Abstract
Ophthalmic surgery requires a highly dexterous and precise surgical approach to work within the small confines of the eye, and the use of robotics offers numerous potential advantages to current surgical techniques. However, there is a lag in the development of a comprehensive training and credentialing system for robotic eye surgery, and certification of robotic skills proficiency relies heavily on industry leadership. We conducted a literature review on the curricular elements of established robotics training programs as well as privileging guidelines from various institutions to outline key components in training and credentialing robotic surgeons for ophthalmic surgeries. Based on our literature review and informal discussions between the authors and other robotic ophthalmic experts, we recommend that the overall training framework for robotic ophthalmic trainees proceeds in a stepwise, competency-based manner from didactic learning, to simulation exercises, to finally operative experiences. Nontechnical skills such as device troubleshooting and interprofessional teamwork should also be formally taught and evaluated. In addition, we have developed an assessment tool based on validated global rating scales for surgical skills that may be used to monitor the progress of trainees. Finally, we propose a graduating model for granting privileges to robotic surgeons. Further work will need to be undertaken to assess the feasibility, efficacy and integrity of the training curriculum and credentialing practices for robotic ophthalmic surgery.
摘要
眼科手术需要高度灵敏和精确的方法在眼部小范围工作, 而机器人的使用为当前的手术技术提供了许多潜在优势。然而, 机器人眼科手术的全面培训和认证体系的发展还很滞后, 机器人技能熟练程度的认证在很大程度上依赖于行业的领先地位。我们对已建立的机器人培训项目的课程内容进行了文献回顾, 并从各个机构获取了临床指南, 概述了眼科手术机器人外科医生的培训和认证的关键组成部分。基于我们的文献回顾和作者与其他机器人眼科专家之间的非正式讨论, 我们建议机器人眼科受训人员的整体培训框架以逐步的、基于能力的方式进行, 从教学式学习, 到模拟练习, 到最后的手术实践。非技术技能, 如设备故障排除和跨专业团队合作, 也应正式教授和评估。此外, 我们还开发了一个基于全球外科技能评价量表的评估工具, 可用于监控受训人员的进度。最后, 我们提出了一个授予机器人外科医生特权的分级模型。未来需要进一步开展相关研究, 评估培训课程的可行性、有效性和完整性, 以及机器人眼科手术的实践认证。
Similar content being viewed by others
Introduction
Ophthalmic microsurgery requires a highly dexterous, steady, and precise surgical approach to work within the small confines of the eye [1]. Ocular tissues such as the retina do not regenerate, making it imperative for surgeons to avoid preventable injuries [2]. The use of robotics in eye surgery offers numerous potential advantages to current surgical techniques, including increased precision and maneuverability, tremor reduction, better ergonomics, and simultaneous utilization of multiple surgical instruments and cameras [3, 4]. Robotic eye surgery was first described in 1989 [5], and since then, multiple ophthalmic robotic procedures have been developed and performed either experimentally or in humans, including corneal transplantation [6], penetrating keratoplasty [7, 8], cataract extraction [9, 10], and pars plana vitrectomy [11, 12].
Surgical subspecialties such as gynecology, urology, and general surgery have already integrated robotics into their surgical approach, and formal training programs to support this have been developed [13,14,15,16,17,18,19,20,21,22,23]. In contrast, robotic ophthalmic surgery still remains in its infancy with extremely limited implementation and no formal training curricula—despite the fact that robotic eye surgery has distinctly different technical and nontechnical elements compared to manual ophthalmic surgery [24, 25]. A competency-based robotics training pathway is needed to ensure the safe and appropriate use of this new technology: reducing the risks of preventable complications, responding to instrument or system malfunctions, assisting with hospital credentialing, and generally mitigating the medico‐legal aspects of inadequate surgical training [26].
The goals of this paper are to (1) review the curricular elements of established training programs in robotic surgery, (2) outline a training and credentialing framework for robotic eye surgery based on the experiences and suggested guidelines in existing robotic surgical training programs, and (3) identify commonalities and distinctive features of robotic ophthalmic surgery as they relate to training and credentialing.
