Abstract
Objective
To evaluate the differences in peripapillary and macular vascular parameters by optical coherence tomography angiography (OCTA) between patients with primary congenital glaucoma (PCG) and healthy controls; and to determine their diagnostic accuracy.
Material and methods
Observational, cross-sectional study including 39 eyes with PCG and 78 healthy eyes. Only one eye per patient was included. All included patients underwent a comprehensive ophthalmic examination and peripapillary and macular analysis were performed by AngioplexTM OCTA (Cirrus HD-OCT 5000) with a 4.5 × 4.5 mm optic nerve head scan and 6 × 6 mm macular scan. Global data and quadrant data from peripapillary vascular parameters and global data and circular sectors data from macular superficial plexus parameters were compared between groups. The glaucoma discrimination capability of these parameters was calculated as areas under the receiver operating characteristics curve (AUC ROC).
Results
Mean age was 14.1 ± 8.7 years for the PCG patients and 11.7 ± 6.2 years for controls (p = 0.093). All vascular peripapillary measurements (global and quadrants; all p < 0.001) and all macular measurements (p < 0.042) excepting perfusion density in the inner circle (p = 0.087), were reduced in the PCG group compared to controls. According to AUC ROC, peripapillary (all ≥ 0.706) and macular parameters (all ≥ 0.699) showed good diagnostic capacity. AUC ROC for the most discriminatory measurements corresponding to blood flux index (0.887) and whole macula vascular density (0.855) were similar (p = 0.085).
Conclusion
Peripapillary and macular vascular parameters by OCTA are decreased in patients with PCG, showing a good capacity to discriminate between normal and glaucomatous eyes.
Similar content being viewed by others
Introduction
Primary Congenital Glaucoma (PCG) is a rare disease affecting children early in life. The diagnosis of PCG is based on clinical criteria. However, in the last years the introduction of new devices such as optical coherence tomography (OCT), has proved to be useful for the management of paediatric glaucoma [1] as it provides objective retinal nerve fibre layer (RNFL) thickness and ganglion cell complex measurements [1].
Retina ganglion cells (RGCs) and their axons are considered the most highly metabolically active structures in humans, with strong vascular supply [2]. Peripapillary capillaries form a distinct network of capillary beds within the RNFL to meet the large energy requirements of the RGCs [3]. Neurovascular and functional mechanisms are thought to be linked, in glaucoma, high intraocular pressure and ischaemia could induce pathological changes due to vulnerability of RGCs [3].
Optical Coherence Tomography Angiography (OCTA) is a new technology that allows rapid examination and offers high-quality images of the retinal vasculature [4, 5]. Using contrast imaging, this technology allows a quantitative assessment of the retinal and choroidal microvasculature which includes the optic nerve head (ONH), the peripapillary region, and the macular region [6, 7], being, therefore, a non-invasive technique safer than conventional angiography (it does not require any injection of a contrast agent) [8]. These advantages offered by OCTA provide clear benefits for the management of children in clinical practice.
Previous studies reported that OCTA optic nerve head (ONH) vessel density measurements have similar diagnostic accuracy as structural parameters such as circumpapillary RNFL thickness by OCT, and lower vessel density is significantly associated with the severity of glaucoma. Lower ONH perfusion has also been related to a thinner macular ganglion cell complex and lower macular vessel density in glaucoma patients [9]. Therefore, changes in vascular parameters have been analysed in several types of glaucoma in adults. However, there is a lack of information about the possible changes in the peripapillary and macular microvasculature in patients with PCG.
The objective of the current study is to evaluate the differences in peripapillary and macular vascular parameters between patients with PCG and healthy controls and to determine their diagnostic accuracy.
Material and methods
Study participants
This was an observational cross-sectional study in which we examined 39 eyes of 39 patients diagnosed with PCG (diagnosed and managed at the National Reference Center for infant glaucoma) and 78 eyes of 78 healthy age-matched Caucasian subjects. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Hospital. Written informed consent was obtained from all participants aged 18 years or older or from the parents or legal guardians of participants younger than this age (each child gave their verbal consent to participate in our study).
