Abstract
Objectives
To investigate whether any microvascular changes are present in optic disc, peripapillary or maculary regions in healthy subjects with a family history of glaucoma.
Methods
A total of 82 healthy subjects including 42 first-degree relatives of primary open-angle glaucoma (POAG) and 40 controls were enroled in this cross-sectional study. Global and sectoral vessel density (VD) measurements excluding large vessels, retina nerve fibre layer (RNFL) and macula ganglion cell (mGCC) thicknesses were obtained from a combined optical coherence tomography-angiography (OCT-A) and spectral-domain OCT system. Effect size (ES) was used for evaluating the magnitude of the statistically significant difference. Area under receiver operating characteristic curves (AUCs) were used to examine the ability of parameters to differentiate first-degree relatives to controls.
Results
There was no significant difference in RNFL and mGCC thicknesses between groups. Although lower VD measurements in optic disc, peripapillary region, and macula were observed in the first-degree relatives, statistically significant mean difference (3.13 ± 0.87, p = 0.001) and large ES (0.80) were in only lower nasal sector of peripapillary region. Inter-eye asymmetry of supero-nasal VD was also statistically higher (3.74 ± 2.55 vs 1.89 ± 1.64) with a large ES in the first-degree relatives (p < 0.001, ES = 0.86). AUC for differentiating first-degree relatives from controls was highest for inter-eye asymmetry of supero-nasal sector VD (0.74, p < 0.001), followed by lower nasal sector VD (0.72, p < 0.001).
Conclusion
Compared to controls, first-degree relatives of patients with POAG were found to have significantly greater inter-eye asymmetry in supero-nasal peripapillary VD and less VD in the lower nasal peripapillary region.
Similar content being viewed by others
Introduction
Glaucoma is one of the leading causes of irreversible vision loss worldwide [1]. Although the pathogenesis of glaucoma is still not exactly understood, elevated intraocular pressure (IOP) and impairment of ocular blood flow are two main contributing factors for its development and progression [2,3,4]. Optical coherence tomography-angiography (OCT-A)—an innovative technology using laser light reflectance of the surface of moving red blood cells—allows the evaluation of retinal microcirculation through different segmented areas in glaucoma with high level of precision. Several studies have researched the differences in the microvasculature of the optic nerve head (ONH), peripapillary region, and macula among glaucoma, glaucoma suspects, and healthy subjects. A gradual reduction in retinal vessel density has been observed from healthy eyes to glaucoma suspect eyes to primary open-angle glaucoma (POAG) eyes [5]. This finding suggests that retinal vascular dropout may occur early in the disease cascade. Therefore, understanding early events in the glaucomatous process is essential to allow of early diagnosis.
A family history of glaucoma is a well-defined risk factor for the development of glaucoma. Epidemiologic studies reported that having a family history of glaucoma increased the possibility of developing glaucoma. It was reported that first-degree relatives of glaucoma patients had a 22% lifetime risk of developing glaucoma, in comparison to 2.3% in relatives of normal controls [6,7,8].
In the current study, we aimed to reveal any microvasculature change in healthy subjects having family history of glaucoma. Based on this, we investigated the difference in microcirculation of optic disc, peripapillary region and macula between healthy subjects with and without POAG in their first-degree relatives.
Materials and methods
This prospective observational study received approval of the Ethics Committee of SANKO University and was adherent to the tenets of the Declaration of Helsinki. Before included in the study, the study protocol was explained in detail. Written informed consent was obtained from each subject.
The study and control groups included healthy individuals with and without POAG in their first-degree relatives, respectively. For the study group, the individuals whose first-degree relative (parents or siblings) had POAG with at least 1-year follow-up in this tertiary hospital were invited for the study. Subjects who were admitted for refractive error or routine ophthalmic examination and did not have a family history of glaucoma were included in the control group. All subjects underwent extensive clinical examination including assessment of best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement with Goldmann applanation tonometry, central corneal thickness measurement (TRK-2P, Topcon Corp, Tokyo, Japan), gonioscopy, dilated fundus examination, visual field testing by Standard Automated Perimetry (Humphrey Field Analyzer;24-2 Swedish interactive threshold algorithm; Carl Zeiss Meditech, Jena, Germany), spectral-domain optical coherence tomography (SD-OCT; Avanti; Optovue, Inc), and OCT-A (AngioVue, Optovue, Inc, Fremont, California, USA) imaging. Visual field testing was performed within 1 week of clinical examination.
