol described by Agrawal et al. [18]. Briefly, the OCT scan was opened using ImageJ software (version 1.53a; National Institutes of Health, Bethesda, MD, USA). First, the scan was converted into an 8-bit type and then binarized to view the choroid-scleral junction using the autolocal threshold tool (Niblack Method). The circumscribed total choroidal area (TCA) was selected as the region between the retinal pigment epithelium and the choroid-scleral junction using the polygon tool (Fig. 1C). This region was saved to the region of interest (ROI) manager. Next, the scan was converted to a Red-Green-Blue colour type. The dark-coloured pixels representing the LA were selected using the colour threshold tool and saved to the ROI manager. Both areas in the ROI manager were selected and merged using an “AND” command (Fig. 1D).
In addition, the peripapillary scans were divided into four sectors by a 90° angle and binarized separately, as described previously by Pellegrini et al. [24] and Park et al. [25]. These sectors consisted of temporal, superior, nasal, and inferior areas (315°–45°, 45°–135°, 135°–225°, and 225°–315° intervals on TSNIT graph, respectively; Fig. 2A–C).
The CVI was calculated as the ratio of the LA to the TCA. The number of light-colored pixels expressing the SA was calculated by subtracting the LA from the TCA. All these measurements were carried out separately by two researchers blinded to the diagnosis of the study participants (MS and MA). The means of both values were included in the statistical analysis.
Statistical analysis
Statistical analyses were executed with the Statistical Package for the Social Sciences (SPSS) software program, version 22 for Windows (SPSS Inc., Chicago, IL, USA). The visual (histograms and probability graphs) and analytical (Kolmogorov–Smirnov/Shapiro–Wilk test) methods were performed to evaluate normality of distribution. The numerical variables were presented as mean value and standard deviation, the categorical variables were presented as proportion. An independent samples t-test was used to compare the choroidal thickness and vascularity parameters between the groups. The Chi-square test was employed to analyse the categorical variables. Correlation coefficients and their significance were calculated using the Pearson test. A p-value of <0.05 was considered statistically significant.
Results
This study enrolled 38 eyes of 38 patients with BD (23 male and 15 female) and 35 eyes of 35 control participants (21 male and 14 female; p = 0.810). The mean age of the BD group was 34.6 ± 5.7 years, while the mean age of the control group was 33.5 ± 5.1 years (p = 0.732). The BCVA value was 20/20 according to the Snellen chart in both groups. The mean disease duration was 7.1 ± 2.5 (5–12) years in the BD group. Of the BD patients, 38 had oral aphthosis, 28 had genital aphthosis, 19 had papulopustular lesions, 14 had erythema nodosum, 7 had vascular involvement, and 5 had neurological involvement. The IOP, AL, spherical equivalent, and CCT did not differ significantly between the groups (p = 0.401, p = 0.573, p = 0.126, and p = 0.378, respectively; Table 1). Furthermore, the macular and peripapillary CT values were not significantly different between the BD and control groups (all p > 0.05; Table 2).
Table 3 shows the choroidal vascular measurements of the submacular and peripapillary regions between the groups. In the macula and peripapillary regions, the TCA was not significantly different between the BD and control groups (all p > 0.05). The SA values were higher in the macula and the temporal, inferior, and global peripapillary sectors in the BD cases compared to the controls (p = 0.017, p = 0.026, p = 0.019, and p = 0.044, respectively). The eyes with BD had significantly lower LA values in the temporal and nasal peripapillary sectors than in the control eyes (p = 0.035 and p = 0.041, respectively). The submacular CVI value was lower in the BD group compared to the control group (67.38 ± 2.91 vs. 70.64 ± 2.80; p = 0.009). The mean CVI values of the temporal, nasal, and inferior peripapillary sectors were lower in the BD group compared with the control group (65.11 ± 3.04 vs. 68.73 ± 2.87, p = 0.002; 65.59 ± 2.88 vs. 67.42 ± 2.56, p = 0.010; and 64.81 ± 2.83 vs. 67.01 ± 2.77, p = 0.008, respectively). Although the mean CVI value of the superior peripapillary sector was lower in the BD group than it was in the control group (66.40 ± 2.83 vs. 67.27 ± 2.69), this value was not statistically significant (p = 0.091).
CVI had intraclass correlation coefficient values (with 95% CI) of 0.901–0.945 interobserver reliability and 0.963–0.995 intraobserver reliability (Table 4).