Methods
An extensive literature search using MEDLINE, EMBASE, Web of Science, and Cumulative Index to Nursing and Allied Health Literature databases, as well as a focused internet search, was performed for all articles relevant to the training curriculum and credentialing process of robotic surgery. All articles from inception to March 2020 were included and no language restrictions were applied. Specific search strategies were used for each database and were tested by an academic librarian. The keywords and MeSH terms used to identify the articles were as follows: “robotic surgery training”, “curriculum,” “medical education”, “competency”, “credentialing”, and “privileging.” Since this study did not involve human subjects, institutional review board approval was not required.
A total of 2807 articles were retrieved from the literature search, and after removing the duplicates (1267), there were 1540 unique citations. Screening of the titles and abstracts to identify eligible studies based on a set of pre-determined inclusion criteria was conducted by one author (BH) and resulted in the exclusion of 1402 articles. Studies were excluded if they were not published in English, if they were not a journal publication, or if they did not have a focus on education, training, curriculum, or credentialing aspects of robotic surgery. The remaining 138 studies underwent a full text screening by two authors (BH and MS). This second round of screening excluded studies that utilized surveys, or if they only focused on a specific surgical procedure or technique. After the exclusion of 94 full text articles, a final total of 44 studies were included and categorized into three main subjects: (1) curriculum framework, (2) training modalities (dry, virtual and wet lab simulations) and assessment, and (3) credentialing processes. Discrepancies were resolved by discussion with a third reviewer (ME). A flow diagram outlining the process of this literature search is shown in Fig. 1.
Results
In total, there were 10 papers on curriculum framework, 26 papers on training modalities and assessment, and 8 papers on credentialing processes. The studies on the overall curricular framework reported performance outcomes following implementation of a robotics surgical training program and included multiple surgical disciplines across different academic training centers in Europe, Canada and the United States. [13,14,15,16,17,18,19,20, 22, 27] The principle training framework remained consistent in all ten of these papers, proceeding in a stepwise proficiency-based manner from didactic online lectures, to hands-on lab training, to finally gradual involvement in the operating room (Fig. 2). Of the eight papers that reported guidelines on their credentialing process, three of them were multidisciplinary and the other five were from general surgery, otolaryngology, gynecology, and thoracic surgery [28,29,30,31,32,33,34,35].
Training curriculum
Didactic learning
Based on our literature review, the da Vinci system (Intuitive Surgical Inc., Sunnyvale, CA, USA) is the most commonly studied robotics platform in the literature. Learning this system typically begins with building the foundational knowledge of robot technology, device functions, and system limitations [26]. Intuitive surgical provides free, interactive online modules to introduce the various components of its robotics platform [36], and each module has questions to assess learning and retention. Upon successful completion of all modules and an online knowledge assessment, a certificate of completion is provided. In addition to industry-provided online training modules, several training programs assign peer-reviewed articles that are to be discussed with the attending staff [13, 37].
Simulation training modalities
The next step in training typically incorporates knowledge application of robotic technology into a practical laboratory setting. Trainees usually participate in brief, in-person training sessions with industry representatives to familiarize themselves with the operation of the robotic consoles and to ensure proper and safe application of techniques [17]. There are three primary training environments that are most frequently used in robotic surgery: dry labs, virtual reality (VR) simulators and wet labs. In dry lab settings, individuals use inanimate models to acquaint themselves with the instruments and to develop dissecting and suturing techniques in a less immersive environment. The major limitation of this approach is the lack of an authentic environment and media that are representative of live or human tissue. VR simulators enable surgeons to practice techniques in a digital environment that more completely recreates entire surgical procedures. Compared to dry lab training, this is a more immersive experience and requires the trainee to practice surgical decision making, however, it still cannot replicate the feel and response of human tissue. Lastly, wet labs allow trainees to develop surgical skills on cadavers or live animal models, although the high costs associated with this training approach can be a limitation.
Studies have shown that individuals who train in a wet lab setting have higher minimal proficiency scores compared to those who train with VR or dry lab simulators [38]. In addition, the total training time for wet lab trainees to reach minimum proficiency was also significantly lower than dry lab training (116 min versus 561 min) [39]. For all three modalities, the most effective training approach was one that included numerous repetitions of a specified task that were independent to the training program duration) [40].
