Abstract
Purpose
To compare the efficacy of topical voriconazole 1% and the combination therapy of 0.02% polyhexamethylene biguanide (PHMB) and 0.02% chlorhexidine for the treatment of Acanthamoeba keratitis (AK).
Methods
This is a prospective, pilot, double-masked randomized comparative study. Twenty-three eyes of 23 patients with microbiologically (smear and/or growth on culture) confirmed AK were randomized to group BG (PHMB 0.02% and chlorhexidine 0.02%) or group VZ (voriconazole 1%). Primary outcome measure was change in geometric mean (GM) of the corneal ulcer size at final visit. Secondary outcome measures were change in visual acuity.
Results
Out of 71 patients with confirmed AK seen during study period, 23 patients were recruited and 18 patients completed minimum 2 weeks of treatment and further analyzed. Ten patients received BG, whereas eight received VZ. Median ulcer size measured as GM of infiltrate decreased from 5.7 mm (IQR, 5.3–6.5 mm) (p = 0.02) to 1 mm (IQR, 0–4.3 mm) in group BG and from 4.5 mm (IQR, 1.8–5.1 mm) (p < 0.05) to 0.7 mm (IQR, 0–1.6 mm) in VZ group. Median visual acuity improved from 1.79 (IQR, 1.48–2.78) to 1.10 (IQR, 0.48–1.79) in BG group (p = 0.02) and from 1.60 (IQR, 1.00–2.78) to 0.80 (IQR, 0.48–1.30) in VZ group (p = 0.18).
Conclusion
These outcomes suggest that topical VZ as a monotherapy in AK treatment is effective and comparable to BG combination therapy but needs trials with larger sample size and longer follow-up to provide conclusive evidence.
Similar content being viewed by others
Introduction
Management of Acanthamoeba keratitis (AK) poses several challenges [1,2,3] such as delayed diagnosis, non-availability of approved drugs, and unpredictable response to medical treatment. Biguanides (BG) or diamidines [4,5,6], the mainstay drugs for this condition, are not available as commercial licensed ophthalmic preparations. They are required to be administered for a prolonged period often resulting in severe ocular surface toxicity and, in some cases, even intraocular damage in the form of dilated fixed pupil and cataract formation [7, 8]. Despite a prolonged treatment, 20–40% patients fail to respond and end up undergoing surgery [9,10,11,12,13,14] for the eradication of infection [15]. Therefore, the search for newer treatment with drugs having [16,17,18,19,20] superior efficacy and reduced ocular surface toxicity is ongoing.
In the past 2–3 decades, voriconazole has been extensively studied primarily for its activity against fungi but has also been studied for Acanthamoeba infection by several authors. The drug has been shown to be effective against both trophozoite and cyst forms in in vitro susceptibility studies [6, 21, 22]. Cabello–Vilchez et al. [20] found its efficacy to be superior to chlorhexidine and proposed it as a first line of management. It has been shown to induce programmed cell death [21] of Acanthamoeba. Monotherapy with topical voriconazole 1% was found to be effective in a rat cornea model of AK [12]. Use of voriconazole as an adjunctive treatment via topical [23] and oral routes [17, 24] has been shown to result in faster resolution of AK in anecdotal human case reports.
Since voriconazole is available as a commercial preparation, both for topical and oral administration, we intended to carry out a study to assess the efficacy of topical voriconazole monotherapy in the treatment of AK. The efficacy was assessed in comparison to the current standard treatment at our center i.e. combination of polyhexamethylene biguanide (PHMB) and chlorhexidine, in a prospective pilot randomized comparative masked study.
Methodology
The study protocol was approved by the Institutional Ethics Committee (LEC 08/16/06/063) and followed the tenets of the Declaration of Helsinki. The study was registered (CTRI/2018/04/013133) with Clinical Trials Registry-India (CTRI) and was conducted at a tertiary eye care center in southern India between August 2016 and March 2018. Being a pilot study and owing to low incidence of the infection, the arbitrary sample size of 30 was decided according to the Rules of thumb [25] as described by Browne et al. [26].