All participants underwent a comprehensive ophthalmologic examination by a single examiner (LMF) in which the variables recorded were best corrected visual acuity (BCVA) (decimal Snellen scale) and refractive state expressed as spherical equivalent (SE; cycloplegic refraction), dilated fundus examination and cup-to-disc ratio determined by the same experienced examiner (LMF). To minimize the effect of refractive error on OCTA testing, eyes with a SE > ±6 dioptres were excluded.
As the control group, 78 healthy children and healthy young adults were recruited among those undergoing a routine ophthalmologic exam, the children of adult patients, and children of staff members who agreed to participate in this study. Eyes were defined as normal (in the absence of other features of glaucoma) if they showed a BCVA ≥ 0.8 (Snellen Scale), an optic nerve cup-to-disc asymmetry ≤ 0.2, and a cup-to-disc ratio < 0.5 without focal neuroretina rim loss, haemorrhage, or pallor. Children were excluded if they had a family history of glaucoma or had been diagnosed with amblyopia or strabismus. All measurements were made in both eyes, but only the data of one eye randomly selected were entered in the analysis.
For the patient group, 39 children were recruited and all fulfilled the diagnostic criteria for PCG according to the Childhood Glaucoma Research Network classification: [10] IOP > 22 mmHg upon diagnosis, clinical evidence of glaucoma such as enlarged corneal diameter, Haab’s striae and glaucomatous appearance of the optic nerve head. Eyes were included if they had sufficient visual acuity for fixation, ≥0.1 using Snellen Scale, good ocular media transparency and no other previous ocular surgery excepting glaucoma surgeries. The presence of nystagmus or leukoma was an exclusion criterion. In this group, information was collected for both eyes when it was possible or both eyes were eligible (index eye and fellow eye).
Visual field was performed for all PCG patients using Tendency oriented perimetric (TOP) strategy with the Octopus visual field. Mean defect (MD) and loss variance (sLV) values were collected.
When both eyes were eligible, one eye was randomly selected using a randomization list obtained from www.randomization.com.
OCTA measurements
Macular and peripapillary scans were acquired using an SD-OCT (Cirrus, HD-OCT 5000; Carl Zeiss Meditec, Inc., Dublin, CA, USA). The Cirrus 5000 has a 68-kHz axial scan repetition rate per second with an axial and transverse resolution of 5 and 15 microns, respectively [11].
The device´s software AngioplexTM (version 10.0, Carl Zeiss Meditec) includes the algorithm “optical microangiography” (OMAG), and offers automatic measurements of vascular parameters in the superficial plexus with FDA approval. AngioplexTM quantifies the superficial retina which is defined from the inner limiting membrane (ILM) to the inner plexiform layer (IPL). Blood flow information is generated by OMAG with 245 A-scans in each B-scan. Eye-tracking offered by this system was used for all exams. All scans were performed by the same operator (LMF) with pupil dilation in a dark room on the same day as the other tests. Poor-quality scans with a signal strength index (SSI) < 8 were excluded from the analysis.
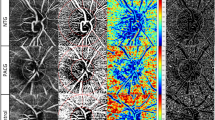
The scan areas and the segmentations are shown in Fig. 1. Peripapillary 4.5 × 4.5 mm scans centred on the optic nerve were performed for each patient. Automatically, AngioplexTM analyse the area located between an inner circle with a radius of 2 mm and an outer circle with a 4.50 mm radius both centred on the ONH, divided into four quadrants: superior, inferior, temporal and nasal. Two peripapillary parameters were calculated and collected: peripapillary perfusion density (pPD) and flux index (FI) for the global and sectors (superior, temporal, inferior and nasal). PD is the total area of perfused radial peripapillary capillary vasculature per unit area in a specific region (%); and FI is a dimensionless parameter between 0 and 1 representing the average decorrelation signal (Fig. 1A, B).
