Abstract
Background/Objectives
To compare the safety and efficacy of cystotome-assisted prechop phacoemulsification surgery (CAPPS) and femtosecond laser-assisted cataract surgery (FLACS) in patients with hard nucleus cataract.
Subjects/Methods
Ninety-six eyes of 64 patients with grade IV hard nucleus cataract were assigned to 1 of the 2 groups (49 CAPPS and 47 FLACS). Follow-up visits were performed at 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year, and the outcome measures comprised ultrasound power, effective phacoemulsification time (EPT), corrected distance visual acuity (CDVA), endothelial cell density (ECD), corneal endothelium cell loss rate (ECL), central corneal thickness (CCT), and intraoperative and postoperative complications.
Results
The ultrasound power and EPT were lower in the CAPPS group (p = 0.03 and <0.0001, respectively). Patients in both groups gained better CDVA postoperatively. The ECD value decreased at each follow-up visit and did not return to the preoperative level; FLACS resulted in greater endothelial cell loss compared to CAPPS. CCT increased immediately after the surgery and decreased thereafter. The mean CCT value returned to the preoperative level 3 months postoperatively in the CAPPS group, while in the FLACS group, CCT value took 6 months to return to the preoperative level. Miosis was more likely to occur in the FLACS group.
Conclusions
Due to its efficacy and cost-effectiveness, CAPPS is worth promoting and applying to clinical work in the future.
Similar content being viewed by others
Introduction
Cataracts are the leading cause of blindness in the world. The World Health Organization estimated that 95 million people worldwide have developed cataracts [1]. Phacoemulsification (ultrasound) was first introduced over 40 years ago and has become the standard method of cataract surgery today in several countries. Of the steps of conventional phacoemulsification surgery (CPS), lens fragmentation is a crucial part. However, this step is quite difficult in cataract patients with a hard nucleus. Thus, finding a method to perform this effectively in hard nucleus cases has become a promising research field in the treatment of cataracts.
Femtosecond laser-assisted cataract surgery (FLACS), a new technology, was introduced in 2009 [2] and was considered to be the next stage in improving cataract surgery outcomes. FLACS can automate over half of the cataract surgery steps, increase precision and reproducibility of anterior capsulotomy, reduce ultrasound power requirement during phacoemulsification, provide better wound architecture and decrease collateral tissue damage [3,4,5]. However, the effect of FLACS on cataracts with a hard nucleus is controversial and even considered to be somewhat poor [6, 7]. In addition, the high cost associated with FLACS may also be a barrier to its wide use.
Recently, we used a cystotome-assisted prechop method in phacoemulsification. This prechop method is simple and safe, and it works well for patients with a hard nucleus. To date, no study has evaluated and compared the clinical outcomes of cystotome-assisted prechop phacoemulsification surgery (CAPPS) and FLACS for hard nucleus cataracts. Therefore, we performed a comparative study to compare CAPPS and FLACS for cataracts with grade IV hard nuclei to discover which is a better treatment for hard nucleus cataracts.
Materials/subjects and methods
Study design
This comparative, retrospective, cohort study was performed at Xiangya Hospital, Central South University, Changsha, China. We retrospectively reviewed cataract patients with grade IV hard nuclei who underwent CAPPS (CAPPS group) and FLACS (FLACS group). All the patients were given the option to choose either of the methods. All surgeries were completed by the same experienced surgeons (Xiaobo Xia and Weitao Song) between April 2016 and December 2018, and the authors did not have access to information that could identify individual participants during or after data collection. The selected patients consented to the medical treatment and to have their medical records used for research before the study. The study protocol was reviewed and approved by the Central South University Xiangya Hospital Medical Ethics Committee and followed the guidelines set forth in the Declaration of Helsinki.
Inclusion criteria were a minimum age of 18 years and age-related cataract with grade IV hard nucleus. The degree of nucleus hardness was classified according to the Emery-Little classification, and the grade standard is shown in Supplementary Table S1.