Operative experience
Most programs have trainees start with observing surgical cases in the operating theater and then progressing to a bedside assistant role, where procedural steps such as patient positioning and port placement, as well as physical and mechanical limitations of the robotic system are taught in the later stage [13, 19, 26, 41]. Once trainees advance to using the console, involvement is gradual, with the surgical case divided into achievable steps. Trainees are then allowed to perform increasingly more complex parts of the procedure at the discretion of the supervising surgeon as competency levels of more simple procedures are successfully attained [13, 19]. As dual console systems become more common, training will likely be enhanced as both the supervising surgeon and trainee are able to operate and interact collaboratively at the same time [42].
Assessment
In order to monitor trainee progress, competency benchmarks and a system for regular formal evaluation of proficiency need to be established. It is important to recognize that different individuals have different learning curves (i.e., the number of cases that need to be performed before there is an acceptable plateau of surgical performance parameters such as operating time, facility of tissue manipulation, complication rate, and conversion to manual techniques) [26]. As such, continuous bidirectional feedback during training is essential to help trainees identify their strengths and weaknesses, while also assisting trainers maintain an effective and supportive coaching and mentoring environment.
One clinical tool for assessing robotic surgical skills is the Global Evaluative Assessment of Robotic Skills (GEARS), which consists of six domains (depth perception, bimanual dexterity, efficiency, force sensitivity, autonomy, and robotic control) scored on a five-point Likert scale [43]. GEARS was created upon consultation with an expert panel of robotic surgeons and has been shown to be valid, reliable, and versatile in providing formative feedback to trainees in various training environments [44]. Alternatively, two other global rating scales that have also been validated for evaluating the skills of surgical trainees are the Global Operative Assessment of Laparoscopic Skills (GOALS) [45] and Objective Structured Assessment of Technical Skills (OSATS) [46]. Given the absence of any assessment tools specific for ophthalmic surgery, it is likely that one will need to be generated. One of our co-authors (MdS) has developed a modified grading scale (Table 1) based on GOALS and OSATS that can be used to evaluate ophthalmic robotic surgical skills and is currently analyzing its reliability and validity in assessing longitudinal performance in a clinical study.
Nontechnical skills
Although the training phases described above focus primarily on technical skills, nontechnical skills such as device troubleshooting, teamwork, and communication are equally as important in robotic surgery. In fact, one study has found 86% of adverse surgical events being unrelated to a surgeon’s technical ability [47], while another study suggests that 75% of negative surgical outcomes or delays from robotic surgeries are due to nontechnical complications such as electrical, system, or software failures [48]. Therefore, it is necessary that a robotics training curriculum include strategies for basic equipment troubleshooting and contingency planning (i.e., when to convert to an open approach) in the event that surgical devices become nonoperational.
As support staff and nursing personnel are key players in good surgical outcomes, team simulations to practice interprofessional teamwork and communication skills should be incorporated in the robotics surgical program. An aviation-style teamwork training program that emphasizes cooperation, problem-solving and situation awareness can help reduce technical errors and misunderstandings in the surgical theater [49]. In addition, the Non-Technical Skills for Surgeons and Oxford Nontechnical Skills Training Tools for the Surgical Team have been found to be effective for evaluating the nontechnical skills of the individual surgeon and the team, respectively [50]. All individuals assigned to robotic surgery should receive training and be assessed for their understanding of the robotic system and troubleshooting options. A pre and post-operative robotic checklist should be developed and adhered to so that device and site-specific operations are standardized and consistently performed [51]. A post-operative team debriefing to evaluate whether the robotic systemic performed as expected, and if there were deviations or modifications, should also be undertaken.
