Abstract
Background
To assess reactivation after initial intravitreal injection of ranibizumab (IVR) for type 1 retinopathy of prematurity (ROP) or worse and the outcome following reinjection of ranibizumab for this reactivation.
Methods
This retrospective study was performed on infants screened for ROP between March 2013 and February 2020 in Mansoura University Children Hospital, Mansoura, Egypt. Infants treated with ranibizumab 0.25 mg/0.025 mL were identified for review of their clinical outcomes. Data of infants with reactivation and IVR re-injection were analysed.
Results
A total of 2318 infants were screened for ROP, 115 (5%) infants (216 eyes) with a mean gestational age of 30 ± 2.5 weeks and mean birth weight of 1290 ± 355.2 g received IVR at mean postmenstrual age (PMA) of 38 ± 3.1 weeks. All treated eyes demonstrated initial regression of ROP. However, ROP reactivation occurred in 5 (2.3%) eyes of 3 patients, at an average of 9.6 ± 2.9 weeks after treatment. None of these eyes had retinal detachment. A second dose IVR was administered and all five eyes showed regression with complete retinal vascularisation, at a mean PMA of 60 ± 5.1 weeks.
Conclusions
IVR is beneficial as an initial and subsequent treatment for type 1 ROP or APROP. A long-term follow-up until complete retinal vascularisation is recommended to avoid disease reactivation.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is a neovascular disorder of the developing retinal blood vessels of preterm infants. It is considered as a leading cause of childhood blindness all over the world, especially in middle-income countries [1]. The disease process is associated with high levels of vascular endothelial growth factor (VEGF) secreted by the avascular retina which in turn leads to neovascularization, retinal detachment and permanent visual loss [2]. Over the past several decades, retinal ablation by cryotherapy or laser has been the gold standard in treating severe ROP [3, 4]. However, they exhibit complications such as peripheral visual field defect and myopic shift. Moreover, there is an approximately 10% risk of retinal detachment or other unfavourable structural outcome despite laser treatment in Early Treatment ROP (ETROP) randomised trial [5]. This encouraged researchers to use (anti-VEGF) for treatment of ROP, enabling continuous vascularisation of the retina without destroying it and potentially minimising the risk of retinal detachment.
The most commonly used VEGF inhibitors are bevacizumab and ranibizumab. However, the safety and efficacy of both remain uncertain [6, 7]. Furthermore, some studies describing pharmacokinetics of anti-VEGF assumed that their VEGF suppression effect may be transient [8, 9]. This might explain reactivation of ROP after a single treatment with an anti-VEGF agent, either with bevacizumab or ranibizumab [10, 11]. Therefore, timely detection and management of reactivation has become a major concern in anti-VEGF therapy for ROP. This study was carried out to assess the reactivation after initial intravitreal injection of ranibizumab (IVR, Lucentis®) for type 1 ROP or worse and the outcome following reinjection of ranibizumab for this reactivation.
Methods
A retrospective review of medical records of preterm infants screened for ROP in Mansoura University children hospital, Mansoura, Egypt during the period from March 2013 to February 2020 was performed. This included infants admitted to neonatal intensive care unit or referred from other hospitals participating in the Egyptian Neonatal Network for ROP screening. We included records of infants with type 1 ROP or aggressive posterior ROP (APROP), who received IVR as initial monotherapy according to ETROP study [12]. In our hospital, the policy for ROP treatment is to use ranibizumab, not laser as a primary line of treatment. Records with incomplete data or patients with a follow-up of less than 6 months after initial IVR injection were excluded. The study followed the Declaration of Helsinki. It was approved by Mansoura faculty of medicine Institutional Review Board (code No R.20.08.985) and registered on www.clinicaltrials.gov (NCT04539106).
Data extracted included gestational age (GA), birth weight (BW), type of pregnancy (single or multiple), laterality of eye involvement, stage and zone of ROP and post menstrual age (PMA) at initial injection and at complete regression. Fundus examination of preterm babies was performed using binocular indirect ophthalmoscopy under topical anaesthesia, and fundus photographs were obtained with a RetCam III digital fundus camera. ROP was diagnosed and classified according to the International Classification of ROP [13]. Diagnosis was confirmed independently by at least two paediatric retina specialists.