The following were the exclusion criteria: (1) endothelial cell density (ECD) < 2000 cells/mm, severe dry eye, corneal scars, corneal dystrophy or any other corneal pathologies; (2) ocular contraindications to FLACS—poorly dilated pupils (<5.0 mm), small hyperopic eyes with steep cornea (difficult to achieve suction), narrow palpebral fissure, severe conjunctival chalasis, nystagmus or lack of cooperation; (3) manifest glaucoma treated with anti-glaucoma drugs or past glaucoma filtration surgery; (4) history of intraocular trauma, surgery or retinal laser procedures; (5) poor cooperation in diagnostic tests or non-compliance at follow-up.
Preoperative assessment and preparation
Before the surgeries, the medical histories of the enrolled patients were recorded. The measurements included the following: corrected distance visual acuity (CDVA); slitlamp (K Series, Keeler Instruments, Inc. Malvern, PA, USA) evaluation, including cornea, lens, cataract grade, and fundus status; tonometry (CT-80, Topcon, Tokyo, Japan), dilated fundus evaluation, corneal topography (Pentacam, Oculus Optikgerate GmbH); axial length and biometry with IOL Masterbiometer (Carl Zeiss Meditec AG, Germany); and OCT (CIRRUS™ HD-OCT 500, Carl Zeiss Meditec AG, Germany). ECD and the percentage of hexagonal cells were analyzed using a non-contact specular microscope (SP 2000P; Topcon, Tokyo, Japan) with the IMAGE-NET imaging system (version 4.0; Topcon). During every visit, three photographs of each cornea were taken and analyzed automatically by IMAGE-NET imaging system, and these examinations were performed by a blinded observer. Anterior chamber depth was also recorded using an Ultrasonic A/B Scan (ODM-2100, MEDA CO., LTD, Tianjin, China). All patients were instructed to apply topical levofloxacin three times for 1 day before the surgery. Pranoprofen was also used three times for 1 day. Moreover, the patients’ pupil was dilated with one drop of tropicamide three times at an interval of 10 min before the surgery.
Surgical technique
All the surgeries were performed by two experienced cataract surgeons. All the patients had implantation of aspheric monofocal IOLs of the same brand (Tecnis ZCB00 intraocular lens, Abbott Medical Optics, Inc.). They were prescribed tobradex four times a day for 2 weeks and pranoprofen four times a day for 1 month.
Cystotome-assisted prechop phacoemulsification
As shown in Fig. 1A, the cystotome was made of a 27-gauge needle by the surgeon, and the length of the cystome was less than 1 mm to be sure not to injure the posterior capsule. After the corneal incisions and capsulorhexis were completed—without hydrodissection and hydrodilineation—the cystotome and the chopper were inserted into the anterior chamber. Figure 1B depicts the key points of the technology: the squeeze force made by the chopper and cystotome transferred to the center of the nucleus and split it. Then, the procedure was repeated until the nucleus was split into four pieces. The prechop should be performed carefully, and the force should be adjusted in order to not harm the posterior capsule and capsulorrhexis edge. Subsequently, the surgeon can perform the conventional phacoemulsification procedure, remove the fragmented crystalline lens using the Stellaris phaco machine (Bausch and Lomb, Rochester, NY, USA) and insert the IOL.
Femtosecond laser-assisted cataract surgery
The Victus Femtosecond Laser Platform (Bausch and Lomb, Rochester, NY, USA) was used in this study. At the beginning of the operation, parameters for the location, structure and depth of the clear corneal incisions were taken into consideration. A radial cut pattern with eight segment cuts was used in the study, and the radial cuts were up to 7 mm long, with a 700-mm posterior safety margin to the posterior capsule. The pre-set energy for anterior capsulotomy was 7000 to 7400 nJ, and nucleus fragmentation varied from 8000 to 9000 nJ. After accurate docking and visualization, the system performed the operation following the standard FLACS procedure [1]. When completing the standard FLACS steps, the surgeon used the Stellaris phaco machine (Bausch and Lomb, Rochester, NY) to complete the phacoemulsification and insert the IOL.
