Abstract
Objectives
We performed a systematic review and meta-analysis to assess the efficacy and safety of the mineralocorticoid receptor antagonist (MRA) treatment for central serous chorioretinopathy (CSC).
Methods
We searched the PubMed, Embase, and the Cochrane Library to identify relevant clinical studies published prior to March 2020. The primary outcome was change in best-corrected visual acuity (BCVA), and the secondary outcomes included the subretinal fluid (SRF), subfoveal choroidal thickness (SFCT), and central macular thickness (CMT).
Results
Five randomized controlled trials (RCT) and four cohort studies met the inclusion criteria with a total of 352 eyes. The MRA treatment was not superior to placebo in BCVA at 1 month (WMD = −0.06, 95% CI −0.15–0.02, P = 0.15, I2 = 86%), 3 months (WMD = −0.04, 95% CI −0.14–0.06, P = 0.44, I2 = 77%) and 6 months (WMD = −0, 95% CI −0.05–0.05, P = 0.92, I2 = 0%). The MRA treatment resulted in significant reduction than the placebo in the SRF (WMD = −60.64, 95% CI −97.91 to −23.37, P = 0.001, I2 = 49%), SFCT (WMD = −39.15, 95% CI −52.58 to −25.72, P < 0.001, I2 = 0%), and CMT (WMD = −60.75, 95% CI −97.85 to −23.65, P = 0.01, I2 = 53%).
Conclusions
Our meta-analysis shows that the MRA treatment can improve anatomical structure in CSC patients, but it is not effective for achieving BCVA gain. The applicant of the MRA is safe and have no severe effect.
摘要
目的: 通过meta分析, 研究醛固酮受体拮抗剂(mineralocorticoid receptor antagonist, MRA) 治疗中心性浆液性脉络膜视网膜病变(central serous chorioretinopathy, CSC)的疗效和安全性。方法: 检索PubMed、Embase和the Cochrane Library数据库, 收集关于MRA治疗CSC的相关临床研究, 检索截止日期为2020年3月。主要结局指标为最佳矫正视力(best-corrected visual acuity, BCVA)的变化, 次要结局指标为视网膜下积液的厚度(subretinal fluid, SRF)、中心凹下脉络膜的厚度(subfoveal choroidal thickness, SFCT)和中心凹下视网膜的厚度(central macular thickness, CMT)。结果: 共纳入5篇随机对照研究和4篇队列研究, 包括352只眼。与空白对照组比较, 使用MRA治疗CSC 1月(WMD = −0.06, 95% CI −0.15–0.02, P = 0.15, I2 = 86%)、3月(WMD = −0.04, 95% CI −0.14–0.06, P = 0.44, I2 = 77%)和6月(WMD = −0, 95% CI −0.05–0.05, P = 0.92, I2 = 0%)的BCVA变化并无显著差异。但使用MRA进行治疗, 可以明显的减少SRF(WMD = −60.64, 95% CI −97.91∼−23.37, P = 0.001, I2 = 49%)、SFCT(WMD = −39.15, 95% CI −52.58∼−25.72, P < 0.001, I2 = 0%) 和CMT(WMD = −60.75, 95% CI −97.85∼−23.65, P = 0.01, I2 = 53%)。结论: 我们的研究结果表明, MRA治疗可以显著的促进CSC患者眼底解剖结构的恢复, 但对于其视力无明显的改善作用。
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSC) is one of the most common vision-threatening retinal disorders, especially for middle-aged male individuals [1]. It is characterized by the accumulation of subretinal fluid (SRF) between the neurosensory retina and the retinal pigment epithelium (RPE), RPE alterations, and choroidal vessels dilation. The pathogenic mechanism of CSC remains unknown. The risk factors of CSC include the Type A personality, use of steroid or psychopharmacologic medications, sleeping disorder, hypertension, H. pylori infection, autoimmune disease, etc. [1]. Some of CSC patients are self-limiting. However, 30–50% of the patients cannot recover without the treatment, which leads to permanent vision loss [2]. Treatments for CSC include laser photocoagulation, transpupillary thermotherapy, subthreshold micropulse laser, photodynamic therapy (PDT), anti-vascular endothelial growth factor drugs, and mineralocorticoid receptor antagonists (MRA) [3,4,5].
