Abstract
Purpose
To study the varied demographic, visual and clinical presentation of patients with nanophthalmos.
Methods
Retrospective chart review of 144 consecutive subjects with nanophthalmos from January 2010 to January 2018 was done. Demographic details, visual acuity, refractive status, clinical parameters and surgical data were collected.
Results
Mean age at presentation was 48.76 ± 15.99 years (5–74 years) and 55.6% were females. Median BCVA was 0.78 (6/36 Snellen equivalent) and median spherical equivalent was +10.0 (5.7–12.5) . Amblyopia was seen in 30.0% patients. Legal blindness was present in 16.7% of the study population. The mean IOP was 18.38 ± 9.38 mmHg. Angle closure disease was present in 67.7% and 35.7% had angle closure glaucoma. Presence of peripheral anterior synechia had higher odds (OR = 3.66; 95% CI, 1.71–7.84) of associated glaucoma. The mean axial length, 17.64 ± 1.74 mm was inversely correlated to the mean Retinochoroidal Sclera(RCS) thickness of 1.99 ± 0.25 mm (r = −0.28, p value < 0.001).All patients who had undergone surgery for glaucoma (n = 11) had associated intra or postoperative complication. Cataract surgery by manual small incision had more complications than Phacoemulsification (p value = 0.001). Occurrence of uveal effusion was significantly lower in eyes which underwent sclerostomy (p = 0.04)
Conclusion
Nanophthalmos is a rare disorder with varying degree of visual impairment & amblyopia. High incidence of angle closure glaucoma was observed. Surgical management for glaucoma is often challenging with frequent complications. Cataract surgery by phacoemulsification had significantly lower complications than SICS and performing a concomitant sclerostomy reduced the occurrence of uveal effusions
Similar content being viewed by others
Introduction
Nanophthalmos or ‘dwarf eye’ is characterized by a small eye where the anterior and posterior segments are reduced in size, with abnormally thickened sclera [1]. It is a rare disease with a prevalence of <1% in most populations [1].
Diagnostically, axial length (AL) below 20.5 mm and Retinochoroidal sclera thickness (RCS) > 1.7 mm with no associated ocular malformations are grouped under this special subtype of microphthalmos [2, 3]. Nanophthalmos occurs due to the developmental arrest of the globe, after the embryonic fissure is closed. This results in the abnormally thickened sclera, short AL, small corneal diameter, and crowding of the anterior chamber secondary to a high lens to eye volume ratio [2,3,4]. Diagnosis and management of nanophthalmos poses certain unique challenges. In the early years, nanophthalmos can affect visual acuity leading to amblyopia and strabismus [5]. In adulthood, angle closure glaucoma, exudative retinal detachment, CME or uveal effusions can threaten visual function [1,2,3,4].
Due to the rarity of the condition, there have been no large-scale studies done on nanophthalmos. The objective of the current study is to report the demographic profile, clinical presentation, ocular parameters and visual morbidity in patients with nanophthalmos which perhaps is the largest series till date.
Methods
We retrospectively analyzed the clinical data of all subjects diagnosed with nanophthalmos at Aravind eye hospital, Madurai over a period of 8 years, between January 2010 and June 2018. The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of our hospital (RET201000192). Eyes with anterior and posterior segment malformations were excluded. Patients with complex microphthalmos, relative anterior microphthalmos and posterior microphthalmos, were excluded from the study.
Nanophthalmos was diagnosed based on AL below 20.5 mm and RCS > 1.7 mm, measured by B-scan (ophthalmic technology incorporation, USA) with no associated ocular malformations. Glaucoma diagnosis was confirmed if the IOP was >21 or ≤21 mmHg, with optic nerve damage in the form of rim thinning, notching, nerve fibre layer defect or asymmetric disc cupping. Angle closure disease was defined according to American academy of ophthalmology preferred practice pattern classification 2015 as primary angle closure suspect (PACS), primary angle closure (PAC) and primary angle closure glaucoma (PACG) [6]. Amblyopia was defined as the reduction of best-corrected visual acuity of one or both eyes that cannot be attributed exclusively to a structural abnormality of the eye [7, 8]. Classification of visual impairment was done according to the International Classification of Diseases 11 [9].