Outcome measures
The ultrasound power and effective phacoemulsification time (EPT) were recorded during the operation, as were the intraoperative complications. After surgery, the patients could choose two professors who were blinded to the study to finish the follow-up visits. The primary postoperative outcome measures were CDVA, ECD, corneal endothelium cell loss rate (ECL), central corneal thickness (CCT), the eye number of different grades of corneal oedema, the grade of anterior chamber flare and intraoperative and postoperative complications. CCT was measured by Pentacam (Oculus Optikgerate GmbH). The grade of corneal oedema was recorded according to the Xie standard [8]. The grade of anterior chamber flare followed the Standardization of Uveitis Nomenclature Working Group [9]. All the outcomes were measured preoperatively and at 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year postoperatively.
Statistical analysis
Statistical analyses were performed using the SAS analytics software (version 9.4, Cary, North Carolina, USA). Differences in baseline variables and intraoperative parameters were assessed with chi-square tests, t-test, or mixed-model analysis of variance (ANOVA), as applicable. Eye-specific data were adjusted for inter-correlation between the two eyes of a single participant using a mixed-effects model with patient numbers as a random effect. In these studies, the repeated-measures analysis of covariance (ANCOVA) model included the factors and covariates of group, value at baseline, time and group-by-time interaction. We used a mixed-model repeated-measures ANCOVA, which adjusted for repeated CDVA, ECD or CCT measures over time for a given eye and the inter-correlation between the two eyes of a single participant. We used a mixed-model measures ANOVA, which adjusted for repeated ECL measures over time for a given eye and the inter-correlation between the two eyes of a single participant. For binomial or multinomial response variables, a generalized linear mixed model was used to compare two treatment groups with patient numbers as a random effect. A p value <0.05 was considered statistically significant.
Results
Ninety-six eyes of 64 patients (49 in the CAPPS group; 47 in the FLACS group) were examined in this study, and none of the patients were lost to follow-up. Demographic data and baseline characteristics of the study population are shown in Supplementary Table S2. The demographic data were similar between patients in the CAPPS and FLACS groups.
Ultrasound power and effective phacoemulsification time
The mean ultrasound power was 13.57 ± 1.46% in the CAPPS group and 14.39 ± 1.54% in the FLACS group. There was a significant difference between the two groups (p = 0.03). EPT was also significantly lower in the CAPPS group (12.36 ± 1.86) than in the FLACS group (14.22 ± 1.27) (p < 0.0001).
CDVA
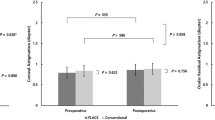
Figure 2 shows the outcomes of CDVA at each follow-up visit. CDVA values were significantly improved from 1 day to 1 year postoperatively in both groups, and the differences were significant (p < 0.0001) (Supplementary Table S3). There were no statistically significant differences in CDVA between groups at each follow-up visit postoperatively (Table 1).
(A) CDVA was significantly improved from 1 day to 1 year in both groups, but there are no significant differences between the two groups. (B) ECD decreased in both groups postoperatively with significant differences from 1 day to 1 year. (C) The ECL percentage was lower in the CAPPS group compared to the FLACS group. (D) CCT increased significantly at 1 day after surgery in both groups. The mean CCT values returned to preoperative levels at 3 months in the CAPPS group, and it took 6 months to return to the preoperative levels in the FLACS group.
ECD
The postoperative changes in ECD are depicted in Fig. 2. ECD decreased in both groups postoperatively with significant differences (p < 0.0001, Supplementary Table S4). While the baseline ECDs were comparable in both groups, the differences in ECD at each follow-up visit between the two groups were statistically significant. We used the following formula: ECD (preoperative) − ECD (postoperative)/ECD (preoperative) = ECL percentage. The ECL percentage was lower in the CAPPS group compared to the FLACS group (Fig. 2), and the differences were highly statistically significant at each follow-up visit postoperatively (Table 2).