Consecutive cases of microbiologically confirmed AK during the study period were included in the study. As we were testing monotherapy with voriconazole for the first time, we excluded advanced cases with widest infiltrate diameter >8 mm, presence of gross thinning or corneal perforation, associated limbitis or scleritis along with those with age <18 years. We also excluded cases with mixed infection, i.e., cases with some other infection along with Acanthamoeba as confirmed on microbiological examination. Although confocal scan [27] is one of the tools to recognize other coexisting infections, it could not be performed in all cases owing to unavailability. Informed consent was obtained from all the cases.
Cases were randomized to either group biguanides (BG) (combination treatment with PHMB 0.02% and chlorhexidine 0.02%) or group VZ (voriconazole 1%) using random allocation table (generated by computer allocation table) by the microbiologist who also dispensed medication to both the groups in look-alike dispensers. These had similar appearances that could not be identified from outside. Both the patient and the evaluating physician were masked to the allocation group. All patients used two bottles (study drug X and study drug Y) with a gap of 1 hour. In group BG, X was PHMB and Y was chlorhexidine, whereas in group VZ, both X and Y were voriconazole. Topical medications were used hourly in day time, whereas every 2 hours during night time for 1 week followed by continuation of two hourly administration for 3 weeks and later reduction to six times a day till the resolution of infection. Topical cycloplegics were added in all cases. Patients were evaluated daily for 1 week and thereafter periodically based on the severity of the disease by single physician who was responsible for including, excluding, or withdrawing patients from the study. As the physician was completely masked to the study drug, decision about withdrawal of any patient was unbiased and done essentially on clinical signs.
The clinical response was assessed by measuring the size of corneal infiltrate and grading the anterior chamber inflammation under slit-lamp examination. The size of the infiltrate was measured in longest and widest perpendicular diameters and geometric mean (GM) was calculated. The severity of anterior chamber inflammation was graded using SUN criteria [28]. Visual acuity of the patients was assessed with Complog system [29] (computerized logMAR visual acuity measurement). Other outcome measure was healing time, i.e., the time for complete resolution of AK as defined by development of corneal scar (GM being 0 mm) without any signs of corneal and anterior chamber inflammation (zero grade). All enrolled cases of both groups were intended to treat for a minimum of 2 weeks duration to assess the clinical response and, if found to be worsened after 2 weeks or any point of time later, cases were withdrawn from the study and shifted to either combination of PHMB 0.02% and chlorhexidine 0.02% or subjected to surgical treatment according to the severity of infection. Treatment duration of minimum 2 weeks was decided based on our previous experience and published literature [30]. However, owing to ethical reasons and pilot nature of this trial, if the keratitis worsened within 2 weeks, appropriate decision was taken in favor of the patient.
The statistical analysis was performed using the software Origin v7.0 (OriginLab Corporation, Northampton, MA, USA). The continuous data were checked for the normality of distribution by Shapiro–Wilk test. The equality of variance was assessed by Levene test. Mean and standard deviation described the parametric data, whereas median and inter-quartile range (IQR) described the non-parametric ones. The categorical data were described in proportions. Between groups, continuous data were compared by either Student’s t-test (parametric data with equal variance) or Mann–Whitney test (parametric data with unequal variance or non-parametric data) and categorical data by Fisher’s exact test. Comparisons between different visits were done by paired t test or Wilcoxon signed-rank test for continuous data and McNemar test for categorical data. Linear regression was performed to evaluate the relationship between pre-treatment infiltrate size and change in infiltrate size. Sensitivity analyses were performed after including cases, which were originally excluded after randomization, by assuming both best and worst outcomes to determine the effect of the loss of those participants on the change in ulcer size. For this analysis, the worst outcome was defined as 2 mm increase in GM of infiltrate and the best one as clinically resolved infection (GM being zero mm). A p value of <0.05 was considered statistically significant.
Results
At the beginning of the study, we planned for 30 patients (15 in each group), however, the recruitment was terminated after inclusion of 23 patients owing to extremely slow recruitment. Despite seeing 71 patients of microbiologically proven AK during the study period, majority of the patients could not be recruited for various reasons (consort flow diagram).