Vessel density measurements of the superficial and deep capillary plexus did not differ significantly between the two groups in the foveal and parafoveal regions (all p > 0.05; Table 5).
The foveal avascular zone (FAZ) measurements were as follows: The mean diametric size of FAZ in the deep capillary plexus was 0.581 ± 0.09 mm2 in the BD group and 0.575 ± 0.09 mm2 in the control group (p = 0.124), while the mean diametric size of FAZ in the superficial capillary plexus superficial capillary plexus was 0.31 ± 0.09 mm2 in the BD group and 0.32 ± 0.12 mm2 in the control group (p = 0.079). While the subfoveal CT showed an excellent positive correlation with the macular TCA (r = 0.856, p < 0.001, Fig. 3), it had no significant correlation with the LA, SA, and CVI (all p > 0.05).
Discussion
BD is a systemic, immune-mediated occlusive vasculitis and has an inflammatory involvement that progresses with recurrent attacks in multiple organ-systems. The eye is the most commonly involved vital organ, and the visual prognosis is typically poor in affected individuals [6]. Regardless of the presence of ocular involvement, vascular tissue is also an important target in BD, and many vascular manifestations cannot be clinically observed [2]. In the present study, we aimed to explore subclinical changes in the choroidal luminal and stromal areas in BD patients without ocular involvement using the image binarization method in EDI-OCT imaging. To the best of our knowledge, this study is the first to evaluate the CVI in non-ocular BD cases.
The choroid consists of blood vessels and SA that contains melanocytes, fibroblasts, resident immunocompetent cells, and supporting collagenous and elastic connective tissue [26]. The quantitative parameters for the choroid have been comprehensively explored in several ocular diseases and have attracted great attention. The most extensively studied of these markers is the CT measurement. Previous studies have reported contradictory outcomes associated with the CT in non-ocular BD patients. Chung et al. found significant choroidal thickening in the eyes of patients with non-ocular BD compared to the control eyes (310.5 ± 81.0 vs. 256.9 ± 67.9 μm) [11]. Similarly, Comez et al. detected increased CT values in the BD group without ocular involvement compared to the control group (348.59 ± 38.10 vs. 302.97 ± 36.03 μm) [12]. Their study concluded that increased CT values may indicate subclinical ocular involvement due to choroidal inflammation. However, Atas et al. [13] and Yuvaci et al. [14]. reported insignificant differences in the subfoveal CT values between non-ocular BD patients and control groups (325.13 ± 64.63 vs. 325.92 ± 60.03 μm and 331.74 ± 79.60 vs. 325.95 ± 80.17 μm, respectively). Conversely, Mittal et al. demonstrated a thinner mean central subfield CT in non-ocular BD patients when compared with controls (227.5 ± 56.93 vs. 306.85 ± 17.85 μm) [15]. Furthermore, in the same study, the central subfield choroidal volume was lower in the eyes with BD than in the control eyes (0.18 ± 0.04 vs. 0.24 ± 0.02 mm3). The authors suggested that over time, the choroid could become thinner as a result of subclinical choroidal involvement attacks without any apparent ocular involvement.
In this study, there was no difference in terms of the CT and TCA between the patient and control groups. The TCA is composed of the circumscribed total choroidal area and contains both vascular and connective tissues. The CT is a measurement that extends from the retinal pigment epithelium to the choroid-scleral junction and includes components of the vascular area and stromal tissue. Therefore, the CT informs data about the vascular areaindirectly [20]. In addition, both inflammatory changes in the SA and the involvement of vascular tissue may affect the CT and TCA results. Subfoveal CT showed an excellent positive correlation with the macular TCA (r = 0.856, p < 0.001) in the present study. The current study also demonstrated an increase in the SA fraction (temporal and inferior peripapillary sectors, global peripapillary area, and macular area), a decrease in the LA fraction (temporal and nasal peripapillary sectors), and a decrease in the CVI in all regions (except for the superior peripapillary sector) in patients with non-ocular BD. In this respect, the CVI may be a more reliable marker for choroidal involvement in these cases than CT. Agrawal et al. proposed that the CVI could be a novel tool for monitoring the progression of panuveitis [18]. Furthermore, when compared to the CT, the CVI was not associated with most physiological variables, including age, axial length, refractive status, IOP, systolic blood pressure, and diurnal variation [20].
When the enucleated eyes of patients with ocular BD were treated with immunohistochemical staining, macrophage, and T lymphocyte-predominant cellular infiltrates were detected in the choroid [27]. Although there was no clinical ocular involvement in participants of the current study, increased values of SA may result from the inflammatory nature of BD. In addition, the vasculitic course of BD may explain the decrease in the LA and CVI observed in the present study.