For all subjects, inclusion criteria were (1) aged ≥18 years, (2) best-corrected visual acuity of 20/20 and a refraction less than 2.0 dioptres sphere and 2.0 dioptres cylinder; (3) IOP less than 21 mm Hg with no history of elevated IOP; (3) open angle on gonioscopy; (4) normal appearing optic disc, intact neuroretinal rim; (5) within normal (p > 5%) measurements for retina nerve fibre layer (RNFL) and macular ganglion cell complex (mGCC) analysis; (6) a reliable (≤15% false-positive and false-negative results and ≤15% fixation losses) Standard Automated Perimetry tests with the Humphrey 24-2 Swedish Interactive Threshold Algorithm with a pattern standard deviation within 95% confidence-interval limits and a glaucoma hemifield test within normal limits. Subjects with a history of intraocular surgery, any retinal or optic disc pathologies, uveitis, ocular trauma, any systemic disease including diabetes mellitus, systemic hypertension, and neurological diseases or subjects using any systemic medication were excluded. Any suspicious or unknown family history for glaucoma was accepted as exclusion criteria for controls.
The OCT AngioVue system, consisting of a combined OCT-A and SD-OCT system, was used for OCT-A image acquisition and processing. The device characterizes the vascular structures of the retina at the capillary level. The split-spectrum amplitude-decorrelation angiography method was used to capture the dynamic motion of the red blood cells and provide a high-resolution 3D visualization of perfused retinal vasculature. The OCT-A characterizes vascular information at each retinal layer as an en-face angiogram, a vessel density map, and vessel density in percentage, calculated as the percentage area occupied by vessels in measured area, using the commercial Optovue Angiovue system software (Optovue, Inc, version 2018.0.0.14). Vessel density within the RNFL was measured from the internal limiting membrane to RNFL posterior boundary after removal of large vessels with radial peripapillary capillary slab. Whole en-face image small vessel density in optic disc OCT-A scan was measured in the entire 4.5 × 4.5 mm image centred on the optic disc, and peripapillary small vessel density was calculated in the region defined as 750 µm wide elliptical annulus extending from the optic disc boundary. Peripapillary small vessel density and corresponding RNFL thickness derived from the same scans were recorded as eight sectors—upper nasal, lower nasal, infero-nasal, infero-temporal, upper temporal, lower temporal, supero-nasal and supero-temporal—provided by the device. Macular superficial vessel density measurements were calculated in a slab from the internal limiting membrane to posterior border of the inner plexiform layer. Macular whole image vessel density measurements were calculated for 6 × 6 mm scans centred on the fovea. Parafoveal vessel density was measured in an annular region with an inner diameter of 1 mm and an outer diameter of 3.0 mm. Image quality was assessed for all OCT-A and OCT scans. Images with a scan quality of ≥8/10 and without segmentation failures or artifacts (e.g. irregular vessel pattern or disc boundary on the en-face angiogram, and local weak signal) were only analyzed. Poor quality images, which were defined as those with a signal strength index (SSI) < 45, were excluded from the analysis.
Only one randomized relative for per POAG patient was included for analysis according to inclusion and exclusion criteria. For both groups, one eye was randomly chosen for analysis. Inter-eye asymmetry values were calculated as the ASdfghtween the right and left eyes; │OD ‒ OS│.
Statistical analysis
Descriptive statistics were given as mean ± standard deviation (SD). The normality of the data was assessed with Kolmogorov−Smirnov test. For two groups comparison, independent samples t-test was used. In order to find optimal cut-off points for some continuous variables in discriminating groups receiver operating characteristic (ROC) curve analysis was performed and area under curve (AUC) was calculated for these points. IBM SPSS Statistics 23 was used for analyzing data. For ROC analysis MedCalc statistical software demo version was used. A p value of <0.05 was considered statistically significant. The effect size was used for evaluating clinical significance in parameters with statistical significance. The effect size was evaluated as Cohen’s suggestion that is d = 0.2 be considered a ‘small’ effect size, 0.5 represents a ‘medium’ effect size and 0.8 a ‘large’ effect size [9]. A post-hoc power analysis was conducted to assess the sufficiency of the sample size.
