Abstract
Background/Objectives
Fluorescein angiography (FA) has been a pivotal tool to study the pathophysiology of retinopathy of prematurity (ROP) in vivo. We examined the course of ROP using FA in order to assess the predictive value of angiographic features.
Subjects/Methods
This is an observational retrospective cohort multi-center study of eyes screened for ROP with binocular indirect ophthalmoscope and with FA. All infants undergoing screening examination for ROP who had retinal vasculature limited to Zone I and posterior Zone II vascularization underwent FA between 31 and 34 weeks postmenstrual age. RetCam fundus imaging and video digital fluorescein angiography were performed in the neonatal intensive care units. Masked grading of the FA images was retrospectively conducted by two ROP expert ophthalmologists. Ten criteria that describe retinovascular and choroidal features on FA were used to assess their predictive value for development of treatment-requiring ROP.
Results
A total of 98 eyes of 56 patients were included for this study. FAs of eyes of premature infants show a wide range of features either at the junction between the vascular and avascular retina and posteriorly to that. Among the angiographic features evaluated, leakage, shunts and hyperfluorescent lesions at the junction between vascular and avascular zone were predictive of the development of treatment-requiring ROP (p < 0.05), but findings in the posterior vascularized retina were not.
Conclusions
FA can add to our understanding of the evolution of vascular abnormalities in the course of ROP and can help predict which eyes will go on to treatment.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is a disease of developing blood vessels in the retina of the premature infant. At present, ROP is characterized by the location, stage and extent of the disease based only on ophthalmoscopic findings. These findings have been described usefully in the different iterations of the International Classification for ROP [1,2,3] (ICROP) which has become a common language among ophthalmologists not only to manage ROP clinically [4,5,6,7], but it has also provided a framework for clinical trials and other studies that improve the care of the premature infant [4, 8]. With the introduction of new retinal imaging techniques such as digital fundus imaging, optical coherence tomography, fluorescein angiography (FA), and scanning laser ophthalmoscopy, there are new opportunities to understand the limitations of using just an ophthalmoscopic description of the retinal findings based on ICROP.
FA has been part of routine assessment of retinal vasculature or to guide treatment in several adult vasoproliferative diseases such as wet age-related macular degeneration and diabetic retinopathy [9, 10]. FA in the evaluation of ROP was introduced in the 1970s by Flynn and Kushner [11, 12] and Cantolino et al. [13], recognizing that ROP is a largely vascular disease from the start. Since the development of new techniques for bedside imaging (most commonly Retcam digital camera [Natus Medical Incorporated, Pleasanton, CA]) over the last two decades, FA has demonstrated, in more detail, vascular abnormalities that are not readily apparent on ophthalmoscopic fundus exams [14]. In 2006, Ng et al. [15] demonstrated that angiograms showing clear retinal vascular pattern could be obtained as part of ROP screening.
In 2011, we presented an FA study to catalog FA findings in eyes with severe ROP that were scheduled to undergo laser photoablation [16]. In the subsequent years, FA has been used to better evaluate the vascular abnormalities of eyes screened for ROP [17]. The present study is a preliminary assessment of FA as a predictive tool for progression to treatment-severity disease in a group of premature infants with incomplete vascularization in zone I or posterior zone II at early ROP examinations.
Materials and methods
This is an observational retrospective cohort multi-center study that evaluates a population of premature infants at risk for ROP born in five neonatal intensive care units (NICUs) in Italy. All infants born between 2004 and 2017 with gestational age (GA) less than 32 weeks and/or birth weight (BW) less than 1500 g, were screened for ROP. FA using RetCam digital camera has been used for ROP examinations since 2004 at the participating sites. During this period, all infants presenting ROP with Zone I and posterior Zone II vascularization with or without ophthalmoscopic diagnosis of ROP underwent FA before 34 weeks postmenstrual age (PMA). At the time of FA session, ophthalmoscopical examination was performed to exclude presence of treatment-requiring ROP by different ophthalmologist. Digital fundus images were captured using the Retcam II/III with a wide-field 130-degree lens after dilatation of the pupils achieved with a mixture of cyclopentolate 0.2% and phenylephrine 1% given as one drop in both eyes every 15 min twice, 30 min before examination. With the use of a lid speculum as necessary, color fundus photography of each eye was performed first, followed by FA of the eye with better visualization or the eye with the most severe form of ROP first and then the fellow eye. FAs were performed using a bolus of 10% fluorescein solution intravenously administered at a dose of 0.1 ml/kg, followed by an isotonic saline flush. Angiographic digital images were obtained at various time intervals and in all retinal quadrants. All scans were anonymized and indexed as sets of individual eyes and were presented to graders in random order. Two ophthalmologists with expertise in ROP and blind to an infant’s BW, GA, PMA and ROP status observed during the hospital course, and unmasked only to dye transit time. They independently graded each anonymized set of FAs for retinal and choroidal lesions using criteria reported in our previous paper [18]. If discordant, a consensus grading was reached by the two graders.