Vascular changes were evaluated non-invasively using OCT-angiography imaging in non-ocular BD cases. Rafaat et al. identified capillary nonperfusion areas, perifoveal capillary arcade disruption, vessel rarefaction, and telangiectasia in the superficial and deep capillary plexus [17]. Comez et al. observed lower flow values not only in the superficial and deep capillary plexus but also in the choriocapillaris area when compared with healthy participants [12]. In current study, we found no significant differences in superficial and deep capillary vessel densities. OCT-angiography allows the noninvasive evaluation of the retina and the choriocapillaris flow. However, this approach is insufficient to analyze the remaining choroidal vascular status. Indocyanine green angiography is currently the most valuable method used in the evaluation of choroidal vascularity [28] but is rarely used during follow-up because it is invasive, requires the use of dye during the procedure, and may therefore cause allergic reactions. With the emergence of the latest developments in imaging methods, such as swept-source technology and enhanced depth imaging mode, more detailed information about the choroid can be obtained non-invasively. The CVI value produced by binarization of the stromal and luminal areas provides clear information about the choroidal vascularity.
The present study had some limitations. First, the cross-sectional method used in this study can not provide accurate data on the development of ocular involvement over time in these patients. The second limiting factor of this research, as in other CVI studies, was that some technical limitations could cause CVI variations [20]. Briefly, these include a lower quality of scans, shadowing in the choroid due to larger retinal vascular patterns, a deficiency in the spatial resolution affecting the easy differentiation of the luminal and stromal components of the choriocapillaris with current imaging technology, and the use of a manual measurement-based system. However, only high-quality scans were contained in this study to minimize variations in the CVI.
In conclusion, the increased SA values in non-ocular BD cases may indicate the presence of subclinical inflammation in the stromal layer, including the connective tissue and immune cells. Reduced LA values could suggest choroidal vasculitic involvement. Although there was no statistically significant difference in the CT and TCA, the decrease in CVI may be a marker of choroidal inflammation/involvement in non-ocular BD. A larger series and longitudinal studies are needed to answer the question of whether these parameters can predict ocular involvement that has not yet been clinically observed.
Summary
What was known before
-
Optical coherence tomography angiography imaging also revealed impaired perfusion and decreased vessel density in the retinal capillary plexus and choriocapillaris of patients with non-ocular Behcet disease.
What this study adds
-
We evaluated choroidal vasculature characteristics in patients with Behcet disease without clinically ocular involvement using the optical coherence tomography-based image binarization. A decreased choroidal vascularity index was detected in the macula and peripapillary regions. This novel non-invasive marker can be used to demonstrate the choroidal involvement/inflammation.
References
Hatemi G, Seyahi E, Fresko I, Talarico R, Hamuryudan V. One year in review 2019: Behcet’s syndrome. Clin Exp Rheumatol. 2019;37:3–17.
Davatchi F, Chams-Davatchi C, Shams H, Shahram F, Nadji A, Akhlaghi M, et al. Behcet’s disease: epidemiology, clinical manifestations, and diagnosis. Expert Rev Clin Immunol. 2017;13:57–65.
H B. Über rezidivierende aphthöse, durch ein Virus verursachte Geschwüre am Mund, am Auge und an den Genitalien. Dermatol Monatsschr. 1937;105:1152–7.
Deuter CM, Kotter I, Wallace GR, Murray PI, Stubiger N, Zierhut M. Behcet’s disease: ocular effects and treatment. Prog Retinal Eye Res. 2008;27:111–36.
Kazokoglu H, Onal S, Tugal-Tutkun I, Mirza E, Akova Y, Ozyazgan Y, et al. Demographic and clinical features of uveitis in tertiary centers in Turkey. Ophthalmic Epidemiol. 2008;15:285–93.
Tugal-Tutkun I. Behcet’s Uveitis. Middle East Afr J Ophthalmol. 2009;16:219–24.
Namba K, Goto H, Kaburaki T, Kitaichi N, Mizuki N, Asukata Y, et al. A major review: current aspects of ocular Behcet’s disease in Japan. Ocul Immunol Inflamm. 2015;23:S1–23.
Reiner A, Fitzgerald MEC, Del Mar N, Li C. Neural control of choroidal blood flow. Prog Retinal Eye Res. 2018;64:96–130.
Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retinal Eye Res. 2012;31:377–406.