CCT
Preoperative CCT values were similar in the two groups (529.27 ± 13.05 and 528.13 ± 12.40 μm, respectively; p = 0.54) and increased significantly at 1 day after surgery in both groups (p < 0.0001). At the 1-week point, the CCT values began to decline (Fig. 2). The mean CCT values returned to preoperative levels at 3 months after surgery in the CAPPS group (p = 0.75), whereas in the FLACS group, it took 6 months to return to the preoperative levels (p = 0.45) (Supplementary Table S5). The differences were highly statistically significant at 1 week, 1 month, 3 months, and 6 months postoperatively between the two groups (Table 3).
Intraoperative and postoperative complications
Supplementary Table S6 displays the intra- and postoperative complications in the two groups. Miosis occurred in eight eyes (17.02%) after the femtosecond laser pretreatment in the FLACS group, while only one case presented in the CAPPS group. In these cases, additional epinephrine hydrochloride (Grandpharma, China co. LTD.) was injected intracamerally during surgery. This difference was significant (p = 0.04). Anterior capsule tear was observed in one eye (2.12%) in the FLACS group. No other adverse events, such as posterior capsule rupture, zonular dehiscence, or vitreous prolapse, occurred during the surgeries.
Significant conjunctival redness or hemorrhage occurred during treatment in two eyes (4.08%) in the CAPPS group and seven eyes (14.89%) in the FLACS group. This difference was not significant (p = 0.11). After surgery, corneal oedema was observed in both groups. There were no significant differences in the number of corneal oedema grading at 1 day and 1 week between the two groups (p = 0.78 and p = 0.21, respectively). Similarly, no statistically significant differences were found in the grading of anterior chamber flare at 1 day and 1 week postoperatively (p = 0.62 and 0.74, respectively). Two of the 49 CAPPS cases and 6 of the FLACS cases developed posterior capsule opacification (PCO) post-surgery, and the difference was not significant (p = 0.17).
Discussion
The treatment of hard nucleus cataract cases is a difficult issue worldwide. This is mainly because the radial suture plane of these lenses tends to have a strong adhesive quality around the posterior epinucleus, forming a dense posterior nucleus plate [10,11,12]. When treating a hard nucleus, more ultrasound power and phacoemulsification time are often required. Damage to the corneal incision, corneal endothelium, iris and other intraocular tissue, and even bullous ketatopathy and other serious surgery complications may occur due to mechanical trauma from sonic waves and thermal injury [8, 13]. Thus, reducing the effective ultrasound power and shortening the EPT are key factors to improve the quality of cataract surgery, especially in hard nucleus cases.
It has been reported that FLACS can effectively reduce intraoperative ultrasound power as compared to CPS [14]. In our study, we found that the ultrasound power and EPT in the CAPPS group were much lower than in the FLACS group, indicating that CAPPS can effectively reduce the ultrasound power and EPT by fracturing the nucleus first.
Endothelial cell damage occurs frequently in cataract surgery, mainly due to ultrasound power and EPT [13]. In previous studies, ECL ranged from 4 to 25%, and can reach as high as 42% in hard nucleus cases [15, 16]. In our study, the ECL percentage in the CAPPS group was much lower than that in the FLACS group. We propose several justifications for this. First, during CAPPS, the prechop of the nucleus decreases the ultrasound power in the surgery. Second, the viscoelastic substance in the anterior chamber can partly absorb the ultrasound energy and reduce the damage to reactive oxygen species [17,18,19], while in FLACS, as the laser beam goes through the aqueous humor easily, endothelial cells are fully exposed to the laser energy. Third, cellular injury and free radicals released during the procedure of lens fragmentation in FLACS may also cause higher ECL; moreover, microcavitation bubbles formed from photodisruption in the cornea and during fragmentation of the lens may have a potent effect on the ECL [20].