Grading parameters
The FA features of vascularized retina recorded by the graders are shown in Fig. 1 and include foveal avascular zone (FAZ) (presence or absence), choroidal filling pattern (linear or lobular), hypofluorescent areas without adjacent hyperfluorescent lesions, and hypofluorescent areas without adjacent hyperfluorescent lesions. The FA features at the junction between vascular and avascular retina included finger-like anastomoses, shunts, vascular tangles, hyperfluorescent lesions (i.e., cotton-wool patches and popcorn lesions), leakage, and capillary obliteration (Fig. 2). For finger-like anastomoses [11], shunts, tangles, hyperfluorescent lesions, leakage, capillary obliterations, hypofluorescent areas with or without hyperfluorescent lesions, a grade from 0 to 3 was assigned based on severity (no lesion, mild, moderate and severe) (Supplementary Fig. 1). When the quality of the image did not permit assigning a grade for a variable, that entry was recorded as ungradable. To evaluate the prediction of treatment-requiring ROP (defined using Early Treatment for Retinopathy of Prematurity criteria) [6] using FA features, only FAs obtained at a single exam before 34 weeks PMA were used in this study. Patients were treated between 24 and 72 h after ophthalmoscopical identification of treatment-requiring ROP by two ophthalmologists. Among eyes that subsequently progressed to treatment severity, only those with the FA obtained at least 5 days prior to treatment were used in this study. Five days cut-off was chosen to be sure that ophthalmoscopical findings were not severe enough for treatment and to avoid potential discrepant diagnoses of treatment-requiring ROP. For those eyes that did not undergo treatment, the last FA before 34 weeks PMA was included in this analysis.
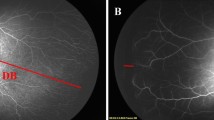
a Absence of foveal avascular zone, b Presence of foveal avascular zone, c Linear pattern of the choroidal vascular circulation, d Lobular pattern of the choroidal vascular circulation, e Hypofluorescent area surrounded by hyperfluorescence, f Hypofluorescent area without surrounding hyperfluorescence.
Informed consent was obtained by the parents for each FA examination as part of the protocol for ROP screening at each site. The study was conducted in accordance with the tenets of the Declaration of Helsinki. The local Institutional Review Board at each institution approved this study and approval number was not provided because it involved only retrospective data analysis.
Statistical analysis
We described the FA features using percentages, and demographic characteristics (BW, GA) using mean (standard deviation) and median (1st and 3rd quartiles). To determine whether early FA features can predict progression to treatment severity, we first analyzed each FA features using univariate logistic regression models followed by multivariate logistic regression models that included FA features with p < 0.10 in the univariate analyses. The multivariate model went through a backward selection of risk factors, and the final multivariate model only retained the FA features with p < 0.05. The final multivariate model was then adjusted by BW and GA to determine their independent associations of early FA features with ROP treatment. The odds ratio (OR) and its 95% confidence intervals (95% CI) for each FA features were calculated from logistic regression models. As the FA features and ROP treatment are eye-specific, these statistical models use eye as unit of analysis, and the inter-eye correlation was accounted for by using generalized estimating equations [19]. The inter-grader agreement for each feature was also calculated using Cohen’s weighted kappa.
