Abstract
Objectives
To analyze the effect of the employment of polyvinylpyrrolidone-iodine (PVP-I) 0.6% eye drop on the clinical course of patients affected by Adenoviral Keratoconjunctivitis (AKC).
Methods
Consecutive patients with clinical signs of AKC and positive results of AdenoPlus test were enrolled from four Italian Centres. Patients were randomized to receive: PVP-I 0.6% eye drops four times/daily for 20 days (Group A) or hyaluronate-based tear substitutes four times/daily for 20 days (Group B). Best-corrected visual acuity (BCVA), optical coherence tomography (OCT) Optovue iVue pachymetry map; corneal haze; conjunctival injection and chemosis; subepithelial corneal infiltrates (SEIs); corneal and conjunctival staining and corneal densitometry were recorded at diagnosis and at every follow-up visit. The primary outcome was the resolution time of AKC.
Results
Overall, 59 AKC patients (34 for Group A and 25 for Group B) completed the study. Patients of Group A showed a significantly shorter resolution time and lower incidence of SEIs compared to patients of Group B. In particular, SEIs were present at the last visit in 3/34 (8.82%) patients of the Group A vs 11/25 (44%) of the Group B (p = 0.005). Patients of Group A showed a significantly lower incidence of corneal haze compared to patients of Group B (0/34 vs 3/25; p = 0.038). No side effects were reported for both groups.
Conclusions
Although further clinical evaluations are needed, according to our data the use of PVP-I 0.6% eye drop in the setting of AKC reduces the risk of SEIs as well as the resolution time of the disease.
Similar content being viewed by others
Introduction
Around the world, infectious conjunctivitis is a common eye disease due to viruses, bacteria, or in some cases chlamydia. Among these, viruses are considered the most common cause, as the adenovirus (AdV) infections are involved in 92% of all keratoconjunctivitis cases [1, 2].
Conjunctivitis is usually a mild eye disorder although it can sometimes become severely disabling with most common symptoms like redness, swelling, tearing, and irritation which often last for 1 to 3 weeks. The corneal involvement distinguishes adenoviral keratoconjunctivitis (AKC) from other forms of viral conjunctivitis. Sometimes conjunctival pseudomembranes occur, while a fairly typical sign observed in many cases is ipsilateral preauricular lymphadenopathy [3].
A diagnosis of AKC is principally based on personal anamnesis, symptoms, and clinical manifestations. Although AKC is a self-limiting disease, most affected patients look for treatment due to visual impairment from persistent subepithelial infiltrates (SEIs), pseudomembranes, and iridocyclitis.
Until the complete resolution of the disease, patients usually complain of high pain even if being under standard treatments such as cold compresses, tear substitutes and topical vasoconstrictors.
Although topical corticosteroids represent a drug of choice when vision-threatening complications occur a recurrence rate of 30% has been reported after tapering the corticosteroids off [4, 5].
Recent studies suggested the employ of povidone-iodine in AKC [6, 7] since it acts as a broad-spectrum antiseptic agent. Povidone-iodine is a polymeric iodophor that reacts with oxygen containing functional groups of bacteria and viruses, causing oxidation of their proteins [8].
Herein, we aimed to evaluate the effect of polyvinylpyrrolidone iodine (PVP-I) 0.6% on the clinical course of AKC and on its late complications such as SEIs.
Methods
Patients
In this prospective multicentre randomized controlled study, 68 patients affected by AKC who presented to four Italian eye centers between June 2019 and September 2019 were enrolled. Nine patients dropped out of the study because they failed to complete the follow-up. Among the treated patients 26 were males (44%), the average age was of 42 years ± 13.5 with a range from 18 to 82. All patients were Caucasian.