Macular 6x6mm scans centred on the fovea were obtained for each patient. AngioplexTM software automatically calculates two parameters from the superficial retinal layer slab in macular scans: macular vascular density ((VD) defined as the total length of blood vessel from the skeletonized image to the total area [in mm 1] ratio); and macular perfusion density (mPD), defined as the total area of perfused vasculature per unit area in the region of measurement (%).
AngioPlexTM subdivides the macular scan into the Early Treatment of Diabetic Retinopathy Study (ETDRS) map: central circle (cC) within the 1-mm central circle on the Early Treatment Diabetic Retinopathy Study (ETDRS) grid, an inner circle (iC) from 1 to 3 mm, an outer circle (oC) from 3 to 6 mm and a full circle, a whole circle (w) (Fig. 1C, D). mPD and macular VD data related to those rings were collected (Fig. 1C, D)
Foveal avascular zone (FAZ) measurements were collected: FAZ area is defined as the macular foveal area without blood flow signal (mm2), the FAZ perimeter (mm), and the acircularity index (AI; i.e., the measured perimeter of FAZ-to-the perimeter of projected circle ratio with the same area as the FAZ, 0–1) of FAZ.
Statistical analysis
Statistical analyses were performed using SPSS software (version 20.0; SPSS, Inc., Chicago, IL, USA). Sex and laterality differences between the normal and glaucoma groups were compared using the Pearson X2 test. The Student’s t-test was used to compare age, Cup-to-disc ratio, SE, BCVA and OCTA measurements between the two groups. Differences between both eyes in clinical variables and OCTA measurements were analysed using student’s t-test. Correlation between main vascular parameters and markers of glaucoma severity was analysed using the Pearson correlation coefficient. Receiver operating characteristics curves (ROC) were constructed and areas under the curve (AUC) were used to assess the capacity of each variable obtained by OCTA to distinguish between glaucomatous and healthy eyes. AUC ROC was compared for the different variables using DeLong Method. Significance was set at P ≤ 0.05.
Results
The study population consisted of 39 PCG patients (mean age, 14.0 (8.6) years) and 78 healthy subjects (mean age, 11.71 (6.20) years) (p = 0.093).
A total of 46 children with PCG were examined however only 39 were included in the study group due to poor quality of exams in three patients (one child with cataract and two ones with corneal opacity) and the impossibility to complete OCTA scans in four cases (poor cooperation in two children, with 4 and 5 years old, nystagmus in one case and poor fixation in one patient with low visual acuity (BCVA = 0.1)).
The clinical and ocular characteristics of the study population are described in Table 1. There were no significant differences between glaucoma patients and healthy subjects in any baseline clinical characteristics, including age, gender and laterality (all P > 0.09). As expected, affected glaucoma eyes had higher SE, cup-to-disc ratio and worse BCVA compared to healthy eyes (all p < 0.001).
The clinical characteristics of the PCG group were: mean IOP 16.52 (3.92) mmHg (range 7–26 mmHg) and mean number of hypotensive ocular medications used 0.72 (0.99); all required glaucoma surgeries and mean glaucoma surgeries were 1.81 (1.25). Of these glaucoma surgeries, in three eyes surgery involved the implant of an Ahmed valve, in 12 eyes surgery was trabeculectomy (median number of trabeculectomies per eye was 1.2 (P25-P75 = 1–2)) and in 17 eyes the surgery was goniotomy (median number of goniotomies per eye was 1.8 (P25-P75 = 1.3–2.6)). Mean MD was 8.38 (7.32) db (ranged from 0.10 to 27.20 db) and mean sLV was 4.47 (2.05) (ranged from 1.20 to 8.40db).
Global mean peripapillary FI 0.39 (0.06) was lower in glaucoma group than 0.46 (0.01) in healthy group (p < 0.001) and global mean peripapillary pPD 42.60 (4.62) was lower in glaucoma group than 46.10 (1.32) in healthy group (p < 0.001) (Table 2a).