Results
A total of 102 infants meeting enrollment criteria had undergone at least one FA by 34 weeks PMA at a participating center. Forty-six infants were excluded from this analysis as the first FA was within 5 days of treatment for ROP. The remaining study cohort of 56 infants included in this analysis had a mean BW of 808 g (range 380–1320) and mean GA of 26.5 weeks (range 23–30). The mean PMA of the time of the FA exam was 32.7 weeks (range 28–34) (Supplementary Table 1). Among the 112 eyes, one eye of 14 infants was excluded due to poor quality of the images. Among the 56 infants, 21 eyes of 13 infants underwent treatment at an average of 10.4 days (range 5–16) after FA. The mean GA of the treated group was 25.0 ± 1.6 weeks and that of the untreated was 26.9 ± 1.3 weeks (p < 0.001) and the mean BW was respectively 691.4 ± 159.2 g and 846.0 ± 192.8 g (p < 0.001). The PMA at the FA exam was 32.1 ± 1.2 weeks for the treated babies and 32.9 ± 1.4 weeks for the untreated (p = 0.010) (Supplementary Table 2). At subsequent examinations, the most severe stage of ROP reached in these eyes is provided in Supplementary Table 3. Among the eyes with most severe ROP in Zone I, 20 of the 29 eyes (69.0%) developed stage 3 ROP while among Zone II eyes, 27 of 69 eyes (39.1%) developed stage 3 ROP. In the entire study cohort, plus disease was observed in 4 of 29 Zone I eyes (6.9%) and 4 of the 69 (2.9%) Zone II eyes. Supplementary Table 4 provides a description of the various FA findings components in 98 eyes. Importantly, characterizing all components in many images was not possible due to varying image quality across the retinal images. Among the 98 study eyes, ungradable images ranged from 29.6% for FAZ and capillary obliteration to 46.9% for choroidal vascular pattern. Finger-like anastomoses were observed in 50.0% (n = 49 eyes) of the 98 eyes and these changes were graded as mild, moderate or severe based on the location and density of the vessels [16]. Shunts were the most common feature (64.3%, n = 63) observed in the eyes of these premature infants. In the severe cases, the shunt can connect all four retinal quadrants suggesting a profound alteration of retinal circulation. Capillary tangles (52.0%, n = 51) are often observed at the V-Av junction with a variable degree of leakage. Hyperfluorescent lesions at the V-Av junction, such as “cotton-wool-like,” are observed in 29 (29.6%) eyes as another sign of an exudative process near the V-Av junction. Perivascular dye leakage itself was frequent (43.9%, n = 43), but only of mild (30.6%) or moderate (13.3%) intensity. FA clearly demonstrated moderate or severe obliteration of capillary network (69.4%, n = 68) that can be interpreted a sign of a remarkable alteration in the development of retinal vasculature. In the 98 eyes, FAZ was present only in 16 eyes (16.3%) (Fig. 1a and b) and absent in 53 eyes (54.1%), while the remaining 29 eyes (29.6%) were ungradable. A massive loss of central retinal capillary bed and/or lack of choriocapillary perfusion were often observed well inside the vascularized retina as hypofluorescent areas: in many cases near these persistent hypo- or non-perfused retinal areas, some hyperfluorescent lesions were also evident (52.0%, n = 51), though hypofluorescent areas without adjacent hyperfluorescence were observed more frequently (64.3%, n = 63).
In univariate analysis examining the association between FA features and the subsequent development of treatment-requiring ROP (Tables 1 and 2), several features at the junction between the V-Av retina were significantly predictive of treatment-requiring ROP, including leakage (p = 0.02), hyperfluorescent lesions (p = 0.01), and shunts (p = 0.04). Regarding the other features noted at the junctional area, only a borderline association was evident for tangles (p = 0.10) and capillary obliteration (p = 0.09). However, no association has been found with those angiographic features in the vascularized retina. In multivariate analysis, shunts were independently associated with the development of treatment-requiring ROP in a dose-response manner (linear trend p = 0.01), and the association remained significant even after adjustment by BW and GA (linear trend p = 0.005) (Table 3). The association between hyperfluorescent lesions at the junction and development of treatment-requiring ROP was also significant (linear trend p = 0.004); however, no significant association was noted after adjustment by BW and GA (Table 3). We found excellent agreement between graders ranging from 86.70% to 100% across the angiographic features, with weighted kappa ranging from 0.96 to 1.00 (Supplementary Table 5). No adverse events related to FA exams have been reported by the safety monitoring service in all centers involved.