Results
A total of 82 healthy subjects including 42 first-degree relatives of POAG and 40 controls were included in the analysis. The demographic and ophthalmic characteristics of the subjects are summarized in Table 1. There was no significant difference between groups with and without POAG in their first-degree relatives in terms of demographic and ophthalmic characteristics. Also, SSI values for optic disc and macula scans did not differ between groups.
Table 2 shows the vessel density and structural measurements of the subjects. In optic disc analysis, vessel density in whole image, peripapillary region, and upper nasal, lower nasal, and supero-temporal sectors of peripapillary region were statistically lower in the first-degree relatives of POAG patients with an effect size varied from 0.48 to 0.80 (p = 0.004, p = 0.009, p = 0.011, p = 0.001, p = 0.028, respectively). In contrast to vessel density, RNFL thickness in indicated peripapillary sectors did not differ between groups. In macula analysis, whole image vessel density was statistically higher in controls than in the first-degree relatives of POAG patients (p = 0.023; effect size = 0.50).
Table 3 demonstrates inter-eye asymmetry of optic disc and macular vessel density measurements. Inter-eye asymmetry of small vessel density in inside disc, and also both lower temporal and supero-nasal sectors of peripapillary region were statistically higher in the first-degree relatives of POAG patients with an effect size varied from 0.44 to 0.86 (p = 0.034, p = 0.043, and p < 0.001, respectively). No significant difference was found in inter-eye asymmetry of vessel density in macula analysis.
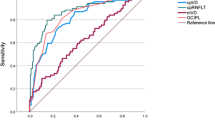
In vessel density analysis, the AUC for differentiating between eyes of first-degree relatives of POAG patients and controls was highest for lower nasal sector of peripapillary region (0.72 ± 0.06, p < 0.001), (Fig. 1a) followed by optic disc whole image vessel density (0.68 ± 0.06, p = 0.002), peripapillary vessel density (0.66 ± 0.06, p = 0.010), supero-temporal vessel density (0.63 ± 0.06, p = 0.036), and upper nasal vessel density (0.62 ± 0.06, p = 0.056).
In inter-eye asymmetry analysis, the AUC for differentiating between eyes of first-degree relatives of POAG patients and controls was highest for inter-eye asymmetry of supero-nasal vessel density (0.74 ± 0.05, p < 0.001) (Fig. 1b), followed by inter-eye asymmetry of inside disc vessel density (0.64 ± 0.06, p = 0.019), and lower temporal vessel density (0.60 ± 0.06, p = 0.111).
Considering the inter-eye asymmetry of supero-nasal vessel density in post hoc analysis, the power for the sample size used was found to be 97%.
Discussion
In the present study, we aimed to observe possible microvascular changes in optic disc, peripapillary region, and macula perfusion by evaluating healthy individuals with a familial glaucoma risk. In our results, although there was a statistical significance for optic disc, peripapillary region in global (whole image and peripapillary) and some sectoral areas (supero-temporal, lower nasal and upper nasal), and macula, the differences did not reach a substantial magnitude except lower nasal sector vessel density of peripapillary region. The effect size of the lower nasal sector vessel density of peripapillary region was found to be 0.80 (95% confidence interval of 0.36 and 1.26) in the current study. The effect size, one of the most important indicators of clinical significance, reflects the magnitude of difference in outcomes between groups; a greater effect size indicates a larger difference between study and control groups [9]. Also, inter-eye asymmetry of supero-nasal vessel density had statistical significance and demonstrated a more notable difference with a large effect size of 0.86 (95% confidence interval of 0.4 and 1.30). In a previous study using sectoral analysis similar to the current study, the effect size of peripapillary sectors which showed a statistically significant difference between the glaucoma and control groups, even varied from 0.51 to 0.92 [10]. Thus, the difference in lower nasal sector vessel density of peripapillary region and inter-eye asymmetry of supero-nasal vessel density seems noticeable, considering both statistical difference and effect size.