Mean peripapillary FI values ranged from 0.38 (0.05) to 0.40 (0.06) in the glaucoma group, being higher, ranging from 0.44 (0.01) to 0.48 (0.02) in the healthy group (all p < 0.001). And mean pPD values ranged from 40.13 (6.36) to 46.47 (3.97) in the glaucoma group, being higher, ranging from 44.39 (2.23) to 48.86 (2.35) in the healthy group (all < 0.001) (Table 2a, Fig. 1A, B).
The macular vascular measurements were significantly lower in affected glaucoma eyes compared to healthy eyes (p < 0.042), excepting perfusion density in the inner circle (iC-mPD) (p = 0.087) (Table 2b). As expected, those values in the macular inner circle were the lowest in both groups for VD (8.99 (3.26) in glaucoma group and 10.80 (4.00) in healthy group) and for pPD (21.41 (6.97) in glaucoma group and 25.32 (8.62) in healthy group) (the inner circle includes FAZ area). In the outer circle and full macular circle, higher VD and pPD differences were found between healthy and PCG group (both p < 0.001). FAZ measurements were similar in both groups (Table 2b) (all p > 0.271).
OCTA was performed in both eyes in a total of 13 patients with PCG (in the rest of patients with PCG, only one eye was examined due to blindness in the fellow eye, impossibility to obtain an exam with good quality or cases with unilateral disease). In these 13 cases, statistical differences were found in BCVA between better eye and worse eye: 0.77 (0.28) vs 0.38 (0.25), p = 0.001. No statistical differences were found in: C/D ratio was 0.45 (0.29) vs 0.57 (0. 26), p = 0.280; mean pPD was 42.57 (4.74) vs 41.74 (4.35), p = 0.652, mean FI was 0.40 (0.05) vs 0.37 (0.07), p = 0.314; mean mVD was 17.08 (1.50) vs 16.69 (1.61), p = 0.553; and mean mPD was 41.99 (3.82) vs 41.00 (4.11), p = 0.555; mean MD was 6.56 (6.75) dB vs 9.96 (9.03) dB, p = 0.288 and mean sLV was 4.44 (2.29) dB vs 4.19 (1.84)dB, p = 0.759; between better and worse eye, respectively.
Table 3 represents the correlation between main vascular parameters and markers of glaucoma severity. Good correlation was found between perimetric parameters and peripapillary vascular parameters (all p < 0.007). However, no statistical correlation was found between BCVA and OCTA peripapillary measurements.
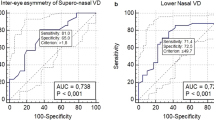
The AUROC for differentiating between PCG and healthy eyes are shown in Table 4. Considering peripapillary parameters (all AUC ROC ≥ 0.706), best AUC ROC was found for global FI (0.867 (0.04)) and pPD (0.887 (0.04)); and for inferior FI (0.886 (0.04)) and pPD (0.890 (0.04)).
All macular parameters (AUC ROC ≥ 0.699) shown good diagnostic capacity. Best AUCs were obtained for outer circle (AUC ROC oC-VD = 0.841 (0.05) and AUC ROC oc-mPD = 0.813 (0.05)) and whole circle parameters (AUC ROC w-VD = 0.855 (0.04) and AUC ROC w-mPD = 0.840 (0.04)). Null discriminatory capacity was obtained for FAZ measurements (AUC ROC FAZ area = 0.527, AUC ROC FAZ perimeter = 0.501 and AUC ROC AI = 0.566).
The parameters blood flux index (FI AUC ROC = 0.887(0.04)) and full VD (w-VD AUC ROC = 0.855 (0.04)) showed the best diagnostic performance differentiating glaucoma eyes from healthy eyes, considering global measurements. AUC ROC for both variables were compared using DeLong Method and no statistical differences were found (p = 0.085).