The data recorded was age, gender, presenting complaints, laterality, uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), spherical equivalent, intraocular pressure (IOP), gonioscopy grading of angles by modified Shaffer along with presence of peripheral anterior synechiae (PAS), slit-lamp biomicroscopy, undilated or dilated optic disc and retinal changes, cycloplegic refraction when available, AL measurement, anterior chamber depth (ACD), lens thickness using IOL master and RCS measurement by B-mode ultra-sonogram. In patients requiring cataract surgery Hoffer Q formula was used to calculate the intraocular lens (IOL) power, and a foldable hydrophilic lens was placed. For those requiring a power more than 40 D a custom-made lens option was given. Number of antiglaucoma medications (AGM), prior laser or surgical intervention(s), surgery details and complications (intraoperative or postoperative complications regardless of severity was documented) and visual field data wherever possible were collected.
Statistical analysis predominantly consisted of descriptive analysis. Descriptive statistics were presented with frequency and percentage for categorical parameters. Mean and standard deviations (SD) were used for normative parametric data while median and interquartile ranges (IQR) were used for non-parametric data. Parametric statistical tests were used if the data was distributed normally and for skewed data non-parametric tests were performed. Normality of the data was checked using Shapiro-Wilk test and box-plot. Visual acuity (Snellen’s equivalent) values were converted into log MAR and median log MAR visual acuity was presented. To find out the association between categorical variable Chi-square test or Fisher’s exact test was performed. Student’s t test was used to find out the significant difference in patients who underwent peripheral iridotomy between different age groups. Paired t test or Wilcoxon sign rank test was used to find out the significant difference between baseline and postoperative IOP, antiglaucoma medications and BCVA. Pearson correlation coefficient was used to find out the correlation between AL and RCS thickness. Logistic regression analysis was used to find out the factors associated with glaucoma such as age, gender, AL, gonioscopic findings, RCS and YAG PI. p value < 0.05 considered as statistically significant. All statistical analysis was done using statistical software STATA 14.0 (TX, USA).
Results
The study included records of 144 patients with nanophthalmos. The demographic details are given in (Table 1). The age at presentation ranged from 5 to 74 years with a mean of 48.76 ± 15.99 years. The common presenting complaints were defective vision (73.6%), routine evaluation (24.3%), pain (0.7%) and others (1.4%).
Visual status at presentation
Median log MAR UCVA was 1.30(3/60) while the log MAR BCVA was 0.78(6/36) at presentation. Median (IQR) of spherical equivalent was +10.0 (5.7–12.5) D at presentation (n = 121/232). Varying degree of visual impairment was seen in 57.6% and Amblyopia was diagnosed in 30.0% of the patients according to the American academy guidelines.
At presentation, 38.2% had moderate visual impairment, 19.4% had severe visual impairment and 16.7% of patients were legally blind. Cause for blindness included; glaucomatous optic atrophy 54.2% (13/24), retinitis pigmentosa 20.8% (5/24), choroidal effusion 12.5% (3/24), Phthisis bulbi 4.2 % (1/24) and spontaneous exudative retinal detachment 8.3% (2/24).
Ocular parameters
The mean AL was 17.64 ± 1.74 mm which inversely correlated to the mean RCS thickness of 1.99 ± 0.25 mm (r = −0.28, p value < 0.001). The average lens thickness was 4.27 ± 0.70 mm. Fundus findings at presentation was hyperaemic small discs (65.8%), glaucomatous disc damage (15.5%), retinitis pigmentosa (6.5%), pale disc (5.6%), macular hypoplasia (3.1%), uveal effusion (2.6%) and disc oedema in (0.9%) eyes.
Prevalence of glaucoma
The mean IOP was 18.38 ± 9.38 mm of Hg. Females had a higher IOP than males though not statistically significant (p value = 0.076). On gonioscopy 88.7% of eyes had closed angles with PAS (23.2%) or without PAS (65.5%). Fifteen eyes were pseudophakic and had open angles. The presence of PAS had 3.66 times higher odds of having angle closure glaucoma (OR, 95%CI = 3.66, 1.71–7.84, p value = 0.001) (Table 2). Acute angle closure crisis was documented in two patients (1.4%). The incidence of angle closure disease was 67.7% in our study and angle closure glaucoma was 35.7% with no age or gender predilection. The ocular parameters like AL, RCS and lens thickness did not increase the occurrence of glaucoma. Peripheral iridotomy was performed in 69.2% of eyes and younger patients were less likely to undergo a prophylactic Yag PI (p = 0.02). Individuals diagnosed with glaucoma were on a mean number of 1.76 ± 1.02 antiglaucoma medications.