Credentialing structure
Surgical complications due to insufficient training and negligent credentialing can increase the risk of medicolegal consequences, and hospitals need to ensure an ethical and transparent model for privileging surgeons [52, 53]. Multiple institutions have developed their own training and credentialing parameters for robotic surgery, however there is no consensus yet on any standardized guidelines. Based on our review of different institutional policies (Table 2) [28,29,30,31,32,33,34,35], we propose the following stepwise algorithm of graduating privileges with continuous evaluation and monitoring of competency at each stage (Fig. 3). The highest minimum number of cases from the suggested guidelines was used in our model, although the minimum number of cases for progression of privileges may differ for ocular surgeries compared to non-eye procedures. It may also differ based on the complexity of the task or the level of automation in hybrid cases of robotic combined with nonrobotic surgeries.
The first level of surgical privileges is provisional: surgeons can perform robotic procedures under the direct supervision of a proctor. In order to be granted provisional privileges, surgeons need to have board certification, hold clinical privileges, and perform an appropriate annual volume of the non-robotic approach to the same procedure. For trainees who have completed a residency program with robotics training, a robotics case list with a minimum of 20 robotic cases, and the program director’s attestation of competency are required. Non-residency-trained surgeons (i.e., those who are already practicing and who wish to obtain robotic privileges) are also required to complete an approved robotic surgery course that includes online modules and hands-on exposure, although the course can be abbreviated for a more streamlined approach.
Both residency-trained and non-residency-trained robotic surgeons should complete initial cases within the first 2 months after training to avoid degradation of surgical skills [30]. Those who have completed at least ten proctored cases and who have received a formal recommendation by the division or department chair would then be able to proceed to the independent privileges level, which allow surgeons to perform robotic procedures without proctor supervision [29, 32, 35]. Most programs require at least 20 cases per year with no absence of cases longer than two months and review of the first ten robotic cases to maintain independent privileges [30, 33,34,35]. A departmental quality assessment (QA)/quality improvement (QI) steering committee should be formed to review case logs and outcomes for the annual renewal of the independent privilege position.
Surgeons who have independent robotic privileges and who have completed an annual minimum of 50 robotic procedures with good outcomes may be considered for proctorship privilege and be responsible for proctoring other trainees. Hospitals should publish standards for surgical outcomes and trends should be followed to identify deviations from the normal [33, 34]. If a surgeon wishes to attempt more complex cases, at least 15 basic cases without complications should be performed and the first two advanced cases should be assisted by another surgeon with privileges to conduct advanced procedures [30]. It is also recommended that surgeons submit a request to the robotic steering committee (similar to an ethics committee review) prior to undertaking more challenging procedures or when contemplating a novel procedure [33]. In the latter scenario, it would be also reasonable to include an assessment from the manufacturer regarding the technical ability of the system to carry out the novel procedure as proposed.
As forms of automated surgery become incorporated into the mainstream, guidelines to support robotic ophthalmic surgery will need to be created and these minimal standards would need to be re-evaluated.
Ophthalmological considerations
Robotic ophthalmic training
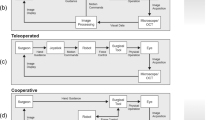
Given that robotic eye surgeries impose particular surgical and engineering challenges that are different from other robotic procedures, the development of training curricula and credentialing structures should take into account the unique intricacies of ophthalmic procedures. Currently, the robotic ophthalmic devices that are most frequently employed are assistive devices for controlling intraocular instruments in vitreoretinal surgeries. Since non-robotic eye surgery still requires microscopes and digital imaging systems for surgical visualization, the transition to ophthalmic robotic surgery will require gaining facility with controlling a single intraocular instrument that is telemanipulated via a handpiece while sitting at the traditional surgical position at the head of the bed. As bimanual robotic systems are currently not available, the surgeon’s other hand functions as it would in traditional surgery—typically holding a light pipe or a second instrument such as a pick or forceps. Under intraocular chandelier lighting, micromovements of the eye created by the instrument in the surgeon’s hand are eliminated, the eye becomes more stable, and the surgeon can focus their entire attention on the task that requires the robotic system.