Although the detailed mechanism has not been clarified exactly, ocular perfusion alterations have been emphasized for the pathogenesis of glaucoma for years [11, 12]. It is not clear whether the alterations in perfusion are a causative factor or a sign of decreased metabolic activity of nerve fibres under stress. Reduced vessel density is accepted as an outcome of impairment ocular perfusion that can lead to RNFL and/or GCC loss chronically [11, 13, 14]. Supporting this hypothesis, a gradual decrease in retinal vessel density in proportion to glaucoma severity has been demonstrated [5, 15,16,17].
Subjects with a family history of glaucoma have a relative risk of 2.1 times [18]. First-degree relatives have an approximately 9-fold increased risk of developing glaucoma, while family history has been found positive in almost half of POAG patients [19]. The measurement of vessel density, which has the potential to detect early pathological changes, may also differ in subjects at risk (i.e. family history) for POAG in which vascular dysregulation is accepted as an important factor for the pathogenesis of the disease [11, 12].
Evaluating vessel density globally as well as in zones might reveal disparities in subjects, particularly without structural or functional damage. A regional variation in the alterations of RNFL thickness and vessel density has been shown by Pradhan et al. [10] in eyes with open-angle glaucoma. The authors investigated the relation between RNFL thickness and vessel density in sectoral analysis and found both RNFL thinning and vessel density decrease in infero-temporal, supero-nasal, and upper nasal sectors in normal hemifield of glaucomatous eyes. Thinner RNFL without reduced vessel density in supero-temporal sector and reduced vessel density with normal RNFL thickness in temporal upper sector was also reported. Moreover, a similar alteration in macula was observed by authors as reduced mGCC in both inferior and superior hemispheres with only reduced vessel density in superior parafoveal region. Therefore, it can be assumed that there may be regional variations in the alterations of structural and vascular changes. Upon this, we evaluated sectoral vessel density measurements in healthy subjects having family history of glaucoma to display regional variations and demonstrated a considerable decrease in lower nasal vessel density measurements of the first-degree relatives of POAG patients. In contrast to the study of Pradhan et al. [10] who used an earlier OCT-A algorithm that included large vessels along with capillaries in its estimation of vessel density, in our study density of capillaries that supply the inner portion of the peripapillary RNFL was analyzed without including large vessels by actual software. We showed that peripapillary capillary vessel density measurements excluding large vessels might be informative even in the subjects with a family history of glaucoma. We also used the 6 × 6 mm scans for macula superficial vessel density measurements; because comparative studies have shown that the 6 × 6 mm scans are better for the detection of glaucomatous changes, compared with the 3 × 3 mm scans [20, 21]. Despite a statistically significance in macula whole image vessel density, the difference did not have a sufficient effect size.
In a study investigating structure-function relationship by Akagi et al. [22], the authors reported no difference in peripapillary vessel density between the perimetrically intact regions of glaucomatous eyes and healthy eyes, despite significant thinner RNFL measurements. In contrast, reduced peripapillary vessel density in perimetrically intact regions of glaucomatous eyes compared with healthy individuals was reported by Pradhan et al. [10]. Chen et al. also showed reduced peripapillary microcirculation in the normal hemisphere of glaucomatous eyes with hemifield defects without a significant difference in RNFL thickness using an optical microangiography, a different OCTA algorithm [23]. Using different methodologies in studies may lead to different results. Both structural and vascular changes precede functional damage in glaucoma. However, it is not clear that whether both structural and vascular changes occur concomitantly or one triggers the other [12, 24]. In our study, we showed that sectorial vessel density difference may occur in risky eyes with undetectable change of RNFL. This may emphasize a considerable observation that peripapillary vessel density reduction may precede RNFL thinning. A previous study of Yarmohammadi et al. [25] who supported this hypothesis reported stronger correlation between visual field mean sensitivity and vessel density in intact hemifield of glaucomatous eyes than that of visual field indices, RNFL and GCC thickness. Similar to the aforementioned study, it was reported that OCT-A can detect early pre-perimetric glaucoma better than structural OCT [5]. It can be speculated that reduced capillary density detected by OCT-A can be a consequence of lower metabolism of dysfunctional ganglion cells that can be very early event of glaucoma process. However, structural OCT detects the RNFL thinning after the apoptosis these ganglion cells undergo. Beyond this, we have observed lower vessel density without RNFL thinning in lower nasal peripapillary sector in the first-degree relatives of POAG patients, but we are still not sure whether this difference is a reduction or an inherited factor. Similar to previous studies [10, 26], nasal peripapillary vessel density measurements were less than that of temporal region in all healthy subjects in our study. This topographical characteristic of nasal versus temporal radial peripapillary capillary and mostly inferior nerve loss predilection in glaucoma may tend the lower nasal area more vulnerable to vascular density differences. These speculations cannot be confirmed according to the findings of this study because of its cross-sectional design. Longitudinal studies are needed to elucidate vascular density changes in first-degree relatives of POAG patients.