Surgical intervention and outcomes
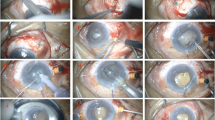
Among the 114 eyes which underwent cataract surgery, 63 (55.3%) underwent only cataract surgery while 51(44.7%) eyes underwent cataract surgery with prophylactic sclerostomy. The average IOL power was 38.15 ± 11.80 dioptres (range, 20.0–74.0). Small incision cataract surgery (SICS) with sclerostomy was done in 12(37.5%) eyes and 20 (62.5%) underwent SICS without sclerostomy. Phacoemulsification with prophylactic sclerostomy was performed in 39 (47.6%) eyes and phacoemulsification without sclerostomy in 43(52.4%) eyes. There was significant improvement in postoperative log MAR BCVA (6/18) (p value = 0.0001) and IOP (15.24 ± 6.74 mm of Hg) (p value = 0.001). Fewer complications were seen in eyes which underwent phacoemulsification (13/81) compared with SICS (10/26) (p = 0.001). Cataract surgery alone had more complications than cataract surgery with concomitant sclerostomy (p value = 0.08) especially uveal effusion which was significantly lower in eyes which underwent sclerostomy (p = 0.04) (Table 3).
Eleven patients underwent surgical treatment for uncontrolled glaucoma. Four underwent trabeculectomy and one of them had a combined trabeculectomy with a sclerostomy. Three patients underwent combined trabeculectomy with IOL implantation & sclerostomy. Glaucoma drainage device (Aqueous Aurolab Drainage Implant—AADI) was done in one patient and three patients underwent Diode cyclophotocoagulation for refractory glaucoma. The mean pre-op IOP & AGM were 42.50 ± 16.03 mm of Hg and 2.62 ± 0.77 AGM. Significant reduction in postoperative IOP was observed (17.31 ± 6.38 mmHg) (p value = 0.002). It was noted that all eyes which underwent glaucoma surgeries had complications (Table 4).
Discussion
Nanophthalmos is a rare clinical spectrum of disorders with a phenotypically small but morphologically normal eyes [1]. They often present as hyperopia or amblyopia in childhood and as angle closure glaucoma, cataract, spontaneous uveal effusions and retinal detachments in adults posing several challenges to the treating clinician.
In our cohort, patients predominantly presented in the fourth decade with 73.6% having defective vision due to cataract as the commonest presenting complaint. Sixteen percent of eyes presented with legal blindness mostly due to glaucomatous optic atrophy. Hyperopia was seen in 65.3% and amblyopia was present in 30.0%. High hyperopia, associated glaucoma, under correction due to unavailability of high powered lenses, spherical aberrations and underlying retinal changes attributed to poor vision.
Crowded optic discs and tortuous retinal vessels were the commonest fundus feature seen in our series which is often related to the dense arrangement of the optic nerve fibres into a small scleral canal [10]. Glaucomatous discs, retinitis pigmentosa, pigmentary retinopathy, macular hypoplasia, uveal effusions, disc oedema, macular striae and retinoschisis were other fundus presentations. Associations of pigmentary retinopathy & macular striae have been reported earlier which could be due to familial inheritance or disproportionate growth of the globe [11].
As nanophthalmic eyes have small globes with normal size lenses, their anterior chambers are also small. This accounts for the increased lens globe volume ratio of 10–30% as opposed to 4% in normals. In our study the mean AL was 17.64 ± 1.74 mm and the mean lens thickness was 4.27 ± 0.70 mm being comparable with a high lens/globe volume ratio. Mean ACD in our cohort was 2.71 ± 0.64 mm which was marginally higher than expected for nanophthalmos. This could be due to the mix of phakic (2.34 ± 0.59 mm), pseudophakic (2.90 ± 0.72) and aphakic lens status seen in our series. We also observed that eyes with shorter AL had greater RCS thickness and this was crucial in deciding whether or not to perform a prophylactic sclerostomy.
Angle closure disease was seen in 67.7% and 35.7% had angle closure glaucoma which is similar to the previous reported incidence of 54–77% [12,13,14]. Nanophthalmos is often associated with varying degrees of angle closure glaucoma. A relative pupillary block secondary to posterior ‘pushing’ mechanism is the most common cause of angle closure glaucoma, which eventually leads to PAS formation [15]. Closed angles were seen in 88.7% with variable degree of PAS. The presence of PAS had 3.66 times higher odds of developing angle closure glaucoma (p value = 0.001) and performing a peripheral iridotomy was more common in older individuals (p value = 0.02). We attribute this to the increasing size of lens with age, causing further shallowing of the anterior chamber and secondary angle closure.
Laser iridotomy may be beneficial in early stages before the development of PAS. Once PAS develops, intraocular surgery may be required. Surgery for glaucoma in these eyes is often considered as a last resort due to high risk of complications [16]. This is due to sudden decompression of the globe during surgery which may trigger the development of massive uveal effusion, leading to secondary retinal detachment, intraocular haemorrhage and malignant glaucoma with loss of vision [1].