Intravitreal ranibizumab injection was performed as follows; topical anaesthetic drop application, lid speculum insertion, standard aseptic eye preparation with 5% betadine and intravitreal injection of 0.25 mg/0.025 ml ranibizumab with a 30-gauge needle 1.5 mm from limbus [14]. In cases with bilateral ROP, both eyes were injected in the same session. Infants were examined on the next day, the next week after injection, then follow up was scheduled according to “American academy of pediatrics recommendation in 2013” until full retinal vascularisation was observed [15]. An informed consent was obtained from the parents of all infants before IVR injection. All cases were injected by the same surgeon (RB) under similar circumstances
Fundus photos taken by Retcam III, were reviewed before and after treatment. Data of infants with reactivation of ROP and IVR re-injection were analysed. Reactivation of ROP was defined as any of the following: recurrent plus disease, recurrent neovascularization at initial or new advancing ridge despite treatment [11].
A systematic literature search was performed involving studies that used IVR as an initial monotherapy for ROP treatment and different modalities for treatment of reactivation, in comparison with the current study.
Statistical analysis
Data was analysed with SPSS (Statistical Package for Social Science) V 21.0. Qualitative data was described using number and percentage. Continuous variables were presented as mean ± SD (standard deviation) for parametric data.
Results
From March 2013 to February 2020, 2318 preterm infants were screened for ROP, out of which 132 (5.7%) infants had treatment requiring ROP according to ETROP classification. Twelve infants were excluded due to incomplete data as well as 5 infants with follow-up examinations not extending to 6 months. One hundred fifteen (5%) infants were included in the study, of which 101 (87.8%) infants had bilateral disease and 14 (12.2%) had unilateral disease. Demographic data and baseline characteristics of treated infants are demonstrated in (Table 1).
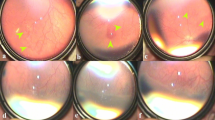
A total of 216 eyes had received IVR as an initial monotherapy at (PMA) of 38 ± 3.1 weeks; out of which 211 (97.7%) eyes showed disease regression with complete retinal vascularisation, at a mean PMA of 53.6 ± 5.1 weeks, while 5 eyes (2.3%) of 3 infants showed reactivation at a mean PMA of 46.7 ± 5.1 weeks, with treatment to reactivation interval of 9.6 ± 2.9 weeks. None of these five eyes had vitreoretinal traction or retinal detachment as a result of reactivation. All eyes with ROP reactivation had APROP in zone I at first diagnosis and received initial IVR at mean PMA of 36.7 ± 2.08 weeks. An adjunctive treatment with second dose IVR was administrated, and all five eyes showed regression and were followed up till complete retinal vascularisation, which occurred at a mean PMA of 60 ± 5.1 weeks. Profile of eyes with ROP reactivation is illustrated in (Table 2). A typical series of fundus photographs for right eye of infant (2) is shown in Fig. 1. A comparison among different studies showing reactivation after initial monotherapy of IVR and how it was managed is summarised in (Table 3).
a First visit before any intervention: there is APROP in Zone 1. b First week following initial IVR representing improvement of plus disease. c, d Twelve weeks after initial IVR: posterior and temporal fundus representing appearance of neovascularization and haemorrhage upon ridge (S3 in posterior Zone2). e One week after second IVR: regression of ridge and retinal neovascularization. f The temporal fundus photograph obtained at last visit shows complete regression with total retinal vascularisation (black arrow pointed to ora serrata).
Discussion
Reactivation of ROP is a serious problem that may result in vitreoretinal traction or retinal detachment. It has been reported following initial treatment with either laser photocoagulation or intravitreal anti-VEGF treatment with either bevacizumab or ranibizumab [10, 11, 16, 17].