Animal experiments indicated that the pathway of MR signal controlled the choroidal vascular bed relaxation, which supported the MRA as a treatment method for CSC [6]. Recently, numerous studies were conducted on whether the MRA therapy (eplerenone or spironolactone) has a better clinical outcome than the others [7]. Some studies claimed that the MRA treatment improved best-corrected visual acuity (BCVA) and reduced the subfoveal choroidal thickness (SFCT) and the SRF in CSC patients [4]. Recently, the VICI trial has reported that the central macular thickness (CMT) was found decreasing after the MRA treatment [8].
The efficiency of the MRA in CSC treatment remains controversial. In order to provide more accurate evidence for clinician about the efficacy and safety of the MRA treatment for CSC patients, we performed an updated meta-analysis of placebo-controlled study and a systematic review to evaluate the efficacy of the MRA for CSC, including all RCT and cohort studies.
Methods
We performed our systematic review and meta-analysis by following the recommendations of the PRISMA statement. The protocol and registration information are available at http://www.crd.york.ac.uk/PROSPERO/ (registration number: CRD42020173466).
Search strategy and eligibility criteria
We searched Embase, PubMed, and the Cochrane Library to identify relevant studies published before March 2020 with a combination of the following MeSH terms: “CSC” or “central serous retinopathy” in combination with “spironolactone” or “eplerenone,” “MRA.” All related articles were retrieved without any language restrictions. Studies were selected based on the following inclusion criteria: (i) RCT and placebo-controlled cohort clinical trials; (ii) patients were diagnosed with CSC and were treated by MRA or placebo; (iii) data on BCVA, SRF, SFCT, or CMT were provided; (iv) sufficient information to extract or calculate the weighted mean difference (WMD) ± standard deviation (SD) of the outcome was contained. Exclusion criteria included: (i) review, case reports, comments, and animal experiments; (ii) self-controlled studies or involvement of treatments with other methods; (iii) full-text manuscripts without available raw data.
Data extraction and quality assessment
Two reviewers extracted the data from the published reports independently. The following information was extracted: the name of the first author, year of publication, study design, country, symptom duration, number of participants, doses and modalities of interventions, follow-up period, OCT device, and outcomes. The risk bias of RCTs was assessed by the Cochrane tool, and cohort studies were assessed by the Newcastle–Ottawa Scale.
Statistical analysis
The data were analyzed with Review Manager 5.30 (Cochrane Collaboration, Oxford, UK). Continuous data were summarized as WMD and SD from the published articles, or the Cochrane Handbook was used to acquire WMD and SD from range, median, and p value. The primary outcome of this meta-analysis was BCVA and the secondary outcomes were SRF, CT, and CMT in OCT. Forest plots were made to visually assess the WMDs and 95% confidence intervals (CI). Heterogeneity was evaluated by the chi-square test and I2 statistics. P < 0.1 or I2 > 50% indicated significant heterogeneity. Subgroup analysis was performed based on the follow-up period and study design. We used the random-effect model to collate data because it was more robust than the fixed-effect model. The sensitivity analysis was accessed using Review Manager 5.30. Publication bias of the BCVA was performed by the Begg’s plot and Egger’s test by Stata software (V.16.0; Stata, College Station, TX, USA).
Results
Search results and study characteristics
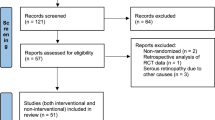
A total of 267 potentially relevant articles were identified. After removing the duplicates, the titles and abstracts of 201 articles were screened by two blinded reviewers. After that, 69 full-text articles were assessed for eligibility. The reasons for further exclusion included: non-comparative studies (n = 29), no placebo-controlled trial (n = 7), irrelevant comparison articles (n = 22), protocol (n = 1), and the lack of data (n = 1). Finally, five RCTs and four cohort studies (352 eyes totally) were identified in our meta-analysis (Fig. 1). The detailed characteristics of nine selected clinical studies were shown in Table 1.