To date, the only approved surgical robotic system for ophthalmology is the CE Mark licensed Preceyes Surgical System (BV, Eindhoven, Netherlands), with which clinical intraocular surgical trials are already underway [54]. This device was designed to approximate the functionality and operational movements required of traditional intraocular forceps, pick, scissors, or sub-retinal needle. However, motion-scaling, a larger handpiece, activation buttons, and suspension of the controller above and adjacent to the surgical field are significant modifications from traditional instrument use—requiring new learning and adjustment of pre-existing techniques. In contrast to the da Vinci system, the Preceyes system has a remote center of motion that allows for robotic programming of tasks to perform. This necessitates the surgeon to master a series of sequential subroutines as each subroutine interaction will be specific to the task and to the level of scaling in relation to the required task. Proficient understanding of the robot will be crucial to comprehend the subroutines and mitigations.
Despite these differences, however, the similarities of the Preceyes Surgical System to traditional vitreoretinal instrument manipulation seem to make learning this robot efficiently achievable. In fact, one of the areas in vitreoretinal surgery where robotics has gained considerable traction alongside conventional manual techniques is macular membrane peeling [55]. There is evidence to suggest that retinal surgeons are able to learn internal limiting membrane peeling within the Eyesi (VRmagic, Mannheim, Germany) simulated vitreoretinal surgical environment after a very short period of training on the Preceyes robot [56]. Based on early simulator data from this study, an appropriately focused course with measurable technical performance endpoints would confirm that attending retinal surgeons have acquired a sufficient degree of proficiency and safety on a device prior to in-human use.
Training with the Preceyes Surgical System can occur using many different simulation options, including a plastic eye model for learning basic movements and cellophane mock-ups for practicing sub-retinal injections and membrane peeling [57]. Moreover, the Preceyes system is compatible with the Eyesi (VRmagic) surgical simulator and can also be easily used in formal wet lab training environments with pig eyes. In contrast to existing non-ocular surgical wet labs, a pig’s eye model is both relatively inexpensive and somewhat representative of a human eye. At the time of writing, the Eyesi (VRmagic) robotics simulation module is not yet commercially available. As a result, one would expect training curricula in the absence of a simulated VR environment to follow the progression as discussed earlier in the paper from didactic to dry lab to wet lab. However, a VR simulation device would allow for the development and assessment of objective surgical metrics. In addition, because early cases with a robotics platform will likely involve a minority of the current retinal surgical workload, a simulator-based skills refresher course would need to be made available for surgeons who experience significant time intervals between robotic cases.
One of the challenges to integrating robotics into ophthalmology is ensuring an appropriate balance of benefits and costs. Specifically, robotics for ophthalmology need to demonstrate versatility in applicability for different surgeries, ability to enable novel therapies, and elimination of redundant personnel. At present, the increased precision of robotics for ophthalmic surgeries comes at the cost of increased surgical time [58]. With increasing surgical experience ideally enhanced by the use of simulators such as the Eyesi, improved surgical skills would lead to greater efficiencies. Similarly, as systems become more widely used in ophthalmic surgery, nursing and support staff will also become more proficient in the set-up and perioperative management of these cases.
Credentialing and privileging of robotic ophthalmic surgeons
Credentialing is the process of appointing a surgeon to medical staff at a healthcare facility and involves reviewing medical staff licensure, insurance, education, training, skills and experience. Privileging is the process of allowing surgeons to undertake specific procedures at certain facilities. Given the preliminary state of robotic ophthalmic surgery, few proficient surgeons, and the availability of only a single CE marked device, credentialing and privileging of new surgeons will likely be a challenge for administrators over the next number of years. As well, the Preceyes Surgical System and any new robotics platform for ophthalmic surgery will require significant training of not only the surgeon, but also support staff, nursing, biomedical engineers, and surgical assistants. It is our hope that documents such as this may give hospitals some understanding of what types of education and experience may be needed of surgeons and other team members prior to implementing a new surgical robotics ophthalmic program.
Conclusions
With its potential to enhance microsurgery, robotics will likely revolutionize ophthalmic surgery in the future. We have highlighted here an evidence-based roadmap of the essential components needed to build a robotics surgical skills curriculum and proposed a graduating model for granting robotic privileges. Several unique features to the training and credentialing of robotic surgeons for ophthalmic surgeries have also been identified. Further work will need to be undertaken to assess the feasibility, efficacy and integrity of training curriculum and credentialing practices for robotic ophthalmic surgery.