Thirty-four patients were treated with Povidone-Iodine 0.6% (Iodim TM, Medivis Italy) eye drops four times a day for 20 days (A group) instead 25 patients used standard treatment with hyaluronate-based tear substitutes (B group). The concentration of PVP-I 0.6% was chosen because of the stability and pH characteristics of the molecule as well as in consideration of the fact that in Italy a medical device with such concentration (for which stability and sterility is guaranteed) is marketed.
The randomization scheme was generated by using the Web site Randomization.com (http://www.randomization.com). The study was conducted following the Declaration of Helsinki and the institutional review boards and the local ethics committee approved the trial.
Rapid diagnostic kit
For the diagnosis of AKC the clinical signs and the positive results of AdV ocular infection test were considered. AdenoPlus test (MT Promedt Consulting GmbH, Germany) uses direct-sampling microfiltration technology: no pretreatment is needed and the antigen could be directly transferred to the immunoassay test strip obtaining an enhanced sensitivity compared with other lateral flow technologies by concentrating antigen in its naive form at the time of collection. So, this technology eliminates any requirement for pretreatment or subsequent dilution and extraction steps [9,10,11].
All human adenovirus (HAdV) types contain the hexon protein that is detected by Adenoplus test. In the laboratory, types 1, 3, 4, 5, 7, 8, 11, 14, 19, 31, 37 were tested and demonstrated a positive antigen-antibody reaction [12].
Follow-up visits
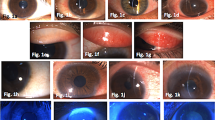
Each patient was observed every 48 h for the first 10 days and then 2 and 3 weeks after the first visit. At every observation we measured OCT pachymetry map, the presence (score 1) or absence (score 0) of corneal haze, conjunctival injection (using three reference images, as illustrated in Fig. 1A, to scoring it from 0 = no conjunctival injection, to 1 = mild conjunctival injection, or 2 = severe conjunctival injection), conjunctival chemosis (with a score from 0 = no chemosis, to 2 = severe chemosis), corneal infiltrates defined by a score (0 = no infiltrates, 1 = less than 10 infiltrates Fig. 1B, 2 = 10 or more infiltrates), corneal and conjunctival staining (scored, consistent with Oxford Scale –ref. [13]), ocular discomfort recorded on a numerical rating scale (NRS) using numbers to rate pain with a score from 0 (no discomfort) to 10 (pain), anterior, central, and posterior corneal densitometry measured by a Scheimpflug Camera (Pentacam System, Oculus, Germany). We also reported best-corrected visual acuity (BCVA) at the time of the first observation, the 10 days visit, and the last evaluation.
Eligibility criteria
Eligibility criteria were an age of 18 years or older with a clinical ocular adenoviral infection as confirmed by a positive AdenoPlus test.
Exclusion criteria
Patients who had ocular diseases like glaucoma or uveitis, who received any topical or systemic medications, used corticosteroid eye drops, and those who had any ocular surgeries in the previous 3 months or who had allergy to iodine were excluded from the study.
Outcome measures
The primary outcome of the study was to evaluate any difference in the resolution time of the infection in both groups of patients. The resolution time was defined according to objective variables evaluation (SEIs decrease or resolution, conjunctival chemosis and injection resolution, corneal transparency recovery) and subjective improvement of the typical distressing symptoms quantified on a NRS of pain. Changes in BCVA, corneal densitometry, and corneal staining during the follow-up were secondary outcome measures.
To perform the statistical analysis we used STATA/IC 15.1 software. Statistical analysis for continuous variables was performed using a two-sample t test with equal variances. Categorical variables were analyzed using the Chi-Square test of variance. A probability value beneath 0.05 was considered as statistically significant.
Results
A total of 68 patients were analyzed in four different Italian eye centers, nine patients dropped out of the study because they did not complete the follow-up and 59 patients completed the study.