Reactivation of ROP following IVR has been related to short systemic half-life and rapid clearing of ranibizumab from the vitreous [18]. However, reactivation rate reported in literature is variable. Some authors reported a relatively low reactivation rate [7], while others found a relatively high reactivation rate of ROP [19]. We reported the lowest reactivation rate (2.3%) after IVR compared to previous studies as shown in (Table 3). This could be due to a relatively mature infants (later GA and higher BW) than some other studies. In Egypt, we still have no guidelines regarding which preterm babies have to be screened. Being a middle-income country with different socioeconomic standards and limited health facilities, we screen all preterm infants (less than 37 weeks of gestation) in order not to miss ROP cases until establishment of an Egyptian screening protocol.
In 2018, Kimyon and Mete in Turkey reported similar GA and BW to ours, albeit a higher recurrence rate (7.1%) [20]. Similarly, several studies with lower GA and BW reported a higher reactivation rate (20.8–83%) [10, 21,22,23]. However, other studies reported high reactivation rate despite average GA or BW [19, 24,25,26]. Another explanation of the lower reactivation rate in our study may be the older PMA (36.7 ± 2.08 weeks) at initial therapy, compared to that reported in previous studies [21, 23, 27]. An early PMA at initial therapy is a risk factor for ROP reactivation, as those infants would have been more ill with a more serious ROP necessitating earlier intervention [21].
In this study, all eyes that had reactivation of ROP were diagnosed initially as APROP in zone I. This is consistent with the results of previous studies [11, 25, 28,29,30]. Feng et al. [28] found that zone I ROP had higher reactivation rate when compared to zone II. Moreover, the extent of retinal neovascularization has been identified by Lyu et al. [25] as an important predictor for prognosis of ROP.
Additionally, we noted that reactivation occurred in the form of stage 2 or 3 with plus disease. Similar observation was made by Ling et al. [21] who found that reactivation after initial anti-VEGF monotherapy mostly involved the return of plus disease and reappearance of neovascularization at the original site of the ridge and/or the advancing edge of retinal vascularisation.
In this study, the treatment to reactivation interval was 9.6 ± 2.9 weeks. A longer mean time of relapse (16 weeks) was reported by the BEAT-ROP study [6], which could be due to different pharmacokinetics of both ranibizumab and bevacizumab and different population included in both studies. Using IVR, Wong et al. [10] observed the shortest reactivation interval (5.9 weeks), that might be related to smaller GA (23.48 weeks) and lower BW (620 g) in their study population, while Zhang et al. [26] recorded the longest reactivation interval of (12.62 ± 7.93 weeks) and this might belong to using a higher dose (0.3 mg in 0.03 mL) of ranibizumab than all other studies.
We noticed that reactivation in both eyes of infant 1 occurred in anterior zone II with treatment to reactivation interval of 12 weeks and occurred in both eyes of infant 3 in posterior zone II with an interval of 6 weeks. This is in agreement with the work by Lyu et al. [25], where reactivation at or near to the initial site of neovascularization occurred significantly earlier than reactivation at a new vascular advancing edge.
For reactivation after initial IVR, some authors used laser photocoagulation [10, 19, 20, 22,23,24,25,26] or IVB [21] as alternative therapy. Their rationale was that using a different treatment modality than the initial one would give a better response. Moreover, laser following intravitreal injection of anti-VEGF provides retreatment on less avascular retina, thus reducing extent of its scar. On the other hand, some authors performed additional IVR either as single therapy [21, 31, 32] or combined with laser photocoagulation [31, 33]. Martínez-Castellanos et al. [33] recommended anti-VEGF re-injection for flat vessels and anti-VEGF re-injection combined with laser for neovascularization. In our practice, we prefer to use IVR as a single retreatment for ROP reactivation after initial IVR therapy. We believe that primary response to the initial treatment with signs of regression indicates success of this treatment modality and disease responsiveness. Reactivation noticed might be due to persistence of risk factors or exposure to a new risk factor that might increase VEGF in the avascular part of the retina, for example poor weight gain and sepsis. In addition, pharmacokinetics of anti-VEGF might explain reactivation following initial regression. Ranibizumab is a small molecule with a relatively short vitreous half-life (5.6 days) [34]. In this study, following IVR reinjection, all five eyes showed regression with successful retinal vascularisation without traction or dragging. Despite the small number of eyes, this result is encouraging and adds to the relatively limited data available on the safety and efficacy of anti-VEGF treatment for ROP. All previous studies have been conducted on European, Asian, or American population. To our knowledge, this is the first study conducted among African (Egyptian) infants. However, our study encountered some limitations, including its retrospective nature. We also acknowledge that being a single centre study may render its results less representative. However, our centre is a large tertiary referral centre and the study had a relatively large sample size.