Meta-analysis results
BCVA
All 9 selected studies reported the outcome of BCVA at different follow-up duration. We evaluated the change in BCVA from baseline to 1 month, 3 months and 6 months with the random-effect model. Subgroup analysis of study design was conducted due to severe heterogeneity among the included studies. The WMD of BCVA change in 1 month between the MRA and the control groups was −0.06 (95% CI: −0.15–0.02), revealing that BCVA was improved by the MRA treatment in CSC patients (Fig. 2a). Nevertheless, the difference between the two groups was not statistically significant (P = 0.15). There was no heterogeneity between data sources in the RCT of all trials at the 1-month follow-up (I2 = 0%). The result also showed that the MRA could not significantly improve BCVA at 3 months (WMD = −0.04, 95% CI −0.14–0.06, P = 0.44, I2 = 77%) and at 6 months (WMD = −0, 95% CI −0.05–0.05, P = 0.92, I2 = 0%), compared to the placebo group (Fig. 2b, c). Subgroup analysis of study design revealed that the BCVA gain of CSC patients in cohort studies (WMD = −0.1) was better than that in RCT studies (WMD = −0.02). Heterogeneity was calculated by the I2 statistics. There was no or low heterogeneity in the studies at 6 months, but the heterogeneity at 3 months was high. Subgroup analysis showed that there was no difference in BCVA outcome between the chronic CSC and acute CSC at 1-month follow-up (Fig. 3a) and 3 months follow-up (Fig. 3b).
SRF
Seven studies reported the change of SRF in the MRA treatment group and the placebo group. The SRF change in the MRA was more than the placebo group significantly (WMD = −60.64, 95% CI −97.91 to −23.37, P = 0.001, I2 = 49%), but there was substantial heterogeneity among the studies for this outcome (Fig. 4a). The subgroup analysis demonstrated that the SRF difference between the two groups was statistically significant at 1 month follow-up with no heterogeneity (WMD = −75.76, 95% CI −112.25 to −39.27, P < 0.00001, I2 = 0%) and 3 months follow-up with high heterogeneity (WMD = −52.47, 95% CI −121.91–16.97, P = 0.14, I2 = 65%).
SFCT
The WMD in SFCT change was −39.15 (95% CI −52.58 to −25.72, P < 0.00001, I2 = 0%), indicating that the SFCT was thinner with the MRA treatment than with the placebo (Fig. 4b). There was no heterogeneity among the studies for this outcome.
CMT
The CMT of patients was observed in three clinical trials during the follow-up. The MRA therapy significantly decreased the CMT (WMD = −60.75, 95% CI −97.85 to −23.65, P = 0.001), and the heterogeneity was I2 = 53% with the random-effect model (Fig. 4c). Subgroup analysis by follow-up duration indicated that CMT was decreased at 1 month follow-up (WMD = −48.65, 95% CI −78.68 to −18.62, P = 0.01, I2 = 0%) and 3 months follow-up (WMD = −83.70, 95% CI −204.59.91–37.19, P = 0.17, I2 = 86%).
Adverse effects
No severe side effect was detected in all nine studies. Some mild side effects were observed, including gastrointestinal disorders [8,9,10], infection, hyperkalaemia, abnormal musculoskeletal and connective tissue [8], neurological symptom (intermittent dizziness) [10], and fatigue sedative effect [11].
Bias
The methodological quality of five RCT studies was assessed by the Cochrane risk of bias tool (Fig. 5). Four observational cohort studies were evaluated by the Newcastle−Ottawa scale (Table 2). Publication bias was assessed by the Egger’s linear regression test and Begg’s funnel plot, and no publication bias was observed.