Analysis did not evidence statistically significant differences among the patients of Group A (PVP-I 0.6% treatment) and Group B (hyaluronate-based tear substitutes) for the continuous variables (nor corneal pachymetry or densitometry) at all assessments. OCT pachymetry did not show significant changes over time between the two groups or among different patients within each group with an average value at the first observation of 562.95 ± 31.22 µm in the Group A and of 570.34 ± 25.38 µm in the Group B. At the last visit the values were respectively 556.19 ± 21.91 µm in the Group A and 566.85 ± 23.25 µm in the B group.
BCVA measured with Snellen charts at the first visit was 0.926 ± 0.102 in the Group A and 0.92 ± 0.119 in the Group B (p = 0.82). BCVA at the 10 days evaluation was 0.970 ± 0.062 in the Group A and 0.956 ± 0.065 in the Group B (p = 0.38). At the end of the study BCVA was 1.0 ± 0 in patients treated with PVP-I and 0.992 ± 0.027 in patients treated with hyaluronate-based tear substitutes (p = 0.09).
Principal data are resumed in Tables 1 and 2.
We observed that d-PVP-I 0.6% four times a day for 20 days statistically decreased the incidence of SEI development (Table 3) and made the resolution of acute adenoviral infection faster than tear substitutes, since reducing objective signs and subjective symptoms.
Moreover, subjective discomfort gets lower faster and in more patients in the A vs B group (Fig. 2A). Corneal staining is significantly reduced in the A group of patients.
At the end of the study 100% of patients of the A group had no corneal haze (Fig. 2B). No patient developed side effects from the use of PVP-I 0.6% eye drops.
Discussion
AdVs represent the etiological agent of 15–70% of eye infections. In subcontinental countries, SEIs may be ascertained in about half of AKC cases. These focal lesions could represent a cellular immune reaction against viral antigens deposited within the corneal stroma under the Bowman membrane, resulting in uncomfortable clinical symptoms and reduced patients’ daily activity. From a histopathological point of view SEIs are related to disruption of collagen fibers in the Bowman layer along with infiltration of lymphocytes, histiocytes, and fibroblasts; these are usually bilateral and often asymmetric and have the potential to cause significant ocular morbidity, vision impairment, photophobia, glare, halos, and foreign body sensation and can persist for months or years once the initial infection [14].
A lot of treatment options have been tried for AKC including palliative therapy, such as cool compresses, lubricant eye drops, and topical steroids. In current opinion steroids, by suppressing corneo-conjunctival inflammation, provide symptomatic relief, but they do not prove capable to shorten the course of the disease. On the other hand, the employment of long-run topical steroids may be also related to side effects such as cataract and glaucoma, not to mention the fact that the topical administration of corticosteroids may also cause prolonged viral seeding [6].
Since at the current state of the art there is no approved and efficient treatment for AKC, we investigated the role of a broad-spectrum antiseptic agent like PVP-I on the course of this kind of infection. A study that used the AdV type 5/New Zealand rabbit ocular model showed that corticosteroids alone can increase viral replication and therefore the length of infectious agent shedding [5].
AdV in fact is able to persist in the host thanks to the employment of immunoevasive strategies, such as inhibiting antiviral response mediated by IFNs, preventing cytolysis of AdV-infected cells by TNFα, promoting intracellular survival by blocking apoptosis of infected cells and evading CTL-mediated cytolysis of infected cells by preventing the display of viral peptide complexed to major histocompatibility complex (MHC) class I molecules on the surface of virally infected cells [7, 15]. Few studies have reported that AdVs retain the power, through the application of multiple pathways, to inhibit the NK response in the early and acute stages of the conjunctivitis: all those features do allow the virus to stay active on the conjunctival surface and provide rise to persistent infection [8, 16].
A decrease in viral titers, virus spread, shortening of the clinical course, and preservation of visual function during AKC has been obtained using Povidone-iodine 0.4% alone or in combination with dexamethasone 0.1% [17, 18]. There is currently an ongoing phase III clinical trial examining the use of a higher concentration povidone-iodine 0.6% alone and in combination with dexamethasone 0.1% versus placebo (NCT02998541) [19].