In conclusion, this study demonstrated that IVR is beneficial as an initial and subsequent treatment for type 1 ROP or APROP. Reactivation is more common with APROP. It can occur as long as there is incomplete retinal vascularisation. Thus, we suggest a long-term follow-up until complete retinal vascularisation to observe any signs of disease reactivation.
Summary Table
What was known before
-
Intravitreal injection of ranibizumab (IVR) has been reported as alternative to conventional laser in primary treatment of severe retinopathy of prematurity (ROP).
-
However, reactivation of the disease has been documented in some studies, and researchers shifted back to laser as a treatment for reactivation.
What this study adds
-
In this study the reactivation rate after initial IVR for type 1 ROP or worse were assessed and the efficacy of reinjection of ranibizumab in reactivated cases were reported.
-
We believe this could be a better alternative to laser photocoagulation for reactivated ROP as it avoids the development of peripheral visual field defect or myopic shift following laser.
References
Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82.
Kwinta P, Bik-Multanowski M, Mitkowska Z, Tomasik T, Pietrzyk JJ. The clinical role of vascular endothelial growth factor (VEGF) system in the pathogenesis of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246:1467–75.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: preliminary results. Arch Ophthalmol. 1988;106:471–9
McNamara JA, Tasman W, Brown GC, Federman JL. Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology. 1991;98:576–80.
Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–50.
Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl J Med. 2011;364:603–15.
Chen SN, Lian I, Hwang YC, ChenYH, Chang YC, Lee KH, et al. Intravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between Ranibizumab and Bevacizumab. Retina. 2015;35:667–74.
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114:2179–82.
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114:855–9.
Wong RK, Hubschman S, Tsui I. Reactivation of retinopathy of prematurity after ranibizumab treatment. Retina. 2015;35:675–80.
Mintz-Hittner HA, Geloneck MM, Chuang AZ. Clinical Management of Recurrent Retinopathy of Prematurity after Intravitreal Bevacizumab Monotherapy. Ophthalmology. 2016;123:1845–55.
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Lemley CA, Han DP. An age-based method for planning sclerotomy placement during pediatric vitrectomy: a 12-year experience. Trans Am Ophthalmol Soc. 2007;105:86–91.
American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus & American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95.
Wang SD, Zhang GM, Shenzhen Screening for Retinopathy of Prematurity Cooperative Group. Laser therapy versus intravitreal injection of anti-VEGF agents in monotherapy of ROP: a Meta-analysis. Int J Ophthalmol. 2020;13:806–15.
Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394:1551–9.
Wu WC, Shih CP, Lien R, Wang NK, Chen YP, Chao AN, et al. Serum Vascular Endothelial Growth Factor After Bevacizumab Or Ranibizumab Treatment For Retinopathy Of Prematurity. Retina. 2017;37:694–701.
Huang Q, Zhang Q, Xu Y, Ji X, Fei P, Peng J, et al. Asymmetric Outcomes of Type 1 Retinopathy of Prematurity after Bilateral Intravitreal Ranibizumab Treatment. J Ophthalmol. 2017;2017:1741386.
Kimyon S, Mete A. Comparison of Bevacizumab and Ranibizumab in the Treatment of Type 1 Retinopathy of Prematurity Affecting Zone 1. Ophthalmologica. 2018;240:99–105.
Ling KP, Liao PJ, Wang NK, Chao AN, Chen KJ, Chen TL, et al. Rates and risk factors for recurrence of retinopathy of prematurity after laser or intravitreal anti-vascular endothelial growth factor monotherapy. Retina. 2020;40:1793–803.
Chan J, Lam C, Kwok M, Wong R, Lee G, Lau W, et al. Risk of recurrence of retinopathy of prematurity after initial intravitreal ranibizumab therapy. Sci Rep. 2016;6:27082.