Although glaucoma is generally bilateral disease, it often shows asymmetric features especially in its earlier stages [24, 27]. Asymmetry has been described as an early sign of glaucomatous damage [28]. In a previous study, greater inter-eye asymmetry of vessel density in optic disc (whole image and circumpapillary) and macula (whole image) scans was demonstrated in glaucoma suspects compared to healthy eyes with no difference in inter-eye asymmetry of RNFL and mGCC thickness [29]. In the current study, inter-eye asymmetry of vessel density in supero-nasal sector was higher statistically with a large effect size in the first-degree relatives of POAG patients compared to controls. Also, ROC analysis of inter-eye asymmetry of vessel density in supero-nasal measurements demonstrated the highest discrimination value in healthy subjects with and without family history of glaucoma (AUC; 0.74, p < 0.001). Inter-eye asymmetry of supero-nasal sector vessel density may be a potential predictive measure in subjects with a family history of glaucoma. However, the course of inter-eye asymmetry of supero-nasal sector vessel density should be evaluated in prospective studies with long-term follow-up.
Studies have shown that the diagnostic abilities of vessel density and the structural measurements increased with increasing severity of glaucoma [23, 30,31,32,33]. As expected, our AUC results were lower than that of other studies. AUC for differentiating the first-degree relatives of POAG patients from controls was highest for inter-eye asymmetry of supero-nasal sector of peripapillary vessel density (0.74, p < 0.001) followed by lower nasal sector of peripapillary vessel density (0.72, p < 0.001). These findings may be a measure to discriminate subjects with and without a family history of glaucoma.
The strength of this study is the strict criteria used in forming the study group. All subjects underwent RNFL, mGCC thickness analysis, and visual field testing to ensure participants with structural or functional damage were excluded from the study. Analyzing only capillaries using actual software of device is another important factor increasing the strength of the current study. The vessel density (capillary vessel density) and RNFL thickness measurements evaluated in this study were based on the measurements automatically provided by the software; as such, the sectors exactly matched with each other (vessel density vs. RNFL thickness). Analyzing capillaries with large vessels masking strategy can increase the capability of vessel density to distinguish eyes of the first-degree relatives of POAG patients from controls. No subjects using any medication were analyzed since many systemic medications can influence vascular density measurements. No significant differences were obtained between the SSI values of both optic disc and macula scans between groups, as the SSI showed a significant association with vessel density measurements [20].
The limitation of this study is its relatively small sample size. Although the sample size per group was small, sufficient power value was obtained with this sample size using post-hoc power analysis. Also, the study had a cross-sectional design, we could not observe the changes in vessel density with time. The true predictive value of the vessel density measurements in first-degree relatives lies in whether they predict the eventual development of glaucoma which can only be shown with a longitudinal study. Another limitation is that only a proportion of first-degree relatives develop glaucoma and it is uncertain whether the current study included these subjects, which has the potential influence on the results of the current study.
In summary, OCTA-derived sectoral vessel density measurements may enhance the clinician’s approach in subjects with a family history of glaucoma without detectable clinical, structural and functional changes. Considering the discriminating ability and statistical significance with a large effect size of inter-eye asymmetry of supero-nasal sector peripapillary vessel density and lower nasal sector of peripapillary vessel density, these indices may be favourable parameters for follow-up of subjects with a positive family history. Future larger studies with long-term follow-up are required to validate our results using the OCTA’s ability of reliably detect progressive changes in vessel density.
Summary
What was known before
-
A gradual reduction in retinal vessel density has been observed from healthy eyes to glaucoma suspect eyes to primary open-angle glaucoma (POAG) eyes.
What this study adds
-
Increased inter-eye asymmetry of supero-nasal sector peripapillary vessel density and reduced lower nasal sector of peripapillary vessel density has been shown in the first-degree relatives of POAG patients without any detectable clinical, structural and functional changes.