After randomization, groups BG and VZ had 11 and 12 patients, respectively. Within BG group, one patient was withdrawn from the study after 1 day of treatment owing to reluctance for admission and follow-up. From VZ group, one patient was withdrawn on third day of recruitment owing to prior missing history of using topical voriconazole. Another three patients in VZ group received treatment only for 7–8 days and were later lost to follow-up. These subjects were excluded from the main analysis. Sensitivity analysis was performed to see the effect of including these subjects assuming best and worst outcomes (see later).
Remaining 18 (78.2%) patients were analyzed for their outcomes at last visit. There were 10 patients in BG group and 8 patients in VZ group. As shown in Table 1, both the groups (BG and VZ) were similar in the demographic details and clinico-microbiological features. Mean age of the patients was 39.8 ± 14.3 years (range, 19–67 years) and male: female ratio was 11:7. Median logMAR visual acuity was 1.80 (IQR, 0.86–2.78). Median duration from the onset of infection to presentation was 30 days (IQR, 7–60 days) in BG group and 20 days (IQR, 10–30 days) in VZ group. Median ulcer size measured as GM of the longest and widest perpendicular diameters of infiltrate was 5.7 mm (IQR, 5.3–6.5 mm) in group BG and 4.5 mm (IQR, 1.8–5.1 mm) in group VZ. Both the groups were similar in proportions of negative and positive smear and culture results.
Table 2 describes the comparison between the outcome of patients from both the groups. At last follow-up, median infiltrate size (in GM) decreased significantly (p = 0.02) to 1 mm (IQR, 0–4.3 mm) in BG group and decreased significantly (p < 0.05) to 0.7 mm (IQR, 0–1.6 mm) in VZ group. The final infiltrate size was comparable between BG and VZ groups (p = 0.62). Statistical power was calculated for the decrease in GM in both the groups and was found to be 95.6% in BG group and 92.1% in VZ group. Median inflammation score decreased significantly (p = 0.03 in BG group and p < 0.05 in VZ group) from 2.0 to 0.5 in both the groups. Median visual acuity improved from 1.79 (IQR, 1.48–2.78) to 1.10 (IQR, 0.48–1.79) in BG group (p = 0.02), whereas the improvement from 1.60 (IQR, 0.80–1.79) to 0.90 (IQR, 0.48–1.30) in VZ group did not achieve statistical significance (p = 0.18).
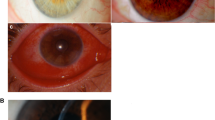
Clinically, although there was complete clinical resolution (Figs. 1 and 2) achieved in 4/10 patients from BG group and 4/8 patients from VZ group, 3/10 (30%) patients from BG group and 3/8 (37.5%) patients from VZ group were still in stage of clinical resolution at their last follow-up. The keratitis in 3/10 (30%) patients from group BG and 1/8 (12.5%) patient from group VZ worsened on the study treatment. Although one of these three patients from BG group worsened at 8 days of treatment and, based on clinical decision, was withdrawn, other two patients initially showed signs of clinical resolution with later worsening. Two of these three exited patients responded with medical treatment completely on follow-up while one patient continued to worsen and required keratoplasty. The patient withdrawn from group VZ owing to worsening of infection responded and healed completely when changed to biguanides.
The median healing time for resolution was 77.5 days in BG and 52 days in VZ group. On evaluating the relationship between pre-treatment infiltrate size (GM) and change in infiltrate size (change in GM) in both groups, it was found that in BG group, there was no significant (p = 0.06) relationship; however, in VZ group, there was a significant (p = 0.008) positive relationship between ulcer GM at presentation and change in GM (R = 0.85; y = 1.27x−2.39).