Erol MK, Coban DT, Sari ES, Bilgin AB, Dogan B, Ozdemir O, et al. Comparison of intravitreal ranibizumab and bevacizumab treatment for retinopathy of prematurity. Arq Bras Oftalmol. 2015;78:340–3.
Arámbulo O, Dib G, Iturralde J, Brito M, Fortes Filho JB. Analysis of the Recurrence of Plus Disease after Intravitreal Ranibizumab as a Primary Monotherapy for Severe Retinopathy of Prematurity. Ophthalmol Retin. 2018;2:858–63.
Lyu J, Zhang Q, Chen CL, Xu Y, Ji XD, Li JK, et al. Recurrence of Retinopathy of Prematurity After Intravitreal Ranibizumab Monotherapy: Timing and Risk Factors. Investig Ophthalmol Vis Sci. 2017;58:1719–25.
Zhang G, Yang M, Zeng J, Vakros G, Su K, Chen M, et al. Comparison Of Intravitreal Injection Of Ranibizumab Versus Laser Therapy For Zone II Treatment-Requiring Retinopathy Of Prematurity. Retina. 2017;37:710–7.
Yi Z, Su Y, Zhou Y, Zheng H, Ye M, Xu Y, et al. Effects of Intravitreal Ranibizumab in the Treatment of Retinopathy of Prematurity in Chinese Infants. Curr Eye Res. 2016;41:1092–7.
Feng J, Qian J, Jiang Y, Zhao M, Liang J, Yin H, et al. Efficacy of Primary Intravitreal Ranibizumab for Retinopathy of Prematurity in China. Ophthalmology. 2017;124:408–9.
Garcia Gonzalez JM, Snyder L, Blair M, Rohr A, Shapiro M, Greenwald M. Prophylactic peripheral laser and fluorescein angiography after bevacizumab for retinopathy of prematurity. Retina. 2018;38:764–72.
Shah PK, Subramanian P, Venkatapathy N, Chan R, Chiang MF, Campbell JP. Aggressive posterior retinopathy of prematurity in two cohorts of patients in South India: implications for primary, secondary, and tertiary prevention. JAAPOS. 2019;23:264.e1–264.
Yang XM, Zhao YX, Wang ZH, Liu L. Effect of anti-VEGF treatment on retinopathy of prematurity in Zone II Stage 3. Int J Ophthalmol. 2018;11:641–4.
Hu Q, Bai Y, Chen X, Huang L, Chen Y, Li X. Recurrence of Retinopathy of Prematurity in Zone II Stage 3+ after Ranibizumab Treatment: A Retrospective Study. J Ophthalmol. 2017;2017:5078565.
Martínez-Castellanos MA, González-H León A, Romo-Aguas JC, Gonzalez-Gonzalez LA. A proposal of an algorithm for the diagnosis and treatment of recurrence or treatment failure of retinopathy of prematurity after anti-VEGF therapy based on a large case series. Graefes Arch Clin Exp Ophthalmol. 2020;258:767–72.
Fidler M, Fleck BW, Stahl A, Marlow N, Chastain JE, Li J, et al. Ranibizumab population pharmacokinetics and free VEGF pharmacodynamics in preterm infants with retinopathy of prematurity in the RAINBOW trial. Transl Vis Sci Technol. 2020;9:43.
Acknowledgements
This work was supported by Science and Technology Development Fund (STDF), as capacity building grant for equipment. The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
The study idea and design were conceived by RMB, WMG and MRB. Material preparation, data collection and analysis were performed by RMB, AEN, EAA and AGE. Preparation of the first draft of the manuscript was written by RMB and AGA. Final review of the manuscript was performed by WMG, AEN, AGA and MRB. All authors approved the final version of the manuscript that was submitted for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bassiouny, R.M., Gaafar, W.M., El Nokrashy, A. et al. Clinical outcome following reinjection of Ranibizumab for reactivation of retinopathy of prematurity. Eye 36, 2137–2143 (2022). https://doi.org/10.1038/s41433-021-01814-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01814-5
This article is cited by
-
Ranibizumab
Reactions Weekly (2023)
-
Comparison of bevacizumab, ranibizumab and aflibercept in retinopathy of prematurity treatment
International Ophthalmology (2022)