Yates et al. evaluated the in vitro antiviral activity of the several P–I concentrations previously used in clinical studies against different HAdV types frequently associated with eye infections [20].
Povidone (polyvinylpyrrolidone, PVP) is a synthetic polymer which acts as a carrier limiting the amount of free iodine present in solution. A peculiar aspect of PVP-I iodophors is that the concentration of free iodine increases with dilution of the complexed PVP-I due to a weakening of iodine linkage with PVP [21, 22]. This feature explains why a 0.6% PVP-I solution is more rapidly bactericidal than a 5% one [23].
No specific antiviral therapy approved by the Food and Drug Administration is accessible to shorten the course of the infection or stop the viral replication. Several virustatic agents such as cidofovir and ganciclovir are suggested in the first week, though there is a lack in definite dose and comparative studies [24].
Cidofovir was reported to have a therapeutic effect in the treatment of AKC in 1% dosage. It was reported to reduce the rate of severe corneal opacities, but therapeutic dosage resulted in high toxicity on the conjunctiva and eyelids with the development of pseudomembranes and lacrimal duct stenosis [25]. Since AdV are species-specific, in vivo models for disseminated AdV infections require the use of a non-human AdV. Moreover, the outcome of antiviral therapy requires early intervention during the viral replicative phase, whereas medical consultation for severe keratoconjunctivitis usually takes place later, once pathology is mainly inflammatory [26].
The results of this study indicate that an ophthalmic solution of PVP-I 0.6% is safe and well-tolerated for the treatment of acute adenoviral conjunctivitis. Outcomes for PVP-I 0.6% showed statistical superiority compared with artificial tears for clinical resolution of the infection probably thanks to a better adenoviral eradication. PVP-I, in facts, is highly effective at producing in vitro virucidal reductions of common ocular AdV types, [17] killing the extracellular adenoviral particles [27].
Moreover, in our study the use of PVP-I 0.6% decreased the incidence of SEI development probably thanks to the reduction of viral titers and consequently the immunological stimulus.
Low concentrations of PVP-I release a higher concentration of free Iodine, which is the only responsible for the antiseptic action. On the basis of previous in vivo and in vitro studies, the chosen dosage for PVP-I 0.6% was one drop four times a day [5, 24].
Adenoviral conjunctivitis usually lasts from 2 to 3 weeks. In our study, the number of patients with complete haze resolution and subjective mild discomfort was significantly higher in the PVP-I group than in the hyaluronate-based tear substitutes group as early as the day 15 visit.
These results suggest that PVP-I may be effective at quicker resolving the viral infection and improving clinical signs, which may improve the quality of life and minimize the spread of infection. A quick recovery from conjunctivitis is also expected to translate into a positive socioeconomic impact because patients are able to return to school or work sooner.
There were no adverse effects during the study. Moreover, the drop tolerability of PVP-I 0.6% was acceptable, with a reduction also of the total discomfort score at the 15 and 21-day visit in the A group patients. This is noteworthy as a result since instillation’s comfort is a component of ocular tolerability, and ocular discomfort will affect patient preference and treatment compliance.
The diagnosis between the various forms of conjunctivitis (bacterial, viral, allergic, and even early stage of acanthamoeba keratitis) can be difficult since different forms of the condition gift analog symptoms. Misdiagnosis of bacterial conjunctivitis might occur in up half of cases, which often ends in inappropriate antibiotic treatment. PVP-I has broad-spectrum antimicrobial action that includes bacteria, viruses, and fungi [28, 29]; that’s why another advantage of its use could moreover extend to the mitigation of the negative effects of misdiagnosis and consequent reduction of the costs and risks associated with unnecessary antibiotic prescriptions [30].
Although the small size of our sample did not allow us to appreciate statistically significant differences in the visual outcome achieved between the two groups, the trend would seem to be a faster functional recovery in group A patients probably due to the lower number of SEIs. This undoubtedly represents an interesting aspect that could be evaluated in further studies.