The increase of CCT often accompanies ECL [21]. The significant difference between the two groups at 1 month and 3 months showed that patients in the CAPPS group can undergo a faster recovery. Notably, it took less time for the CAPPS group to return to the preoperative CCT value, indicating that CAPPS can effectively reduce corneal swelling and recovery time.
Visual acuity is the most important concern of cataract patients. In our study, the CDVA improved greatly in both the CAPPS and FLACS groups. The CDVA at 1 month, 3 months, 6 months, and 1 year is better than it was at 1 day and 1 week, mainly because of the recovery of cornea oedema. As in previous studies, FLACS can raise visual acuity postoperatively [3, 5, 22], and our results indicate that CAPPS has a comparable effect to the FLACS group.
Miosis is a significant problem after femtosecond pretreatment, and the incidence rate may range from 1.23% to 32% [23,24,25]. A small pupil may lead to serious complications, such as dropped lens nucleus, posterior capsule rupture, iris damage, and even cystoid macular oedema during the surgery [26]. A study found prostaglandin E2 and total prostaglandin levels were elevated in the aqueous humor [27]; thus, it was suggested that surgeons can use NSAIDs to prevent intraoperative miosis [25]. In our study, although dilated with pranoprofen 1 day before the surgery, 8 patients had miosis in the FLACS group, while there was one such patient in the CAPPS group, suggesting that miosis is more likely to occur in FLACS, which is consistent with previous reports.
PCO is one of the most common postoperative complications after cataract surgery [28], and in this context, whether FLACS may induce more PCO is still debatable [29,30,31]. In our study, the incidence rate of PCO in both groups was comparable. Further research is needed to explore the PCO rate associated with these two surgeries.
Since the prechop technology was first described by Akahoshi [32], several prechop technologies have been introduced for the clinic, such as Fukasaku Hydrochop Cannula [33] and two modified cystotomes for middle prechop [34]. These technologies have been suitable for III–V-degree hard nucleus cataract patients [35]. The cystotome-assisted prechop method in phacoemulsification used in our study is a relatively novel technology. There are some advantages to CAPPS. First, there is no need for additional specialized instruments, such as the Akahoshi Combo Prechopper. Surgeons can complete the prechop with the same cystotome to transfer in and out of the anterior chamber after finishing the creation of capsulorhexis. Second, the CAPPS procedure requires only two hands to operate. In the course of the split nucleus procedure, it does not require building the occlusion in the endonucleus with precise pedal control and a high vacuum, which have a relatively long learning curve for phaco beginners. Moreover, it is much easier to acquire compared to other prechop procedures.
There are a number of limitations to this study. First, due to the high cost of FLACS [36], we need to respect the choice of patients; this makes our cohort study lack randomization. Second, with the development of technology and the popularity of cataract surgery, it is difficult to enroll more patients with hard nuclei. Hence, more studies are still needed in this regard.
In summary, both surgeries can achieve excellent visual acuity postoperatively. Compared to FLACS, the application of CAPPS in grade IV hard nucleus patients can reduce ultrasound power and endothelial cell loss, achieve faster recovery in central cornea thickness and decrease the incidence rate of intraoperative miosis. In addition, it requires no specialized instruments except a surgeon-bent cystotome, and the learning curve is relatively short. Thus, because of its efficacy and cost-effectiveness, CAPPS is worth promoting and applying clinically in the future.
Summary
What was known before
-
Hard nucleus in cataract surgery can often be challenging to manage and may lead to higher rates of complication.
-
Several surgical techniques available and commonly used to manage the Hard nucleus in cataract surgery.
What this study adds
-
Compared with FLACS, the application of CAPPS in grade IV hard nucleus patients can reduce complications.
-
CAPPS is efficient and cost-effective in patients with hard nucleus cataracts.
References
Liu Y-C, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390:600–12.
Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. J Refract Surg. 2009;25:1053–60.