Discussion
The number of clinical studies on the efficiency of the MRA medications for CSC has increased recently. However, the results were not consistent. In our meta-analysis, compared with placebo, the MRA was noted to be beneficial on the anatomical outcomes, including SRF, SFCT, and CMT. However, there was no statistical difference between the BCVA improvement of the two groups. To the best of our knowledge, this is the first systematic review and meta-analysis of placebo-controlled comparative studies which evaluates the efficacy and safety of the MRA treatment for CSC, including RCT and non-RCT studies.
The MRA, including eplerenone and spironolactone, has been used as the treatment option in multiple prospective and retrospective studies of CSC recently. No significant difference in efficacy was found in eplerenone and spironolactone except mild increasing side effects in spironolactone [12]. There was one meta-analysis of RCT on the MRA treatment, but our visual outcome was contrary to this previous meta-analysis [13]. The reason for this opposite result might be the data of the latest clinical RCT study with large sample size and the cohort studies. Another meta-analysis of the MRA for CSC has recently been published [14]. However, this previously published meta-analyses did not exclude patients receiving PDT treatment and used them as a control group. PDT is a treatment option for CSC, which might have an impact on the evaluation of the MRA effectiveness. In addition, they did not include the VICI trials, which was a landmark clinical trial, and this might reduce the reliability of these findings. Another shortcoming is that the main outcomes of this trial were CMT and SRF, which can only indicate anatomical changes. According to the treatment objective, BCVA should be more suitable to serve as the main outcome of the efficacy. Given the drawbacks of these articles, we conducted a meta-analysis and systematic review to evaluate the efficacy and safety of the MRA in CSC.
Our meta-analysis demonstrated that the MRA was superior in BCVA improvement than the placebo in 1 month, 3 months, and 6 months. Considering the significant heterogeneity, the subgroup analysis of study design and disease type were performed in BCVA outcome. In subgroup analysis, the BCVA improvement was better in the cohort group compared with the RCT group, but there was no statistical difference between them. Subgroup analyses revealed that BCVA at 1 month and 3 months was better in the acute CSC group than in the chronic group. Sensitivity analysis was conducted when we detected high heterogeneity in BCVA outcomes (I2 > 50%). The study of Zucchiatti had a great impact on the heterogeneity [15]. One possible reason was that patients with acute CSC was included. Some researchers found that the improvement in BCVA was statistically significant in the treatment-naïve group [16,17,18]. However, the VICI trial presented negative visual outcomes improvement in a 3-year follow-up of eplerenone treatment for chronic CSC [19].
The results of our meta-analysis showed a significant improvement in anatomical outcomes, including the decrease of SRF, SFCT, and CMT. There were many studies reported anatomical improvement with eplerenone and/or spironolactone treatment [18, 20]. The long-term follow-up revealed that anatomical outcomes were significantly improved in the first year [19]. Nevertheless, some retrospective studies showed no significant reduction in SRF at any follow-up [21].
Compared with the placebo, the MRA treatment was superior in morphological recovery. However, the result of BCVA improvement was not consistent with anatomical improvement. One potential explanation for this is that morphological recovery may be independent of the RPE integrity [22]. The treatment objective for CSC is to preserve the outer neurosensory retinal layers and absorb SRF to avoid irreversible damage to the photoreceptors [4]. Even though the MRA have a great effect on anatomical improvement, the pre-existing damage of retina may be irreversible [4].
The RPE plays an important role in the pathophysiology of CSC, which leads to the accumulation of SRF [4]. The decrease of SRF may restore the normal anatomical photoreceptor–RPE interaction and contribute to decreased retinal thickness in CSC. Recently, CSC was classified in the pachychoroid disease [23]. Therefore, the change of choroid thickness might evaluate the disease conditions. Our meta-analysis indicated the MRA significantly reduced SRF, SFCT, and CMT as compared with placebo. The potential explanations for this finding were described as follows. It has been reported that the pathogeny of CSC might be the inappropriate mineralocorticoid receptor (MR) activation, which could bind to the glucocorticoids and mineralocorticoid [6]. MR is expressed in the retina, including endothelial cells, pericytes, ganglion cells, Müller cells, microglia and RPE, and its activation could induce the accumulation of fluid in the outer retina through potassium channel Kir4.1 and the water channel AQP4 [24]. These actions appeared to be relevant to CSC treatment. The effect of aldosterone on choroid was partially mediated by KCa2.3 in choroidal endothelial cells, which induced the thickening of the choroid and the dilatation of choroidal vessels [25]. This mechanism on retina and choroid could explain that the MRA had beneficial effects on anatomical improvement.