Discussion
Multiple studies have confirmed the reductions in microvasculature in peripapillary, macular and whole image scans in different types of glaucoma in adults using OCTA: POAG (in eyes with both pre-perimetric and perimetric POAG) [7], pseudoexfoliative glaucoma [12] and angle-closure glaucoma [13]. In this study, a reduction of all peripapillary vascular parameters in the superficial plexus was detected in patients with PCG, and in all macular measurements, excepting iC-mPD values, using AngioplexTM.
First, in our study blood flux index was analysed in ONH scans. Horizontal FI was higher than vertical FI in both groups and global FI and quadrant values were decreased in PCG group compared with the healthy group. Results of other studies relating ocular blood flow to glaucoma are difficult to compare due to the variety of techniques applied, different parameters of ocular hemodynamic investigated, and differences in glaucoma populations studied. Jia et al. [14, 15] showed that the optic disc flow index was reduced by 25% in glaucomatous eyes (0.161 ± 0.008) compared with healthy eyes (0.121 ± 0.026).
Second, peripapillary and macular PD results were reduced in the glaucoma group excepting iC-macular perfusion density (including FAZ). Scarce information exists in the literature about perfusion density. Peripapillary perfused capillary density has been demonstrated to be more significantly decreased in pseudoexfoliative glaucoma compared with POAG eyes and in pseudoexfoliative syndrome compared with control eyes [16, 17].
However, interestingly, in our study, a homogeneous FI reduction was observed in all peripapillary quadrants, while pPD showed higher reduction in vertical quadrants. These results support the idea of previous studies in children with PCG, suggesting that glaucoma damage in PCG children could differ from that produced in adults, typically with a global loss of ganglion cell complex thickness and circumpapillary RNFL thickness that was particularly marked in the superior and inferior quadrants [1, 18]. Indeed, these results highlight that both vascular parameters seem to be reporting complementary information but are not interchangeable.
Third, macular vascular density was measured in our study [11]. Whole macular values were 18.32 mm−1 in the healthy group compared to only 16.89 mm−1 in the glaucoma group. Similar to our results, LI et al. [13] reported in adults, the mean VD were 15.19 mm−1 in early glaucoma and 10.89 mm−1 in severe glaucoma. Similar but slightly higher whole macular vascular density was reported from 20.81 mm−1 to 22.01 mm−1 in healthy children aged from 4 to 15 years old. An explanation for this might be that our subjects were aged from 4 to 29 years, and in previous reports, macular VD was positively correlated with age and in subjects <15 years old while negatively correlated with age in adult subjects [19, 20].
The outer circle was the densest in both groups and the inner circle showed the lowest VD values in both groups due to the inclusion of the FAZ in this measurement. The FAZ was analysed separately in our study and similar values for FAZ measurements were found between GCP and control group. In children, the quantitative analysis of FAZ has been reported to be necessary for evaluation of macular perfusion status in several congenital and acquired retinopathies, including retinopathy of prematurity, familial exudative vitreoretinopathy or Coats, and others. This lack of differences detected in FAZ area could be explained by the absence of all retinal layers involved in glaucoma (except for the photoreceptor layer) [2, 21]. In addition, these results support the idea that poor visual acuity in PCG is not related to the poor development of FAZ.
Our results remark that PCG is a bilateral disease but asymmetric. We found statistical differences in BCVA between better and worse eye in the PCG group. No statistical differences were found in other parameters such as cup-to-disc ratio between the better and worst eye, probably due to the low number of included eyes. However, this result could be clinically relevant.
On the other hand, our results highlight the statistical correlation found, between vascular parameters and markers of severity of PCG. Both, pPD and FI showed a negative correlation with MD and sLV. So, as expected, those cases with severe glaucoma and severe defects of visual fields are associated with lower peripapillary perfusion and flux index. Higher cup-to-disc ratio is associated with lower perfusion and FI, however, no correlation was found either between BCVA and OCTA vascular parameters or BCVA and severity markers. These results manifest the lack of association between BCVA and severity of glaucoma. It is well known that visual prognosis in PCG is affected by many factors: amblyopia, nystagmus, strabismus, corneal state (Haab striae, leukoma, oedema…), the presence of cataract and retinal anomalies, among others.