Discussion
We have presented a systematic retrospective analysis of clinical data and FAs from a subset of the premature babies judged clinically and ophthalmoscopically at high-risk of severe ROP born in five different clinical centers in Italy. The aim of this study is to determine the utility of this imaging technology as a predictive tool. No adverse events related to FA exams have been reported in this and our previous studies [16, 18, 20], thus showing that FA can be safely accomplished as a part of ROP screening in the NICU in high-risk infants. Strengths of this study include the provision of greater detail about retinal vasculature development using FA, a multi-center design, and the masked evaluation of images by ROP experts. In addition, we found a high intergrader agreement for the diagnosis of ROP requiring treatment. A potentially serious limitation of this study was that we selected only high-risk infants among the entire premature population screened for ROP. Furthermore, we reported a high percentage of ungradable image components, ranging from 29.6% to 46.9% among the different types of lesions we had selected for analysis. This highlights the difficulty of such procedures in premature infants and is likely due to many factors, including lid aperture and eye size, media opacities, difficulty of examination in awake babies and the time-dependence of some FA features, since fluorescein dye penetrates different parts of the vascular system at different times. To minimize recall bias, angiograms were reviewed at least a year after the acquisition and the graders were blinded to any patient data except for PMA at the exam.
In our study, the univariate analysis shows that three features at the junction between the V-Av retina (leakage, hyperfluorescent lesions, and shunts) are statistically correlated with the evolution into treatment-requiring ROP. All of them are practically invisible without FA. It is important to note that only the findings in the peripheral retina are able in this study to identify eyes that go on to treatment, supplementing our understanding of the severity of these peripheral retinal vascular abnormalities. This is in some way in contrast with the fact that, according to the findings of the ETROP study [8], the decision to treat babies is mainly based on the presence or absence of plus disease, a posterior pole vascular change that typically develops between 34 and 38 weeks of PMA [21].
Study images were obtained from our infants at a mean PMA of 32.7 weeks, probably just at the beginning or even before the appearance of plus disease. We speculate that these peripheral vascular changes detected earlier by FA could be a prelude to the posterior pole plus disease. The blood flow in the retinal periphery could be altered in the presence of vascular anomalies, especially shunts, that are correlated with evolution to severe disease. We can speculate that this phenomenon could contribute to the subsequent development of vascular tortuosity and dilatation. In earlier reports [11, 22,23,24], changes are explained by the reduced capillary resistance and increased retinal blood flow in the presence of arteriovenous shunts bypassing the capillary network. This process may lead to dilatation of the retinal venules first, since they are distensible, and then arteriolar tortuosity. Further longitudinal studies are needed to support this hypothesis.
As we have previously shown, FA in the eyes of premature infants permits observation of the choroidal circulation which could be characterized as showing lobular and perhaps more mature filling pattern to a linear and likely a more immature one (Fig. 1c and d). Choroidal circulation can be appreciated during early phase of FA, especially when it comes to linear filling, that can coexist in some minor extent with lobular pattern as well. ROP is observed at the junction between the vascular and avascular (V-Av) retina and FA provides an excellent visualization of vascular abnormalities at the transition of the V-Av junction. Interestingly, although it is well known that FA makes evident the FAZ [25], in our cohort 29.6% of eyes were ungradable. At this age the velocity of retinal circulation is extremely variable [16], we can speculate that faster circulation in some eyes likely makes it difficult to evaluate both the posterior pole and the periphery at the same FA session.
One of the exclusion criteria was the time of the FA exam within 5 days from the treatment. Patients were treated between 24 and 72 h after diagnosis of treatment-requiring ROP which means that these angiographic findings are present at least two days before the appearance of ophthalmoscopic signs meeting criteria for treatment. As result, this exclusion criteria introduces an intrinsic limitation of having fewer later exams for the treated group, hence the treated cohort had a lower mean PMA at the exam of 0.8 weeks. We cannot exclude that these findings are related to earlier PMA. Further studies with larger cohorts are required to answer this question.