Assuming best outcome, median change in infiltrate size (in GM) was 5.3 mm (IQR, 0.5–6.1 mm) in BG group that was comparable (p = 0.77) to 5 mm (IQR, 0.5–5.9 mm) with exclusion. Similarly, median change in infiltrate size (in GM) was 5.1 mm (IQR, 1.8–5.8 mm) in VZ group that was comparable (p = 0.47) to 3 mm (IQR, −0.4 to 5.1 mm) with exclusion. Assuming worst outcome, median change in infiltrate size (in GM) was 4.7 mm (IQR, −1 to 5.9 mm) in BG group that was comparable (p = 0.86) to 5 mm (IQR, 0.5–5.9 mm) with exclusion. Similarly, median change in infiltrate size (in GM) was 0.7 mm (IQR, −1.4 to 3.2 mm) in VZ group that was comparable (p = 0.30) to 3 mm (IQR, −0.4 to 5.1 mm) with exclusion. Hence, sensitivity analyses based on including the unfavorable cases showed no significant difference from when excluding those cases, providing confidence in the findings. After the resolution, all patients were asked to follow-up regularly for a duration of 1 year to watch for any possible relapse of Acanthamoeba infection. Although 2/8 cases completed their 1 year follow-up after resolution, median duration of follow-up was 85 days (IQR, 30–180) days.
Discussion
Voriconazole [31] is well established as an adjuvant or monotherapy in the management of fungal keratitis [32]. As shown in in vitro trials [20, 21, 24, 33], there was an inhibitory role of voriconazole when used as the sole agent on Acanthamoeba cysts as well as trophozoites. In clinical cases [17, 23], it has been used as adjuvant treatment (both oral and topical) for refractory cases, but in contrast, in vitro experiments performed by Talbott et al. [34] showed antagonism between voriconazole and chlorhexidine. We realized that results of in vitro testing cannot be directly extrapolated to clinics owing to variability in the methodology of trials [35], different host-immune responses, and variation in the strains of Acanthamoeba used. Based on these information in the literature, we performed this pilot trial and observed an efficacious role of voriconazole as monotherapy in some of our patients. Topical voriconazole has its additional benefit than oral one owing to less-systemic toxicity [36] and better affordability by patients. We also compared their outcome with BG in combination as the later were considered as standard treatment for AK.
We analyzed all cases who received any of the study drug (BG or VZ) for minimum of 2 weeks and assessed their outcome. Although treatment duration of 2 weeks might not be appropriate for decision making in infection like Acanthamoeba, it was considered for our study in view of a pilot trial and ethical reason to assess timely management and plan alternative intervention in case of worsening. No patient in our series in either group showed signs of toxicity of the ocular surface, which is in concordance with the earlier reports of safety of voriconazole and BG in the dosage used for the treatment of AK [7, 37]. The clinical response in terms of resolution and worsening of keratitis was almost similar in both the groups. The outcome of BG group in the present study in terms of clinical resolution (7/10) was similar to the study [9] done by Duguid IG et al. [9]. Although relapse of infection is one of the complications in the medical management of AK on reduction or stoppage of the treatment, none of the patients from our study had recurrence reported on follow-up. This observation could be because of lesser duration of follow-up as well. One other observation related to the positive relationship in between pre-treatment size of ulcer and its change with treatment in case of VZ and not with BG, needs further studies to see its clinical significance.
So, this study has the limitations like heterogeneity of the groups in terms of previous treatment and severity of the disease, smaller sample size, and shorter treatment period. Yet as a proof of concept, this study provides important information related to efficacy of topical voriconazole as a monotherapy and additional studies with multicentric trial with larger sample size with lesser limitations will be able to add more evidence related to the utility of voriconazole.
Summary
What was known before
-
Voriconazole has been used as an adjuvant therapy for the management of Acanthameoba keratitis.
-
In animal trial, it has been used as a primary therapy and single therapy with good outcome in terms of eradication of infection.
-
It has proven good in vitro efficacy against Acanthamoeba.
What this study adds
-
We are trying topical voriconazole as a first-line therapy.
-
This will increase the options of management.
-
As voriconaole is commercially available and less toxic to ocular surface, it may be good alternative for the treatment of AK.
References
Carnt N, Robaei D, Minassian DC, Dart JKG. Acanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complications. Br J Ophthalmol. 2018;102:1431–35.
Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487–99 e2.
Sharma S, Garg P, Rao GN. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol. 2000;84:1103–8.
Tirado-Angel J, Gabriel MM, Wilson LA, Ahearn DG. Effects of polyhexamethylene biguanide and chlorhexidine on four species of Acanthamoeba in vitro. Curr Eye Res. 1996;15:225–8.