The association between severity of glaucoma and vascular damage has been reported in POAG [13]. Changes in peripapillary and macular vascular parameters had a strongly positive correlation with structural parameters (GCC thickness and RNFL thickness) and a negative correlation with the severity of glaucoma, which meant the more severe the glaucoma was, the lower vascular parameters were [22, 23]. As in POAG, it is expected that in PCG structural and functional parameters are related to reduced vascular parameters as found in this preliminary study, so new studies are required to evaluate these associations with the severity of the PCG.
In this study, the best macular and papillary diagnostic parameters were FI and full VD and showed excellent and similar discriminatory capacity. Despite this, macular vascular parameters showed slightly lower diagnostic accuracy (range from 0.699 to 0.840) than papillary vascular parameters (range from 0.706 to 0.890) in differentiating between glaucoma patients from healthy subjects. These findings are in accordance to previous reports. In adults, macular VD showed moderate diagnostic performance, which is poorer than measurements in the peripapillary region [24]. Yarmohammadi et al. [25] reported the best papillary AUC ROC 0.84 for whole image vessel density and the best macular AUC ROC 0.69 for parafoveal vessel density, using Angiovue OCTA (Optovue, Fremont, CA, USA). This lower diagnostic accuracy may be attributed to the fact that the measurements of the macular region are concentrated in the 4.5 mm foveal centre that may remain intact in the early stages of the disease.
Peripapillary FI showed excellent AUC ROC in our study. Chen et al. [26] demonstrated similar values for the peripapillary FI with a diagnostic performance for the detection of POAG suspect of AUC ROC = 0.76 and POAG of AUC ROC = 0.93.
All ONH sectors in our study showed excellent diagnostic performance, however, the best values were for the inferior quadrants for both parameters FI and PD. It is remarkable that in our study, the best quadrant discriminatory parameter was inferior pPD. Despite poor information exist in the literature about PD, it is a preliminary result to consider future lines of investigation. Interestingly, published data for structural parameters (RNFL and GCC analysis) in adults agree that the best macular and papillary variable for a diagnosis of glaucoma are those of the inferior temporal quadrant [27].
On the other hand, comparing the diagnostic abilities of peripapillary OCTA vessel densities and OCT RNFL thickness in POAG in the literature, some studies have found them similar [26] (the AUCs of both peripapillary vessel density and RNFL thickness have ranged between 0.85 and 0.95). However, other studies have reported a better diagnostic ability of RNFL thickness (0.91) compared with peripapillary vessel density in POAG (0.88) [24]. Similar to the peripapillary measurements, the diagnostic ability of superficial macular vessel density was found to be similar or inferior to that of macular GCC thickness depending on the authors in POAG [24, 25]. However, in glaucoma suspect eyes the diagnostic capability was found even highest for macular VD (0.70) than for RNFL thickness (0.65) [25].
Although the diagnosis for PCG is based on clinical signs, OCTA offers the opportunity to quantify the microvasculature and objective the damage secondary to PCG.
Indeed, this study demonstrates significant differences between healthy eyes and PCG eyes, and good correlation between main vascular parameters and severity markers were reported. These results suggest OCTA could be useful in clinical practice to determine the severity of PCG and to detect changes to the microvasculature over time.
New studies are needed to clarify the relationship between vascular parameters and glaucoma severity.
On the other hand, no previous studies were reported in PCG and no normative database exist either in children or adults using Angioplex OCTA. So, our results could be useful information to incorporate to the clinical practice as a reference.