At present, FA in a NICU setting is time consuming and not universally available, thus limiting the generalizability of our findings to clinical care at present. However, the use of this technology continues to spread among the pediatric ophthalmologists and retina specialists around the world. The recent report by Mansukhani et al. [26] describes the differences on FA between eyes treated with anti-VEGF and eyes that regressed without treatment and interestingly they found frequent abnormal vascular patterns in both groups. When these findings are considered along with those of the current report that included eyes treated with laser photocoagulation, there is further insight into the structural effects of severe ROP and its treatment. As this technology becomes more feasible in the care of premature infants with ROP, the information obtained using FA may well change clinical approaches and provide greater understanding of subtleties of acute phase ROP using different techniques.
FA may also be useful in understanding pathogenesis [17], guiding laser photocoagulation [16], and describing vessel growth in follow-up phases of treated and untreated eyes [18, 20]. FA allows the clinician to identify more subtle extraretinal neovascularization, which is the hallmark of stage 3 ROP and a pivotal point in the determination of treatment in Zone I and posterior Zone II. Techniques such FA could play a central role in understanding mechanical and functional implication of angiogenesis in ROP. By using FA, we are able to assess neovascularization, vessel leakage, loss of capillary beds and other hypo-perfused areas of the retina and choroid that could represent another driving force of VEGF-induced clinical signs of ROP. The details now documented using FA that are not detected on color fundus photography or binocular indirect ophthalmoscopy will likely lead to new technologies applications. Integration of FA, as well as other different imaging techniques, into the management of ROP at specific levels of risk could be an important future direction of investigation as it has been for other retinal diseases. Furthermore, the question remains how these findings will contribute to our understanding of retinal vascular development and neuroretinal migration and differentiation. Answering these questions has the potential to open new therapeutic options and perspectives in ROP and also identify those infants who require increased surveillance as they have a high likelihood of developing severe ROP.
Summary
What was known before
-
Fluorescein angiography (FA) can show several signs of vasculopathies undetected by ophthalmoscopy nor by other imaging techniques
What this study adds
-
Early FA can unveil signs that preclude evolution to treatment-requiring retinopathy of prematurity
References
International Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102:1130–4. http://www.ncbi.nlm.nih.gov/pubmed/6547831.
Aaberg T, Ben-Sira I, Charles S, Clarkson J, Cohen BZ, Flynn J. et al. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1987;105:906–12. http://archopht.jamanetwork.com/article.aspx?doi=10.1001/archopht.1987.01060070042025.
International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9. http://archopht.jamanetwork.com/article.aspx?doi=10.1001/archopht.123.7.991.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 1988;106:471–9. http://archopht.jamanetwork.com/article.aspx?doi=10.1001/archopht.1988.01060130517027.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119:1110–8. http://insights.ovid.com/crossref?an=00132578-200204000-00018.
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity. Arch Ophthalmol. 2003;121:1684–94. http://archopht.jamanetwork.com/article.aspx?doi=10.1001/archopht.121.12.1684.
Reynolds JD, Dobson V, Quinn GE, Fielder AR, Palmer EA, Saunders RA. et al. Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol. 2002;120:1470–6. http://archopht.jamanetwork.com/article.aspx?doi=10.1001/archopht.120.11.1470.
The Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010;128:663–71. http://archopht.jamanetwork.com/article.aspx?doi=10.1001/archophthalmol.2010.72.
Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials Macular Photocoagul Study group. Arch Ophthalmol. 1991;109:1109–14. http://www.ncbi.nlm.nih.gov/pubmed/1714270.
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC. et al. Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125:1608–22. http://www.ncbi.nlm.nih.gov/pubmed/29776671.
Flynn JT, O’Grady GE, Herrera J, Kushner BJ, Cantolino S, Milam W. Retrolental fibroplasia: I. Clinical observations. Arch Ophthalmol. 1977;95:217–23. http://archopht.jamanetwork.com/article.aspx?doi=10.1001/archopht.1977.04450020019002.