References
Roizenblatt M, Edwards TL, Gehlbach PL. Robot-assisted vitreoretinal surgery: current perspectives. Robotic Surg: Res Rev. 2018;5:1.
Cleary K, Nguyen C. State of the art in surgical robotics: clinical applications and technology challenges. Comput Aided Surg. 2001;6:312–28.
Hubschman JP, Wilson J, Tsao TC, Schwartz S. Robotic eye surgery. Ophthalmology. 2010;117:857.
Douglas R. Robotic surgery in ophthalmology: reality or fantasy? Br J Ophthalmol. 2007;91:1.
Kim Y, Spelman FA. SMOS: stereotaxical microtelemanipulator for ocular surgery. Images of the Twenty-First Century. Proceedings of the Annual International Engineering in Medicine and Biology Society. Seattle, WA, USA: IEEE; 1989.
Tsirbas A, Mango C, Dutson E. Robotic ocular surgery. Br J Ophthalmol. 2007;91:18–21.
Chammas J, Sauer A, Pizzuto J, Pouthier F, Gaucher D, Marescaux J, et al. Da Vinci Xi robot–assisted penetrating keratoplasty. Transl Vis Sci Technol. 2017;6:21.
Bourges J, Hubschman J, Burt B, Culjat M, Schwartz SD. Robotic microsurgery: corneal transplantation. Br J Ophthalmol. 2009;93:1672.
Bourcier T, Chammas J, Becmeur PH, Sauer A, Gaucher D, Liverneaux P, et al. Robot-assisted simulated cataract surgery. J Cataract Refract Surg. 2017;43:552–7. https://doi.org/10.1016/j.jcrs.2017.02.020
Chammas J, Sauer A, Bourcier T. Robotic surgery—a new way to perform cataract surgery. Acta Ophthalmol. 2017;95. https://doi.org/10.1111/j.1755-3768.2017.03585
Roizenblatt M, Edwards TL, Gehlbach PL. Robot-assisted vitreoretinal surgery: current perspectives. Robotic Surg. 2018;5:1–11.
Channa R, Iordachita I, Handa JT. Robotic vitreoretinal surgery. Retina. 2017;37:1220–8.
Moit H, Dwyer A, De Sutter M, Heinzel S, Crawford D. A standardized robotic training curriculum in a general surgery program. JSLS. 2019;23:e2019.00045 https://doi.org/10.4293/JSLS.2019.00045
Shay SG, Chrin JD, Wang MB, Mendelsohn AH. Initial and long-term retention of robotic technical skills in an otolaryngology residency program. Laryngoscope. 2019;129:1380–5.
Rusch P, Kimmig R, Lecuru F, Persson J, Ponce J, Degueldre M, et al. The Society of European Robotic Gynaecological Surgery (SERGS) Pilot Curriculum for robot assisted gynecological surgery. Arch Gynecol Obstet. 2018;297:415–20.
Mark Knab L, Zenati MS, Khodakov A, Rice M, Al-Abbas A, Bartlett DL, et al. Evolution of a novel robotic training curriculum in a complex general surgical oncology fellowship. Ann Surg Oncol. 2018;25:3445–52.
Fastenberg JH, Gibber MJ, Smith RV. Introductory TORS training in an otolaryngology residency program. J Robot Surg. 2018;12:617–23.
Sobel RH, Blanco R, Ha PK, Califano JA, Kumar R, Richmon JD. Implementation of a comprehensive competency-based transoral robotic surgery training curriculum with ex vivo dissection models. Head Neck. 2016;38:1553–63.
Seder CW, Cassivi SD, Wigle DA. Navigating the pathway to robotic competency in general thoracic surgery. Innovations. 2013;8:184–9.
Arain NA, Dulan G, Hogg DC, Rege RV, Powers CE, Tesfay ST, et al. Comprehensive proficiency-based inanimate training for robotic surgery: reliability, feasibility, and educational benefit. Surg Endosc. 2012;26:2740–5.
Mirheydar H, Jones M, Koeneman KS, Sweet RM. Robotic surgical education: a collaborative approach to training postgraduate urologists and endourology fellows. JSLS. 2009;13:287–92.