In conclusion, according to our results, using PVP-I 0.6% in the first few days of clinically significant AKC might help to reduce the risk of SEI as a complication. Diluted povidone-iodine ought to be started as soon as possible when we suspect AKC: in fact, it decreases the incidence of SEI, presumably by decreasing the virus load. We tend to believe that it might be a useful, cheap, safe and easy to access aid in the management of AKC in common clinical practice.
Summary
What was known before
-
There are no approved treatments for AKC 2. PVP-I acts as a broad-spectrum antiseptic agent.
What this study adds
-
PVP-I 0.6% is safe and well-tolerated for the treatment of acute adenoviral conjunctivitis.
-
PVP-I may be effective at quicker resolving the viral infection and improving clinical signs, which may improve the quality of life and minimize the spread of infection 3. Diluted povidone-iodine ought to be started as soon as possible when we suspect AKC.
References
Mueller AJ, Klauss V. Main sources of infection in 145 cases of epidemic keratoconjunctivitis. Ger J Ophthalmol. 1993;2:224–7.
Aoki A, Isobe K, Ohno S. Nationwide surveillance program of epidemic conjunctivitis in Japan. In: Bialasiewicz AA, Schaal KP, editors. Infectious diseases of the eye. Philadelphia: Butterworth-Heinemann; 1994. p. 309–16.
Hillenkamp Jost, Reinhard T, Ross RS, Böhringer D, Cartsburg O, Roggendorf M, et al. Topical treatment of acute adenoviral keratoconjunctivitis with 0.2 cidofovir and 1% cyclosporine. A pilot study. Arch Ophthalmol. 2001;119:1497–1491.
Varu DM, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, et al. American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Conjunctivitis Preferred Practice Pattern. Ophthalmology. 2019;126:P94–P169. https://doi.org/10.1016/j.ophtha.2018.10.020.
Pepose JS, Ahuja A, Liu W, Narvekar A, Haque R. Randomized, controlled, phase 2 trial of povidone-iodine/dexamethasone ophthalmic suspension for treatment of adenoviral conjunctivitis. Am J Ophthalmol. 2018;194:7–15.
OYong K, Killerby M, Pan CY, Huynh T, Green NM, Wadford DA, et al. Outbreak of epidemic keratoconjunctivitis caused by human adenovirus type D53 in an eye care clinic - Los Angeles County, 2017. Morb Mortal Wkly Rep. 2018;67:1347–9.
Harvey SA, Romanowski EG, Yates KA, Gordon YJ. Adenovirus directed ocular innate immunity: the role of conjunctival defensin-like chemokines (IP-10, I-TAC) and phagocytic human defensin-α. Investig Ophthalmol Vis Sci. 2005;46:3657–65.
Chigbu DI, Labib BA. Pathogenesis and management of adenoviral keratoconjunctivitis. Infect Drug Resist 2018;11:981–93.
Sambursky R, Trattler W, Tauber S, Starr C, Friedberg M, Boland T, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol 2013;131:17–22.
Kam KY, Ong HS, Bunce C, Ogunbowale L, Verma S. Sensitivity and specificity of the AdenoPlus point-of-care system in detecting adenovirus in conjunctivitis patients at an ophthalmic emergency department: a diagnostic accuracy study. Br J Ophthalmol. 2015;99:1186–9.
Holtz KK, Townsend KR, Furst JW, Myers JF, Binnicker MJ, Quigg SM, et al. An assessment of the adenoplus point-of-care test for diagnosing adenoviral conjunctivitis and its effect on antibiotic stewardship. Mayo Clin Proc Innov Qual Outcomes 2017;1:170–5.
Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4 T-cell epitope. Hum Gene Ther 2002;10:1167–78.
Workshop, Dry eye. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007;5:108–52.
Bialasiewicz A. Adenoviral Keratoconjuunctivitis. Sultan Qaboos Univ Med J. 2007;7:15–23.