Likewise, in our series though we observed significant IOP control postoperatively, we encountered multiple complications like shallow AC, increased anterior chamber reaction, aqueous misdirection and supra-choroidal haemorrhage following glaucoma surgery. This emphasizes the need for a slow decompression or a staged procedure to manage glaucoma as opposed to combined surgery.
The beneficial effect of IOP control following cataract surgery alone in nanophthalmic eyes could be related to the deepening and widening of the anterior chamber angle [17].
Cataract surgery in nanophthalmic eyes is often challenging with poor visual outcome and potential complications such as uveal effusion, retinal detachment, and corneal decompensation [18, 19]. In our study 25.4% (29) of eyes had complications either during or after cataract surgery and uveal effusion accounted for half of these complications. Uveal effusion was seen in 10.8% (25 /232) of eyes and 72.0% of them were seen after an intraocular surgery. Nanophthalmic eyes are prone to develop uveal effusion either from the thickening of sclera and the reduced scleral permeability to proteins, or from the dense collagen around the vortex veins and the resulting compression of venous drainage channels [12, 20, 21]. Phacoemulsification surgery had significantly less complications than SICS (p = 0.001) with significant visual improvement seen postoperatively in both groups (p value < 0.001).
Performing a cataract with sclerostomy had significantly reduced the occurrence of uveal effusions than cataract surgery alone (p = 0.04). This echoes previous reports of good outcomes with phacoemulsification in nanophthalmic eyes due to the recent evolution in cataract surgery with smaller incisions, better chamber stability with advanced phaco machines and highly viscous ophthalmic visco-surgical devices [18, 22, 23]. A randomized control trial comparing the outcomes of cataract surgery with and without prophylactic sclerostomy has also pointed towards lesser complications in eyes undergoing sclerostomy [24]. Scleral window surgery creates an outflow pathway for the supra-choroidal fluid accumulating during or after surgery thereby reducing complications.
To summarize, nanophthalmos was associated with high incidence of PACG, with glaucomatous optic atrophy being the commonest cause for blindness at presentation. The presence of PAS had higher odds of associated glaucoma. Filtering surgeries in these eyes are fraught with serious vision threatening complications requiring additional surgical intervention. Despite meticulous management the visual outcomes remain guarded due to preexisting amblyopia, glaucoma and retinal pathology. Cataract surgery by phacoemulsification was beneficial in lowering complications and performing a prophylactic sclerostomy reduced the occurrence of uveal effusions.
Summary
What was known before
-
Angle closure glaucoma is commonly seen in patients with nanophthalmos.
-
Spontaneous, intraoperative and postoperative uveal effusions are common.
What this study adds
-
Presence of peripheral anterior synechiae is a risk factor for developing glaucoma.
-
Glaucoma surgeries in nanophthalmos is risky and associated with increased rate of complications.
-
Phacoemulsification with prophylactic sclersotomy minimizes risk of intraoperative and postoperative uveal effusions.
References
Singh OS, Simmons RJ, Brockhurst RJ, Trempe CL. Nanophthalmos: a perspective on identification and therapy. Ophthalmology. 1982;89:1006–12.
Liebmann JM, Weinreb RN, Ritch R. Angle-closure glaucoma associated with occult annular ciliary body detachment. Arch Ophthalmol. 1998;116:731–5.
Trelstad RL, Silbermann NN, Brockhurst RJ. Nanophthalmic sclera. Ultrastructural, histochemical, and biochemical observations. Arch Ophthalmol. 1982;100:1935–8.
Yue BY, Duvall J, Goldberg MF, Puck A, Tso MO, Sugar J. Nanophthalmic sclera. Morphologic Tissue Cult Stud Ophthalmol. 1986;93:534–41.
Sener EC, Mocan MC, Saraç OI, Gedik S, Sanaç AS. Management of strabismus in nanophthalmic patients: a long-term follow-up report. Ophthalmology. 2003;110:1230–6.
American Academy of Ophthalmology Glaucoma Panel. Preferred practice pattern guidelines. primary angle closure glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2015. https://www.aao.org/preferred-practice-pattern/primary-angle-closure-ppp-2015.
American Academy of Ophthalmology. Basic and Clinical Science Course, Section 6: Pediatric ophthalmology and strabismus 2016–2017. San Francisco, CA: American Academy of Ophthalmology; 2016. p. 33.
The Pediatric Eye Disease Investigator Group. A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002;120:268–78.