Geller EJ, Schuler KM, Boggess JF. Robotic surgical training program in gynecology: how to train residents and fellows. J Minim Invasive Gynecol. 2011;18:224–9.
Ahmed K, Khan R, Mottrie A, Lovegrove C, Abaza R, Ahlawat R, et al. Development of a standardised training curriculum for robotic surgery: a consensus statement from an international multidisciplinary group of experts. BJU Int. 2015;116:93–101.
Bourla DH, Hubschman JP, Culjat M, Tsirbas A, Gupta A, Schwartz SD. Feasibility study of intraocular robotic surgery with the da Vinci surgical system. Retina. 2008;28:154–8.
Fine HF, Wei W, Goldman R, Simaan N. Robot-assisted ophthalmic surgery. Can J Ophthalmol. 2010;45:581–4.
Schreuder H, Wolswijk R, Zweemer RP, Schijven MP, Verheijen R. Training and learning robotic surgery, time for a more structured approach: a systematic review. BJOG: Int J Obstet Gynaecol. 2012;119:137–49.
Foell K, Finelli A, Yasufuku K, Bernardini MQ, Waddell TK, Pace KT, et al. Robotic surgery basic skills training: Evaluation of a pilot multidisciplinary simulation-based curriculum. Can Urol Assoc J. 2013;7:430–4. https://doi.org/10.5489/cuaj.222
Estes SJ, Goldenberg D, Winder JS, Juza RM, Lyn-Sue J. Best practices for robotic surgery programs. JSLS. 2017;21:e2016.00102.
Ballantyne GH, Kelley WE Jr. Granting clinical privileges for telerobotic surgery. Surg Laparosc Endosc Percutan Tech. 2002;12:17–25.
AAGL Advancing Minimally Invasive Gynecology Worldwide. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21:157–67.
Erickson BK, Gleason JL, Huh WK, Richter HE. Survey of robotic surgery credentialing requirements for physicians completing OB/GYN residency. J Minim Invasive Gynecol. 2012;19:589–92.
Bhora FY, Al-Ayoubi AM, Rehmani SS, Forleiter CM, Raad WN, Belsley SG. Robotically assisted thoracic surgery: proposed guidelines for privileging and credentialing. Innovations. 2016;11:386–9.
Green C, Levy J, Martino M, Porterfield Jr. J. The current state of surgeon credentialing in the robotic era. Ann Laparosc Endosc Surg. 2020;5. https://doi.org/10.21037/ales.2019.11.06
Lenihan JP Jr. Navigating credentialing, privileging, and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54:382–90.
Gross ND, Holsinger FC, Magnuson JS, Duvvuri U, Genden EM, Ghanem TA, et al. Robotics in otolaryngology and head and neck surgery: Recommendations for training and credentialing: a report of the 2015 AHNS education committee, AAO-HNS robotic task force and AAO-HNS sleep disorders committee. Head Neck. 2016;38:E151–E158.
Intuitive. DaVinci Education. https://www.intuitive.com/en-us/products-and-services/da-vinci/education. Accessed 4 July 2020.
Winder JS, Juza RM, Sasaki J, Rogers AM, Pauli EM, Haluck RS, et al. Implementing a robotics curriculum at an academic general surgery training program: our initial experience. J Robot Surg. 2016;10:209–13.
Valdis M, Chu MW, Schlachta C, Kiaii B. Validation and comparison of novel robotic cardiac surgery simulation-based training programs. Can J Cardiol. 2015;31:S268.
Valdis M, Chu MW, Schlachta C, Kiaii B. Evaluation of robotic cardiac surgery simulation training: A randomized controlled trial. J Thorac Cardiovasc Surg. 2016;151:1498–.e2.
Walliczek U, Förtsch A, Dworschak P, Teymoortash A, Mandapathil M, Werner J, et al. Effect of training frequency on the learning curve on the da Vinci Skills Simulator. Head Neck. 2016;38:1762.
Badani KK, Hemal AK, Peabody JO, Menon M. Robotic radical prostatectomy: the Vattikuti Urology Institute training experience. World J Urol. 2006;24:148–51.