Wei X, Balne PK, Meissner KE, Barathi VA, Schmetterer L, Agrawal R. Assessment of flow dynamics in retinal and choroidal microcirculation. Surv Ophthalmol. 2018;63:646–64.
Chung YR, Cho EH, Jang S, Lee SY, Lee ES, Lee K. Choroidal thickness indicates subclinical ocular and systemic inflammation in eyes with Behcet disease without active inflammation. Korean J Ophthalmol. 2018;32:290–5.
Comez A, Beyoglu A, Karakucuk Y. Quantitative analysis of retinal microcirculation in optical coherence tomography angiography in cases with Behcet’s disease without ocular involvement. Int Ophthalmol. 2019;39:2213–21.
Atas M, Yuvaci I, Demircan S, Guler E, Altunel O, Pangal E, et al. Evaluation of the macular, peripapillary nerve fiber layer and choroid thickness changes in Behcet’s disease with spectral-domain OCT. J Ophthalmol. 2014;2014:865394.
Yuvaci I, Sirakaya E, Pangal E, Atas M, Bayram N, Guler E, et al. Relationship between erythrocyte sedimentation rate and choroidal and retinal thickness in Behcet’s disease. Arquivos Brasileiros de Oftalmologia. 2019;82:263–9.
Mittal A, Velaga SB, Falavarjani KG, Nittala MG, Sadda SR. Choroidal thickness in non-ocular Behcet’s disease—a spectral-domain OCT study. J Curr Ophthalmol. 2017;29:210–3.
Goker YS, Yilmaz S, Kiziltoprak H, Tekin K, Demir G. Quantitative analysis of optical coherence tomography angiography features in patients with nonocular Behcet’s disease. Curr Eye Res. 2019;44:212–8.
Raafat KA, Allam R, Medhat BM. Optical coherence tomography angiography findings in patients with nonocular Behcet disease. Retina. 2019;39:1607–12.
Agrawal R, Salman M, Tan KA, Karampelas M, Sim DA, Keane PA, et al. Choroidal vascularity index (CVI)—a novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS ONE. 2016;11:e0146344.
Iovino C, Pellegrini M, Bernabei F, Borrelli E, Sacconi R, Govetto A, et al. Choroidal vascularity index: an in-depth analysis of this novel optical coherence tomography parameter. J Clin Med. 2020;9:595.
Agrawal R, Ding J, Sen P, Rousselot A, Chan A, Nivison-Smith L, et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog Retinal Eye Res. 2020;77:100829.
International Team for the Revision of the International Criteria for Behçet’s Disease (ITR-ICBD). The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28:338–47.
Lin Z, Huang S, Huang P, Guo L, Bian H, Zhong Y. Analysis of choroidal thickness in ocular hypertensive patients using enhanced depth imaging optical coherence tomography. Lasers Med Sci. 2018;33:111–21.
Zha Y, Zhuang J, Du Y, Cai J, Zheng H. Evaluation of peripapillary choroidal distribution in children by enhanced depth imaging optical coherence tomography. BMC Ophthalmol. 2018;18:173.
Pellegrini M, Giannaccare G, Bernabei F, Moscardelli F, Schiavi C, Campos EC. Choroidal vascular changes in arteritic and nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2019;205:43–9.
Park JW, Suh MH, Agrawal R, Khandelwal N. Peripapillary choroidal vascularity index in glaucoma—a comparison between spectral-domain OCT and OCT angiography. Investig Ophthalmol Vis Sci. 2018;59:3694–701.
Nickla DL, Wallman J. The multifunctional choroid. Prog Retinal Eye Res. 2010;29:144–68.
Charteris DG, Barton K, McCartney AC, Lightman SL. CD4+ lymphocyte involvement in ocular Behcet’s disease. Autoimmunity. 1992;12:201–6.
Yannuzzi LA. Indocyanine green angiography: a perspective on use in the clinical setting. Am J Ophthalmol. 2011;151:745–51.
Acknowledgements
In memory of Mert Simsek.
Author information
Authors and Affiliations
Contributions
MS was responsible for the study design, data collection and analysis, writing of the manuscript and creation of the figures. MA was responsible for manuscript writing and review. RKU was responsible for parts of the data collection and manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simsek, M., Aksoy, M. & Ulucakoy, R.K. Evaluation of retinal and choroidal microcirculation in Behçet’s disease. Eye 36, 1494–1499 (2022). https://doi.org/10.1038/s41433-022-01932-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-01932-8