References
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–11. https://doi.org/10.1001/jama.2014.3192.
Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M. et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121:1322–32. https://doi.org/10.1016/j.ophtha.2014.01.021.
Tobe LA, Harris A, Hussain RM, Eckert G, Huck A, Park J. et al. The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fiber layer structure in patients with open-angle glaucoma over an 18-month period. Br J Ophthalmol. 2015;99:609–12. https://doi.org/10.1136/bjophthalmol-2014-305780.
Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the Advanced Glaucoma Intervention Study. Ophthalmology. 2008;115:1123–9. https://doi.org/10.1371/journal.pone.0170476. e3.
Akil H, Huang AS, Francis BA, Sadda SR, Chopra V. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS One. 2017;12:e0170476. https://doi.org/10.1371/journal.pone.0170476.
Wolfs RC, Klaver CC, Ramrattan RS, van Dujin CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol. 1998;116:1640–5. https://doi.org/10.1001/archopht.116.12.1640.
Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B, BESs Study Group. Risk factors for incident open-angle glaucoma: The Barbados eye studies. Ophthalmology. 2008;115:85–93. https://doi.org/10.1016/j.ophtha.2007.03.017.
Nemesure B, He Q, Mendell N, Wu SY, Hejtmancik JF, Hennis A, Leske MC, Barbados Family Study Group. Inheritance of open-angle glaucoma in the Barbados family study. Am J Med Genet. 2001;103:36–43. https://doi.org/10.1002/ajmg.1498.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1998.
Pradhan ZS, Dixit S, Sreenivasaiah S, Rao HL, Venugopal JP, Devi S. A sectoral analysis of vessel density measurements in perimetrically intact regions of glaucomatous eyes: an optical coherence tomography angiography study. J Glaucoma. 2018;27:525–31. https://doi.org/10.1097/IJG.0000000000000950.
Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93. https://doi.org/10.1016/s1350-9462(02)00008-3.
Flammer J, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 2007;52:162–73. https://doi.org/10.1016/j.survophthal.2007.08.012.
Mansouri K, Rao HL, Hoskens K, D’Alessandro E, Flores-Reyes EM, Mermoud A, et al. Diurnal variations of peripapillary and macular vessel density in glaucomatous eyes using optical coherence tomography angiography. J Glaucoma. 2018;27:336–41. https://doi.org/10.1097/ijg.0000000000000914.
Lévêque PM, Zéboulon P, Brasnu E, Baudoin C, Labbé A. Optic disc vascularization in glaucoma: Value of spectral-domain optical coherence tomography angiography. J Ophthalmol. 2016;2016:6956717. https://doi.org/10.1155/2016/6956717.
Shin JW, Lee J, Kwon J, Choi J, Kook MS. Regional vascular density-visual field sensitivity relationship in glaucoma according to disease severity. Br J Ophthalmol 2017;101:1666–72. https://doi.org/10.1136/bjophthalmol-2017-310180.
Geyman LS, Garg RA, Suwan Y, Trivedi V, Krawitz BD, Mo S, et al. Peripapillary perfused capillary density in primary open-angle glaucoma across disease stage: an optical coherence tomography angiography study. Br J Ophthalmol. 2017;101:1261–8. https://doi.org/10.1136/bjophthalmol-2016-309642.
Kumar RS, Anegondi N, Chandapura RS, Sudhakaran S, Kadambi SV, Rao HL, et al. Discriminant function of optical coherence tomography angiography to determine disease severity in glaucoma. Invest Ophthalmol Vis Sci. 2016;57:6079–88. https://doi.org/10.1167/iovs.16-19984.
Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44:3783–9. https://doi.org/10.1167/iovs.03-0077.
Awadalla MS, Fingert JH, Roos BE, Chen S, Holmes R, Graham SL, et al. Copy number variations of TBK1 in Australian patients with primary open-angle glaucoma. Am J Ophthalmol. 2015;59:124–30. https://doi.org/10.1016/j.ajo.2014.09.044.
Rao HL, Riyazuddin M, Dasari S, Puttaiah NK, Pradhan ZS, Weinreb RN, et al. Diagnostic abilities of the optical microangiography parameters of the 3x3 mm and 6x6 mm macular scans in glaucoma. J Glaucoma. 2018;27:496–503. https://doi.org/10.1097/ijg.0000000000000952.