The medication effects of the MRA may be affected by the following factors. Patients with thicker choroid, smaller SRF, less RPE detachment, and less intraretinal hyperreflective foci at OCT were associated better anatomical outcomes in the eplerenone treatment [17]. However, the presence of choroidal neovascularization at OCTA and the absence of hotspot at ICGA were predictive biomarkers of unfavorable response to the MRA treatment [22]. Patients with serum potassium level >5.5 mEq/L or a creatinine clearance ≤30 mL/min should not be treated by the MRA, because the MRA could induce hyperkalaemia, which may cause cardiac arrhythmia [4].
The aim was to update the present evidence by analyzing all qualified relevant studies with precise meta-analysis. However, there are several limitations to this meta-analysis. First, limited number of studies were available, and the insufficient quality of the data affected the final results. Second, other subgroup analyses (treatment dosage or duration) and publication bias could not be performed because of the limited number of studies, Drug choice, dosage, and interval of the medications differ in included articles. Limited data on anatomical outcomes could lead to differences and corresponding deviations.
In this systematic review and meta-analysis of comparative studies, the BCVA gain was not associated with the MRA treatment statically. Nevertheless, compared to placebo, the MRA treatment could contribute to the decrease of SRF, SFCT and CMT, and resume the anatomic structure of the retina. The goal for the treatment of CSC is to improve the visual acuity, preserve the outer neurosensory retinal layers, and avoid the irreversible damage to the photoreceptors [4]. Based on result of our meta-analysis, we recommend ophthalmologists to discontinue the MRA treatment for CSC and consider other interventions.
References
Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retinal Eye Res. 2015;48:82–118.
Chen L, Zhang P. Advances in the treatment of central serous chorioretinopathy. Int Eye Sci. 2020;20:79–82.
Iacono P, Toto L, Costanzo E, Varano M, Parravano MC. Pharmacotherapy of central serous chorioretinopathy: a review of the current treatments. Curr Pharm Des. 2018;24:4864–73.
van Rijssen TJ, van Dijk EHC, Yzer S, Ohno-Matsui K, Keunen JEE, Schlingemann RO, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Progr Retinal Eye Res. 2019;73:100770.
Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015:Cd011841.
Zhao M, Celerier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Investig. 2012;122:2672–9.
Fusi-Rubiano W, Saedon H, Patel V, Yang YC. Oral medications for central serous chorioretinopathy: a literature review. Eye (London, England). 2020;34:809–24.
Lotery A, Sivaprasad S, O’Connell A, Harris RA, Culliford L, Ellis L, et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:294–303.
Rübsam A, Thieme CE, Schlomberg J, Winterhalter S, Müller B, Joussen AM, et al. Therapy rationale for mineralocorticoid-receptor antagonists, acetazolamide and a switch of therapy in nonresponders in central serous chorioretinopathy. J Ocul Pharmacol Therapeutics. 2017;33:141–8.
Rahimy E, Pitcher JD 3rd, Hsu J, Adam MK, Shahlaee A, Samara WA, et al. A randomized double-blind placebo-control pilot study of eplerenone for the treatment of central serous chorioretinopathy (ECSELSIOR). Retina. 2018;38:962–9.
Pichi F, Carrai P, Ciardella A, Behar-Cohen F, Nucci P. Comparison of two mineralcorticosteroids receptor antagonists for the treatment of central serous chorioretinopathy. Int Ophthalmol. 2017;37:1115–25.