World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). WHO; 2018. http://www.who.int/classifications/icd/en/.
Khairallah M, Messaoud R, Zaouali S, Ben Yahia S, Ladjimi A, Jenzri S. Posterior segment changes associated with posterior microphthalmos. Ophthalmology. 2002;109:569–74.
Relhan N, Jalali S, Pehre N, Rao HL, Manusani U, Bodduluri L. High-hyperopia database, part I: clinical characterisation including morphometric (biometric) differentiation of posterior microphthalmos from nanophthalmos. Eye. 2016;30:120–6.
Ritch R, Chang BM, Liebmann JM. Angle closure in younger patients. Ophthalmology. 2003;110:1880–9.
Singh OS, Sofinski SJ. Nanophthalmos: guidelines for diagnosis and therapy. In: Albert DM, Jakobiec FA, editors. Principles and practice of ophthalmology. Philadelphia: W.B. Saunders; 1994. p. 1528–1540.
Wladis EJ, Gewirtz MB, Guo S. Cataract surgery in the small adult eye. Surv Ophthalmol. 2006;51:153–61.
Burgoyne C, Tello C, Katz LJ. Nanophthalmia and chronic angle-closure glaucoma. J Glaucoma. 2002;11:525–8.
Yalvac IS, Satana B, Ozkan G, Eksioglu U, Duman S. Management of glaucoma in patients with nanophthalmos. Eye. 2008;22:838–43.
Seki M, Fukuchi T, Ueda J, Suda K, Nakatsue T, Tanaka Y, et al. Nanophthalmos: quantitative analysis of anterior chamber angle configuration before and after cataract surgery. Br J Ophthalmol. 2012;96:1108–16.
Jung KI, Yang JW, Lee YC, Kim S-Y. Cataract surgery in eyes with nanophthalmos and relative anterior microphthalmos. Am J Ophthalmol. 2012;153:1161–1168.e1.
Parrish RK, Donaldson K, MellemKairala MB, Simmons RJ. Nanophthalmos, relative anterior microphthalmos, and axial hyperopia. In: Steinert RF, editor. Cataract surgery. 3rd edition. Saunders, Readfield, ME; 2009. p. 389–400.
Yamani A, Wood I, Sugino I, Wanner M, Zarbin MA. Abnormal collagen fibrils in nanophthalmos: a clinical and histologic study. Am J Ophthalmol. 1999;127:106–8.
Shiono T, Shoji A, Mutoh T, Tamai M. Abnormal sclerocytes in nanophthalmos. Graefes Arch Clin Exp Ophthalmol. 1992;230:348–51.
Faucher A, Hasanee K, Rootman DS. Phacoemulsification and intraocular lens implantation in nanophthalmic eyes: report of a medium-size series. J Cataract Refract Surg. 2002;28:837–42.
Wu W, Dawson DG, Sugar A, Elner SG, Meyer KA, McKey JB, et al. Cataract surgery in patients with nanophthalmos: results and complications. J Cataract Refract Surg. 2004;30:584–90.
Rajendrababu S, Babu N, Sinha S, Balakrishnan V, Vardhan A, Puthuran GV, et al. A randomized controlled trial comparing outcomes of cataract surgery in nanophthalmos with and without prophylactic sclerostomy. Am J Ophthalmol. 2017;183:125–33.
Acknowledgements
The authors would like to acknowledge Dr Pradeep Yammanuru Ramulu, Professor of Ophthalmology at Wilmer Eye Institute, for his inputs on improving the paper. Dr Shilpa More; MD, Dr Hiruni Wijesinghe; MD, Glaucoma clinic, Aravind Eye Hopsital, Madurai; Dr Kavitha; MD, Glaucoma clinic, Aravind Eye Hopsital, Pondicherry for their valuable suggestions. Mrs Kumaragurupari; Chief Librarian, Aravind Eye Hospital, Madurai provided invaluable support in literature review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajendrababu, S., Shroff, S., Uduman, M.S. et al. Clinical spectrum and treatment outcomes of patients with nanophthalmos. Eye 35, 825–830 (2021). https://doi.org/10.1038/s41433-020-0971-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0971-4
This article is cited by
-
Outcomes of combined phacoemulsification, anterior vitrectomy, and sclerectomy in nanophthalmic eyes with glaucoma
Eye (2023)
-
Medical Therapy and Scleral Windows for Uveal Effusion Syndrome: A Case Series and Literature Review
Ophthalmology and Therapy (2023)
-
Truncation mutations in MYRF underlie primary angle closure glaucoma
Human Genetics (2023)