Penteado RC, Bowd C, Proudfoot JA, Moghimi S, Manalastas PIC, Ghahari E, et al. Diagnostic abilities of optical coherence tomography angiography macula vessel density for the diagnosis of glaucoma using difference scan sizes. J Glaucoma. 2020;29:245–51. https://doi.org/10.1097/ijg.0000000000001447.
Akagi T, Iida Y, Nakanishi H, Terada N, Morooka S, Yamada H, et al. Microvascular density in glaucomatous eyes with hemifield visual field defects: an optical coherence tomography angiography study. Am J Ophthalmol. 2016;168:237–49. https://doi.org/10.1016/j.ajo.2016.06.009.
Chen C, Bojikian KD, Wen JC, Zhang Q, Xin C, Mudumbai RC, et al. Peripapillay retinal nerve fiber layer vascular microcirculation in eye with glaucoma and single-hemifield visual field loss. JAMA Ophthalmol. 2017;135:461–8. https://doi.org/10.1001/jamaophthalmol.2017.0261.
Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. https://doi.org/10.1016/s0140-6736(04)16257-0.
Yarmohammadi A, Zangwill LM, Diniz-Filho A, Saunders LJ, Suh MH, Wu Z, et al. Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology 2017;124:709–19. https://doi.org/10.1016/j.ophtha.2017.01.004.
Mansoori T, Sivaswamy J, Gamalapati JS, Agraharam SG, Balakrishna N. Measurement of radial peripapillary capillary density in the normal human retina using optical coherence tomography angiography. J Glaucoma. 2017;26:241–6. https://doi.org/10.1097/IJG.0000000000000594.
Lee SY, Lee EK, Park KH, Kim DM, Jeoung JW. Asymmetry analysis of macular inner retinal layers for glaucoma diagnosis: swept-source optical coherence tomography study. PLoS One. 2016;11:e0164866 https://doi.org/10.1371/journal.pone.0164866.
Poinoosawmy D, Fontana L, Wu JX, Bunce CV, Hitchings RA. Frequency of asymmetric visual field defects in normal-tension and high-tension glaucoma. Ophthalmology. 1998;105:988–91. https://doi.org/10.1016/s0161-6420(98)96049-3.
Hou H, Moghimi S, Zangwill LM, Shoji T, Ghahari E, Manalastas PIC, et al. Inter-eye asymmetry of optical coherence tomography angiography vessel density in bilateral glaucoma, glaucoma suspect, and healthy eyes. Am J Ophthalmol. 2018;190:69–77. https://doi.org/10.1016/j.ajo.2018.03.026.
Wang X, Jiang C, Ko T, Kong X, Yu X, Min W, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2015;253:1557–64. https://doi.org/10.1007/s00417-015-3095-y.
Rao HL, Pradhan ZS, Weinreb RN, Reddy HB, Riyazuddin M, Dasari S, et al. Regional comparisons of optical coherence tomography angiography vessel density in primary open-angle glaucoma. Am J Ophthalmol. 2016;171:75–83. https://doi.org/10.1016/j.ajo.2016.08.030.
Rao HL, Leite MT, Weinreb RN, Zangwill LM, Alencar LM, Sample PA, et al. Effect of disease severity and optic size on diagnostic accuracy of RTVue spectral domain optical coherence tomograph in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:1290–6. https://doi.org/10.1167/iovs.10-5546.
Wan KH, Lam AKN, Leung CK. Optical coherence tomography angiography compared with optical coherence tomography macular measurements for detection of glaucoma. JAMA Ophthalmol. 2018;136:866–74. https://doi.org/10.1001/jamaophthalmol.2018.1627.
Author information
Authors and Affiliations
Contributions
The conception and design of the study; PO, EO and PGK. Data collection; PO and EO. Analysis of the date: PGK. Writing of the manuscript; PO and EO. Creation of the figures: PGK. Manuscript review; PO, EO and PGK.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özyol, P., Özyol, E. & Günel-Karadeniz, P. Evaluation of vessel density in healthy subjects with family history of glaucoma. Eye 37, 82–87 (2023). https://doi.org/10.1038/s41433-021-01894-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01894-3