Narasimhan S, Madhavan HN, K LT. Development and application of an in vitro susceptibility test for Acanthamoeba species isolated from keratitis to polyhexamethylene biguanide and chlorhexidine. Cornea. 2002;21:203–5.
Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol. 2006;53:121–6.
Ehlers N, Hjortdal J. Are cataract and iris atrophy toxic complications of medical treatment of acanthamoeba keratitis? Acta Ophthalmol Scand. 2004;82:228–31.
Herz NL, Matoba AY, Wilhelmus KR. Rapidly progressive cataract and iris atrophy during treatment of Acanthamoeba keratitis. Ophthalmology. 2008;115:866–9.
Duguid IG, Dart JK, Morlet N, Allan BD, Matheson M, Ficker L, et al. Outcome of acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology. 1997;104:1587–92.
Illingworth CD, Cook SD. Acanthamoeba keratitis. Surv Ophthalmol. 1998;42:493–508.
Awwad ST, Parmar DN, Heilman M, Bowman RW, McCulley JP, Cavanagh HD. Results of penetrating keratoplasty for visual rehabilitation after Acanthamoeba keratitis. Am J Ophthalmol. 2005;140:1080–4.
Parthasarathy A, Tan DT. Deep lamellar keratoplasty for acanthamoeba keratitis. Cornea. 2007;26:1021–3.
Aichelburg AC, Walochnik J, Assadian O, Prosch H, Steuer A, Perneczky G, et al. Successful treatment of disseminated Acanthamoeba sp. infection with miltefosine. Emerg Infect Dis. 2008;14:1743–6.
Bagga B, Garg P, Joseph J, Mohamed A, Kalra P. Outcome of therapeutic deep anterior lamellar keratoplasty in advanced Acanthamoeba keratitis. Indian J Ophthalmol. 2020;68:442–6.
Kaiserman I, Bahar I, McAllum P, Srinivasan S, Elbaz U, Slomovic AR, et al. Prognostic factors in Acanthamoeba keratitis. Can J Ophthalmol. 2012;47:312–7.
Walochnik J, Obwaller A, Gruber F, Mildner M, Tschachler E, Suchomel M, et al. Anti-acanthamoeba efficacy and toxicity of miltefosine in an organotypic skin equivalent. J Antimicrob Chemother. 2009;64:539–45.
Tu EY, Joslin CE, Shoff ME. Successful treatment of chronic stromal acanthamoeba keratitis with oral voriconazole monotherapy. Cornea. 2010;29:1066–8.
Gupta S, Shrivastava RM, Tandon R, Gogia V, Agarwal P, Satpathy G. Role of voriconazole in combined acanthamoeba and fungal corneal ulcer. Cont Lens Anterior Eye. 2011;34:287–9.
Polat ZA, Obwaller A, Vural A, Walochnik J. Efficacy of miltefosine for topical treatment of Acanthamoeba keratitis in Syrian hamsters. Parasitol Res. 2012;110:515–20.
Cabello-Vilchez AM, Martin-Navarro CM, Lopez-Arencibia A, Reyes-Batlle M, Sifaoui I, Valladares B, et al. Voriconazole as a first-line treatment against potentially pathogenic Acanthamoeba strains from Peru. Parasitol Res. 2014;113:755–9.
Martin-Navarro CM, Lopez-Arencibia A, Sifaoui I, Reyes-Batlle M, Valladares B, Martinez-Carretero E, et al. Statins and voriconazole induce programmed cell death in Acanthamoeba castellanii. Antimicrob Agents Chemother. 2015;59:2817–24.
Rocha-Cabrera P, Reyes-Batlle M, Martin-Navarro CM, Dorta-Gorrin A, Lopez-Arencibia A, Sifaoui I, et al. Detection of Acanthamoeba on the ocular surface in a Spanish population using the Schirmer strip test: pathogenic potential, molecular classification and evaluation of the sensitivity to chlorhexidine and voriconazole of the isolated Acanthamoeba strains. J Med Microbiol. 2015;64:849–53.