The main limitation of our study was the relatively small number of cases of PCG. This is a rare disease and all those children with PCG but with an opacity of the ocular media, poor fixation, poor vision, lack of cooperation and very early paediatric ages cannot be considered for our study. Therefore, this creates a selection bias towards eyes with less severe glaucoma. In addition, due to the cross-sectional, noninterventional design of the study, firstly it was not possible to evaluate the impact of ocular hypotensive eye drops on vascular measurements. Secondly, we could only identify microvascular superficial changes, and we were not able to establish any cause-and-effect relationships between peripapillary and macular vascular parameters with the severity of glaucoma damage. Therefore, longitudinal studies investigating the temporal sequence of OCTA changes are needed to address whether those changes are a cause or consequence of ONH damage in glaucoma. Thirdly, the possible effect of AL on OCTA measurements was not considered. It is still unclear if AL could artefact our results. While the magnification effect has been demonstrated for OCT thickness measurements, several authors reported no correlation between vascular parameters and AL [20]. On the contrary, Cheng et al. [26] in the Hong Kong Children Eye Study, observed a reduction of VD in shorter AL in a very large population.
In summary, OCTA can provide quantifiable information about the microvasculature of the peripapillary and macular retina in patients with PCG. This is the first study to offer clear evidence that a decrease in peripapillary and macular vascular parameters occurs in PCG, and both parameters showed a high discriminatory capacity for glaucoma detection.
Therefore, OCTA can be an additional tool to determine the damage of glaucoma in PCG patients.
Summary
What was known before
-
Utility of OCTA measurements has been reported in adults.
-
Multiple studies have confirmed the reductions in microvasculature in peripapillary, macular and whole image scans in different types of glaucoma in adults using OCTA.
What this study adds
-
OCTA can provide quantifiable information about the microvasculature of the peripapillary and macular retina in patients with primary congenital glaucoma.
-
This is the first study to offer clear evidence that a decrease in peripapillary and macular vascular parameters occurs in PCG, and both parameters showed a high discriminatory capacity for glaucoma detection.
References
Morales-Fernandez L, Jimenez-Santos M, Martinez-de-la-casa JM, Sanchez-Jean R, Nieves M, Saenz-Frances F, et al. Diagnostic capacity of SD-OCT segmented ganglion cell complex versus retinal nerve fiber layer analysis for congenital glaucoma. Eye 2018;32:1338–44.
Werner AC, Shen LQ. A review of OCT angiography in glaucoma. Semin Ophthalmol. 2019;34:279–86.
Yu PK, Balaratnasingam C, Xu J, Morgan WH, Mammo Z, Han S, et al. Label-free density measurements of radial peripapillary capillaries in the human retina. PLoS One. 2015;10:e0135151.
de Carlo TE, Bonini Filho MA, Chin AT, Adhi M, Ferrara D, Baumal CR, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122(Jun):1228–38.
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express Feb 13. 2012;20:4710–25.
J Jia Y, Morrison JC, Tokayer J, Tan O, Lombardi L, Baumann B, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express Dec 1. 2012;3:3127–37.
Wang X, Jiang C, Ko T, Kong X, Yu X, Min W, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graef Arch Clin Exp. 2015;253(Sep):1557–64.
Fernández-Vigo JI, Kudsieh B, Shi H, De-Pablo-Gómez-de-Liaño L, Serrano-Garcia I, Ruiz-Moreno JM, et al. Normative database of peripapillary vessel density measured by optical coherence tomography angiography and correlation study. Curr Eye Res. 2020;45:1430–7.
Manalastas PI, Zangwill L, Daga F, Christopher M, Saunders L, Shoji T, et al. The association between macula and ONH Optical Coherence Tomography Angiography (OCTA) vessel densities in glaucoma, glaucoma suspect and healthy eyes. J Glaucoma. 2018;27:227–32.
Beck A, Chang TC, Freedman S Weinreb RN, Grajewski A, Papadopoulos M, et al. Section 1: definition, classification, differential diagnosis. In: Weinreb RN, Grajewski A, Papadopoulos M, Grigg J, Freedman S, editors. World glaucoma association consensus series-9: childhood glaucoma. Amsterdam, The Netherlands: Kugler Publications; 2013. p. 3–10.
Li S, Yang X, Li M, Sun L, Zhao X, Wang Q, et al. Developmental changes in retinal microvasculature in children: a quantitative analysis using optical coherence tomography angiography. Am J Ophthalmol. 2020;112:231–9.