Schweitzer C, Brezin A, Cochener B, Monnet D, Germain C, Roseng S, et al. Femtosecond laser-assisted versus phacoemulsification cataract surgery (FEMCAT): a multicentre participant-masked randomised superiority and cost-effectiveness trial. Lancet. 2020;395:212–24.
Donaldson KE, Braga-Mele R, Cabot F, Davidson R, Dhaliwal DK, Hamilton R, et al. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39:1753–63.
Day AC, Burr JM, Bennett K, Bunce C, Doré CJ, Rubin GS, et al. Femtosecond laser-assisted cataract surgery versus phacoemulsification cataract surgery (FACT): a randomized noninferiority trial. Ophthalmology. 2020;127:1012–9.
Assaf AH, Aziz BF. Ultrasound-energy consumption during phases of phacoemulsification of nuclear cataracts using femtosecond laser: a comparative study. Clin Ophthalmol. 2020;14:2829–35.
Emery J, Little J. Phacoemulsification And Aspiration Of Cataracts: Surgical Techniques, Complications, And Results (St. Louis, Mosby, 1979).
Xie L, Yao Z, Huang Y, Ying L. [Corneal endothelial damage and its repair after phacoemulsification]. Zhonghua Yan Ke Za Zhi. 2004;40:90–3.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Foster GJL, Allen QB, Ayres BD, Devgan U, Hoffman RS, Khandelwal SS, et al. Phacoemulsification of the rock-hard dense nuclear cataract: options and recommendations. J Cataract Refract Surg. 2018;44:905–16.
Kamoi K, Mochizuki M. Phaco forward-chop technique for managing posterior nuclear plate of hard cataract. J Cataract Refract Surg. 2010;36:9–12.
Falabella P, Yogi MS, Teixeira A, Jopetibe F, Sartori J, Schor P. Retrochop technique for rock-hard cataracts. J Cataract Refract Surg. 2013;39:826–9.
Walkow T, Anders N, Klebe S. Endothelial cell loss after phacoemulsification: relation to preoperative and intraoperative parameters. J Cataract Refract Surg. 2000;26:727–32.
Chen X, Yu Y, Song X, Zhu Y, Wang W, Yao K. Clinical outcomes of femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery for hard nuclear cataracts. J Cataract Refract Surg. 2017;43:486–91.
Kim EC, Byun YS, Kim MS. Microincision versus small-incision coaxial cataract surgery using different power modes for hard nuclear cataract. J Cataract Refract Surg. 2011;37:1799–805.
Mencucci R, Ponchietti C, Virgili G, Giansanti F, Menchini U. Corneal endothelial damage after cataract surgery: microincision versus standard technique. J Cataract Refract Surg. 2006;32:1351–4.
Takahashi H. Free radical development in phacoemulsification cataract surgery. J Nippon Med Sch. 2005;72:4–12.
Augustin AJ, Dick HB. Oxidative tissue damage after phacoemulsification: influence of ophthalmic viscosurgical devices. J Cataract Refract Surg. 2004;30:424–7.
Jurowski P, Gos R, Owczarek G, Gralewicz GZ. Corneal endothelial cells’ protection against thermal injury: influence of ophthalmic viscoelastic substances in experimental study on rabbits. Eur J Ophthalmol. 2005;15:674–9.
Kim EK, Cristol SM, Geroski DH, McCarey BE, Edelhauser HF. Corneal endothelial damage by air bubbles during phacoemulsification. Arch Ophthalmol. 1997;115:81–88.
Mursch-Edlmayr AS, Bolz M, Luft N, Ring M, Kreutzer T, Ortner C, et al. Intraindividual comparison between femtosecond laser-assisted and conventional cataract surgery. J Cataract Refract Surg. 2017;43:215–22.
Day AC, Burr JM, Bennett K, Doré CJ, Bunce C, Hunter R, et al. Femtosecond laser-assisted cataract surgery compared with phacoemulsification cataract surgery: randomized noninferiority trial with 1-year outcomes. J Cataract Refract Surg. 2020;46:1360–7.