Bang S, Edell E, Eghrari AO, Gottsch JD. Treatment with voriconazole in 3 eyes with resistant Acanthamoeba keratitis. Am J Ophthalmol. 2010;149:66–9.
Hou TY, Chen YC, Hsu CC. Rapid resolution of stromal keratitis with the assistance of oral voriconazole in resistant acanthamoeba keratitis. Taiwan J Ophthalmol. 2017;7:224–6.
Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25:1057–73.
Browne RH. On the use of a pilot sample for sample size determination. Stat Med. 1995;14:1933–40.
Wang YE, Tepelus TC, Vickers LA, Baghdasaryan E, Gui W, Huang P, et al. Role of in vivo confocal microscopy in the diagnosis of infectious keratitis. Int Ophthalmol. 2019;39:2865–74.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature Working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Laidlaw DA, Tailor V, Shah N, Atamian S, Harcourt C. Validation of a computerised logMAR visual acuity measurement system (COMPlog): comparison with ETDRS and the electronic ETDRS testing algorithm in adults and amblyopic children. Br J Ophthalmol. 2008;92:241–4.
Garg P, Kalra P, Joseph J. Non-contact lens related Acanthamoeba keratitis. Indian J Ophthalmol. 2017;65:1079–86.
Pearson MM, Rogers PD, Cleary JD, Chapman SW. Voriconazole: a new triazole antifungal agent. Ann Pharmacother. 2003;37:420–32.
Sharma N, Sahay P, Maharana PK, Singhal D, Saluja G, Bandivadekar P, et al. Management algorithm for fungal keratitis: the TST (Topical, Systemic, and Targeted Therapy) protocol. Cornea. 2019;38:141–5.
Gueudry J, Le Goff L, Compagnon P, Lefevre S, Colasse E, Aknine C, et al. Evaluation of voriconazole anti-Acanthamoeba polyphaga in vitro activity, rat cornea penetration and efficacy against experimental rat Acanthamoeba keratitis. J Antimicrob Chemother. 2018;73:1895–8.
Talbott M, Cevallos V, Chen MC, Chin SA, Lalitha P, Seitzman GD, et al. Synergy testing of antiamoebic agents for acanthamoeba: antagonistic effect of voriconazole. Cornea. 2019;38:1309–13.
Sunada A, Kimura K, Nishi I, Toyokawa M, Ueda A, Sakata T, et al. In vitro evaluations of topical agents to treat Acanthamoeba keratitis. Ophthalmology. 2014;121:2059–65.
Levine MT, Chandrasekar PH. Adverse effects of voriconazole: over a decade of use. Clin Transplant. 2016;30:1377–86.
Lau D, Fedinands M, Leung L, Fullinfaw R, Kong D, Davies G, et al. Penetration of voriconazole, 1%, eyedrops into human aqueous humor: a prospective open-label study. Arch Ophthalmol. 2008;126:343–6.
Funding
This study was funded by Hyderabad eye research foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bagga, B., Sharma, S., Gour, R.P.S. et al. A randomized masked pilot clinical trial to compare the efficacy of topical 1% voriconazole ophthalmic solution as monotherapy with combination therapy of topical 0.02% polyhexamethylene biguanide and 0.02% chlorhexidine in the treatment of Acanthamoeba keratitis. Eye 35, 1326–1333 (2021). https://doi.org/10.1038/s41433-020-1109-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-1109-4
This article is cited by
-
Response to ‘Comment on: ‘A randomized masked pilot clinical trial to compare the efficacy of topical 1% Voriconazole ophthalmic solution as monotherapy to combination therapy with topical 0.02% Polyhexamethylene biguanide and 0.02% Chlorhexidine in the treatment of Acanthamoeba keratitis’’
Eye (2022)
-
Comment on: ‘A randomized masked pilot clinical trial to compare the efficacy of topical 1% voriconazole ophthalmic solution as monotherapy with combination therapy of topical 0.02% polyhexamethylene biguanide and 0.02% chlorhexidine in the treatment of Acanthamoeba keratitis’
Eye (2022)
-
Update on the Management of Acanthamoeba Keratitis
Current Ophthalmology Reports (2022)