Park JH, Yoo C, Girard MJA, Mari JM, Kim YY. Peripapillary vessel density in glaucomatous eyes: comparison between pseudoex- foliation glaucoma and primary open-angle glaucoma. J Glaucoma. 2018;27:1009–16.
Li Z, Xu Z, Liu Q, Chen X, Li L. Comparisons of retinal vessel density and glaucomatous parameters in optical coherence tomography angiography. PLoS ONE. 2020;15:e0234816.
Jia Y, Wie E, Wang X, Zhang X, Morrison JC, Parikh M, et al. Optical coherence tomography angiography of optic disc perfusion in Glaucoma. Ophthalmology. 2014;121(July):1322–32.
Jia Y, Morrison JC, Tokayer J, Tan O, Lombardi L, Baumann B, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express Dec 1. 2012;3:3127–37.
Suwan Y, Geyman LS, Fard MA, Tantraworasin A, Chui TY, Rosen RB, et al. Peripapillary perfused capillary density in exfoliation syndrome and exfoliation glaucoma versus POAG and healthy controls: an OCTA study. Asia Pac J Ophthalmol. 2018;7:84–9.
Güngör SG, Sezenöz AS, Öztürk C, Gökgöz G, Akman A. Peripapillary and macular vessel density measruemnt with optical coherence angiography in exfoliation syndrome. J Glaucoma. 2020;30:71–7.
Srinivasan S, Addepali UK, Rao HL, Garudadri CS, Mandal AK. Spectral domain optical coherence tomography in children operated for primary congenital glaucoma. Br J Ophthalmol. 2014;98:162–5.
Zhang Y, Zhang B, Fan M, Gao X, Wen X, Li Z, et al. The vascular densities of the macula and optic disc in normal eyes from children by optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2020;258(Feb):437–44.
Fernández-Vigo JI, Kudsieh B, Shi H, Arriola-Villalobos P, Donate-López J, García-Feijóo J, et al. Normative database and determinants of macular vessel density measured by optical coherence tomography angiography. Clin Exp Ophthalmol. 2020;48:44–52.
Pilotto E, Frizziero L, Crepaldi A, Della Dora E, Deganello D, Longhin E, et al. Repeatability and reproducibility of foveal avascular zone area measurement on normal eyes by different optical coherence tomography angiography instruments. Ophthalmic Res. 2018;59:206–11.
Shin JW, Lee J, Kwon J, Choi J, Kook MS. Regional vascular density- visual field sensitivity relationship in glaucoma according to disease severity. Br J Ophthalmol. 2017;101:1666–72.
Mansoori T, Sivaswamy J, Gamalapati JS, Balakrishna N. Radial peripapillary capillary density measurement using optical coherence tomography angiography in early glaucoma. J Glaucoma. 2017;26:438–43.
Rao HL, Kadambi SV, Weinreb RN, Puttaiah N, Pradhan ZS, Rao DAS, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol. 2017;101:1066–70.
Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalasas PI, Fatehee N, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci. 2016;57(Jul 1):451–9.
Chen CL, Zhang A, Bojikian KD, Wen JC, Zhang Q, Xin C, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography-based microangiography. Invest Ophthalmol Vis Sci. 2016;57:OCT475–85.
Martinez-de-la-Casa JM, Cifuentes-Canorea P, Berrozpe C, Sastre M, Polo V, Moreno-Montañes J, et al. Diagnostic ability of macular nerve fiber layer thickness using new segmentation software in glaucoma suspects. Invest Ophthalmol Vis Sci. 2014;55:8343–8.
Funding
The authors declare no funding sources
Author information
Authors and Affiliations
Contributions
This manuscript is own’s work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morales-Fernandez, L., Pérez-García, P., Fernández-Vigo, J.I. et al. Peripapillary and macular vascular parameters by optical coherence tomography angiography in primary congenital glaucoma. Eye 37, 267–273 (2023). https://doi.org/10.1038/s41433-021-01919-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01919-x