Flynn JT, Cassady J, Essner D, Zeskind J, Merritt J, Flynn R. et al. Fluorescein angiography in retrolental fibroplasia: experience from 1969–1977. Ophthalmology. 1979;86:1700–23. https://linkinghub.elsevier.com/retrieve/pii/S0161642079353295.
Cantolino SJ, O’Grady GE, Herrera JA, Israel C, Justice J, Flynn JT. Ophthalmoscopic monitoring of oxygen therapy in premature infants. Fluorescein angiography acute retrolental fibroplasia. Am J Ophthalmology. 1971;72:322–31. http://www.ncbi.nlm.nih.gov/pubmed/5109723.
Anon. Color photography vs fluorescein angiography in the detection of diabetic retinopathy in the diabetes control and complications trial. Arch Ophthalmol. 1987;105:1344–51. http://archopht.jamanetwork.com/article.aspx?articleid=636912.
Ng EYJ, Lanigan B, O’Keefe M. Fundus fluorescein angiography in the screening for and management of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2006;43:85–90. http://www.ncbi.nlm.nih.gov/pubmed/16598974.
Lepore D, Molle F, Pagliara MM, Baldascino A, Angora C, Sammartino M, et al. Atlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurity. Ophthalmology. 2011;118:168–75. https://doi.org/10.1016/j.ophtha.2010.04.021.
Klufas MA, Patel SN, Ryan MC, Patel Gupta M, Jonas KE, Ostmo S. et al. Influence of fluorescein angiography on the diagnosis and management of retinopathy of prematurity. Ophthalmology. 2015;122:1601–8. http://linkinghub.elsevier.com/retrieve/pii/S0161642015004054.
Lepore D, Quinn GE, Molle F, Orazi L, Baldascino A, Ji MH. et al. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2017;125:1–9. http://linkinghub.elsevier.com/retrieve/pii/S0161642017312241.
Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. http://www.ncbi.nlm.nih.gov/pubmed/3719049.
Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity report on fluorescein angiographic findings. Ophthalmology. 2014;121:2212–9. https://doi.org/10.1016/j.ophtha.2014.05.015.
Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB. et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmol. 1991;98:1628–40. http://www.ncbi.nlm.nih.gov/pubmed/1800923.
Saunders RA, Bluestein EC, Sinatra RB, Wilson ME, O’Neil JW, Rust PF. The predictive value of posterior pole vessels in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2018;32:82–5. http://www.ncbi.nlm.nih.gov/pubmed/7629674.
Schaffer DB, Palmer EA, Plotsky DF, Metz HS, Flynn JT, Tung B. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1993;100:230–7. http://www.ncbi.nlm.nih.gov/pubmed/8437832.
Davitt BV, Wallace DK. Plus disease. Surv Ophthalmol. 2009;54:663–70. http://linkinghub.elsevier.com/retrieve/pii/S0039625709000666.
Ibayashi H, Nishimura M, Yamana T. Avascular zone in the macula in cicatricial retinopathy of prematurity. Am J Ophthalmol. 1985;99:235–9. https://linkinghub.elsevier.com/retrieve/pii/0002939485903502.
Mansukhani SA, Hutchinson AK, Neustein R, Schertzer J, Allen JC, Hubbard GB. Fluorescein angiography in retinopathy of prematurity: comparison of infants treated with bevacizumab to those with spontaneous regression. Ophthalmol. Retin. 2019: 1–8. https://doi.org/10.1016/j.oret.2019.01.016.
FA team of the Italian ROP study Group
Antonio Baldascino1, Velia Porcaro6, Patrizia Papacci6, Giovanni Vento6, Elena Gusson7, Giorgio Marchini7, Silvia Pignatto8, Paolo Lanzetta8, Elena Piozzi9, Marco Mazza9, Giovanni Marsico9, Giuseppina Fortunato10, Roberto Caputo10, Fernando Molle1
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the FA team of the Italian ROP study Group are listed below Summary.
Rights and permissions
About this article
Cite this article
Lepore, D., Ji, M.H., Ying, Gs. et al. Early angiographic signs of retinopathy of prematurity requiring treatment. Eye 35, 3094–3101 (2021). https://doi.org/10.1038/s41433-020-01305-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01305-z