Rajaiya J, Chodosh J. New paradigms in infectious eye disease: adenoviral keratoconjunctivitis. Arch Soc Esp Oftalmol. 2006;81:493–8.
Burgert HG, Ruzsics Z, Obermeier S, Hilgendorf A, Windheim M, Elsing A. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318.
Freyler H, Sehorst W. The fate of corneal infiltrations in cases of epidemic keratoconjunctvitis. Wien Klin Wochenschr. 1976;28:341–3.
Lachapelle J-M, Castel O, Casado AF, Leroy B, Micali G, Tennstedt D, et al. Antiseptics in the era of bacterial resistance: a focus on povidone iodine. Clin Pract. 2013;10:579–92.
Kovalyuk N, Kaiserman I, Mimouni M, Cohen O, Levartovsky S, Sherbany H, et al. Treatment of adenoviral keratoconjunctivitis with a combination of povidone-iodine 1.0% and dexamethasone 0.1% drops: a clinical prospective controlled randomized study. Acta Ophthalmol. 2017;95:e686–e692.
Yates KA, Shanks RMQ, Kowalski RP, Romanowski EG. The in vitro evaluation of povidone-iodine against multiple ocular adenoviral types. J Ocul Pharmacol Ther. 2019;35:132–6.
Pinto RD, Lira RP, Abe RY, Zacchia RS, Felix JP, Pereira AV, et al. Dexamethasone/povidone eye drops versus artificial tears for treatment of presumed viral conjunctivitis: a randomized clinical trial. Curr Eye Res. 2015;40:870–7.
BASF SE—Care Chemical Division. PVP-iodine grades. Technical information, August 2010, https://industries.basf.com/bin/bws/documentDownload.en.8805242644949.
Musumeci R, Bandello F, Martinelli M, Calaresu E, Cocuzza CE. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur J Ophthalmol. 2019;29:673–7.
Kaufman HE. Adenovirus advances: new diagnostic and therapeutic options. Curr Opin Ophthalmol. 2011;22:290–3.
Kaufman HE, Haw WH. Ganciclovir ophthalmic gel 0.15%: safety and efficacy of a new treatment for herpes simplex keratitis. Curr Eye Res. 2012;37:654–60.
Hillenkamp J, Reinhard T, Ross RS, Böhringer D, Cartsburg O, Roggendorf M, et al. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology. 2002;109:845–50.
Lenaerts L, Naesens L. Antiviral therapy for adenovirus infections. Antivir Res. 2006;71:172–80.
Monnerat N, Bossart W, Thiel MA. Povidone-iodine for treatment of adenoviral conjunctivitis: an in vitro study. Klin Monbl Augenheilkd. 2006;223:349–52.
Sunil KP, Raja BP, Jagadish RG, Uttam A. Povidone iodine-revisited. Indian J Dent Adv. 2011;3:617–20.
Visscher KL, Hutnik CM, Thomas M. Evidence-based treatment of acute infective conjunctivitis: breaking the cycle of antibiotic prescribing. Can Fam Physician. 2009;55:1071–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ricciardelli, G., Giannaccare, G., Di Zazzo, A. et al. Efficacy and tolerability of polyvinylpyrrolidone-iodine 0.6% treatment in adenoviral keratoconjunctivitis: a Prospective Randomized Controlled Study. Eye 36, 160–166 (2022). https://doi.org/10.1038/s41433-020-01344-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01344-6
This article is cited by
-
Prophylaxis of Ocular Infection in the Setting of Intraocular Surgery: Implications for Clinical Practice and Risk Management
Ophthalmology and Therapy (2023)
-
Antiseptics and the Ocular Surface: In Vitro Antimicrobial Activity and Effects on Conjunctival and Corneal Epithelial Cells of a New Liposomal Ocular Spray Containing Biosecur® Citrus Extract
Ophthalmology and Therapy (2022)