Kapoor KG, Wagner AL. Mineralocorticoid antagonists in the treatment of central serous chorioretinopathy: a comparative analysis. Ophthalmic Res. 2016;56:17–22.
Wang SK, Sun P, Tandias RM, Seto BK, Arroyo JG. Mineralocorticoid receptor antagonists in central serous chorioretinopathy: a meta-analysis of randomized controlled trials. Ophthalmol Retina. 2019;3:154–60.
Zhang B, Chou Y, Zhao X, Yang J, Chen Y. Efficacy of mineralocorticoid receptor antagonist for central serous chorioretinopathy: a meta-analysis. Int Ophthalmol. 2020;40:2957–67.
Zucchiatti I, Sacconi R, Parravano MC, Costanzo E, Querques L, Montorio D, et al. Eplerenone versus observation in the treatment of acute central serous chorioretinopathy: a retrospective controlled study. Ophthalmol Ther. 2018;7:109–18.
Farooq O, Habib A, Shah MA, Ahmed N. Effect of oral eplerenone in anatomical and functional improvement in patients with chronic central serous chorioretinopathy. Pak J Med Sci. 2019;35:1544–7.
Borrelli E, Zuccaro B, Zucchiatti I, Parravano M, Querques L, Costanzo E, et al. Optical coherence tomography parameters as predictors of treatment response to eplerenone in central serous chorioretinopathy. J Clin Med. 2019;8:1271.
Chin EK, Almeida DRP, Roybal CN, Niles PI, Gehrs KM, Sohn EH, et al. Oral mineralocorticoid antagonists for recalcitrant central serous chorioretinopathy. Clin Ophthalmol. 2015;9:1449–56.
Petkovsek DS, Cherfan DG, Conti FF, Hom GL, Ehlers JP, Babiuch AS, et al. Eplerenone for the treatment of chronic central serous chorioretinopathy: 3-year clinical experience. Br J Ophthalmol. 2020;104:182–7.
Gergely R, Kovács I, Schneider M, Resch M, Papp A, Récsán Z, et al. Mineralocorticoid receptor antagonist treatment in bilateral chronic central serous chorioretinopathy. Retina. 2017;37:1084–91.
Ghadiali Q, Jung JJ, Yu S, Yannuzzi LA. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a retrospective study analyzing the clinical and multimodal imaging response to therapy. Investig Ophthalmol Vis Sci. 2014;55:2080.
Sacconi R, Baldin G, Carnevali A, Querques L, Rabiolo A, Marchini G, et al. Response of central serous chorioretinopathy evaluated by multimodal retinal imaging. Eye. 2018;32:734–42.
Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, Kneba M, Grzybowski A. Impairment of visual acuity and retinal morphology following resolved chronic central serous chorioretinopathy. BMC Ophthalmol. 2019;19:160.
Golestaneh N, Picaud S, Mirshahi M. The mineralocorticoid receptor in rodent retina: ontogeny and molecular identity. Mol Vis. 2002;8:221–5.
Bousquet E, Zhao M, Daruich A, Behar-Cohen F. Mineralocorticoid antagonists in the treatment of central serous chorioetinopathy: review of the pre-clinical and clinical evidence. Exp Eye Res. 2019;187:107754.
Funding
Supported by Sichuan Province Science and Technology Support Program (2018FZ0031 and 2019YJ0129).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, J., Zhang, Y. & Zhang, M. Efficacy and safety of the mineralocorticoid receptor antagonist treatment for central serous chorioretinopathy: a systematic review and meta-analysis. Eye 35, 1102–1110 (2021). https://doi.org/10.1038/s41433-020-01338-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01338-4
This article is cited by
-
The choroidal nervous system: a link between mineralocorticoid receptor and pachychoroid
Acta Neuropathologica (2023)
-
Mineralocorticoid receptor antagonists for chronic central serous chorioretinopathy: systematic review and meta-analyses
International Journal of Retina and Vitreous (2022)