Fernandes E, Elli E, Giulianotti P. The role of the dual console in robotic surgical training. Surgery. 2014;155:1–4.
Goh AC, Goldfarb DW, Sander JC, Miles BJ, Dunkin BJ. Global evaluative assessment of robotic skills: validation of a clinical assessment tool to measure robotic surgical skills. J Urol. 2012;187:247–52.
Aghazadeh MA, Jayaratna IS, Hung AJ, Pan MM, Desai MM, Gill IS, et al. External validation of Global Evaluative Assessment of Robotic Skills (GEARS). Surg Endosc. 2015;29:3261–6.
Vassiliou MC, Feldman LS, Andrew CG, Bergman S, Leffondré K, Stanbridge D, et al. A global assessment tool for evaluation of intraoperative laparoscopic skills. Am J Surg. 2005;190:107–13.
Reznick R, Regehr G, MacRae H, Martin J, McCulloch W. Testing technical skill via an innovative “bench station” examination. Am J Surg. 1997;173:226–30.
Gawande AA, Zinner MJ, Studdert DM, Brennan TA. Analysis of errors reported by surgeons at three teaching hospitals. Surgery. 2003;133:614–21.
Alemzadeh H, Raman J, Leveson N, Kalbarczyk Z, Iyer RK. Adverse events in robotic surgery: a retrospective study of 14 years of FDA data. PLoS ONE. 2016;11:e0151470.
McCulloch P, Mishra A, Handa A, Dale T, Hirst G, Catchpole K. The effects of aviation-style non-technical skills training on technical performance and outcome in the operating theatre. Qual Saf Health Care. 2009;18:109–15.
Wood TC, Raison N, Haldar S, Brunckhorst O, McIlhenny C, Dasgupta P, et al. Training tools for nontechnical skills for surgeons-a systematic review. J Surg Educ. 2017;74:548–78.
Ahmed K, Khan N, Khan MS, Dasgupta P. Development and content validation of a surgical safety checklist for operating theatres that use robotic technology. BJU Int. 2013;111:1161–74.
Ferrarese A, Pozzi G, Borghi F, Marano A, Delbon P, Amato B, et al. Malfunctions of robotic system in surgery: role and responsibility of surgeon in legal point of view. Open Med. 2016;11:286–91.
Lee YL, Kilic GS, Phelps JY. Medicolegal review of liability risks for gynecologists stemming from lack of training in robot-assisted surgery. J Minim Invasive Gynecol. 2011;18:512–5.
Edwards TL, Xue K, Meenink HCM, Beelen MJ, Naus GJL, Simunovic MP, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng. 2018;2:649–56.
Nuzzi R, Brusasco L. State of the art of robotic surgery related to vision: brain and eye applications of newly available devices. Eye Brain. 2018;10:13–24.
Maberley DAL, Beelen M, Smit J, Meenink T, Naus G, Wagner C, et al. A comparison of robotic and manual surgery for internal limiting membrane peeling. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:773–8.
PRECEYES BV. PRECEYES surgical system. 2019. Accessed 12 August 2020.
de Smet MD, Naus GJL, Faridpooya K, Mura M. Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol. 2018;29:248–53. https://doi.org/10.1097/ICU.0000000000000476
Author information
Authors and Affiliations
Contributions
DM: conceptualization, methodology, validation, writing—original draft, writing—review & editing, visualization, project administration, supervision. ME: methodology, project administration. MS: methodology, investigation, writing - review & editing. MdS: conceptualization, writing—review & editing. BH: conceptualization, methodology, validation, investigation, data curation, writing—original draft, writing—review & editing, visualization, project administration.
Corresponding author
Ethics declarations
Conflict of interest
MdS is a co-founder, employee and patent holder of Preceyes BV. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. None of the other authors report a conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, B., de Smet, M.D., Sodhi, M. et al. A review of robotic surgical training: establishing a curriculum and credentialing process in ophthalmology. Eye 35, 3192–3201 (2021). https://doi.org/10.1038/s41433-021-01599-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01599-7
This article is cited by
-
Robot-assisted subretinal injection system: development and preliminary verification
BMC Ophthalmology (2022)