Bali SJ, Hodge C, Lawless M, Roberts TV, Sutton G. Early experience with the femtosecond laser for cataract surgery. Ophthalmology. 2012;119:891–9.
Roberts TV, Lawless M, Bali SJ, Hodge C, Sutton G. Surgical outcomes and safety of femtosecond laser cataract surgery: a prospective study of 1500 consecutive cases. Ophthalmology. 2013;120:227–33.
Yeoh R. Intraoperative miosis in femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2014;40:852–3.
Hashemi H, Seyedian MA, Mohammadpour M. Small pupil and cataract surgery. Curr Opin Ophthalmol. 2015;26:3–9.
Schultz T, Joachim SC, Kuehn M, Dick HB. Changes in prostaglandin levels in patients undergoing femtosecond laser-assisted cataract surgery. J Refract Surg. 2013;29:742–7.
Wormstone IM, Wormstone YM, Smith AJO, Eldred JA. Posterior capsule opacification: what’s in the bag? Prog Retin Eye Res. 2020;82:100905.
Tran DB, Vargas V, Potvin R. Neodymium:YAG capsulotomy rates associated with femtosecond laser-assisted versus manual cataract surgery. J Cataract Refract Surg. 2016;42:1470–6.
Manning S, Barry P, Henry Y, Rosen P, Stenevi U, Young D, et al. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. J Cataract Refract Surg. 2016;42:1779–90.
Rostami B, Tian J, Jackson N, Karanjia R, Lu K. High rate of early posterior capsule opacification following femtosecond laser-assisted cataract surgery. Case Rep Ophthalmol. 2016;7:213–7.
Akahoshi T. Phaco prechop: manual nucleofracture prior to phacoemulsification. Operative Techniques in Cataract and Refractive. 1998;1:69–91.
Henriques JS, Alió JL, Akahoshi T, Escaf L, Fukasaku H, Walewska A. Prechopping surgical techniques. Tech Ophthalmol. 2009;7:139–45.
Berger A, Contin IN, Nicoletti G, Baltar Pazos PF, Baltar Pazos HS, Gomes JÁP. Middle prechop: fracturing the middle portion of the nucleus. J Cataract Refract Surg. 2012;38:564–7.
Vasavada AR, Nath V, Raj S, Vasavada V, Vasavada S. Technology and intraocular lenses to enhance cataract surgery outcomes-annual review (January 2013 to January 2014). Asia Pac J Ophthalmol. 2014;3:308–21.
Abell RG, Vote BJ. Cost-effectiveness of femtosecond laser-assisted cataract surgery versus phacoemulsification cataract surgery. Ophthalmology. 2014;121:10–16.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81770927, 81974132, and 82000896).
Author information
Authors and Affiliations
Contributions
All authors have contributed to the creation of this manuscript for important intellectual content and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41433_2021_1900_MOESM1_ESM.docx
Supplementary Table S1. Grades of the lens nucleus hardness with the Emery-Little classification strategy in phacoemulsification
41433_2021_1900_MOESM3_ESM.docx
Supplementary Table S3. Differences of corrected distance visual acuity in the baseline and postoperatively visit values of both group
41433_2021_1900_MOESM4_ESM.docx
Supplementary Table S4. Differences of endothelial cell density in the baseline and postoperatively visit values of both group
41433_2021_1900_MOESM5_ESM.docx
Supplementary Table S5. Differences of central corneal thickness in the baseline and postoperatively visit values of both group
Rights and permissions
About this article
Cite this article
He, Y., Wang, C., Zhou, X. et al. Comparison of clinical outcomes between cystotome-assisted prechop phacoemulsification surgery and femtosecond laser-assisted cataract surgery for hard nucleus cataracts. Eye 37, 235–241 (2023). https://doi.org/10.1038/s41433-021-01900-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01900-8